Brain volume increase and neuronal plasticity underly predator-induced morphological defense expression in daphnia longicephala
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Predator-induced phenotypic plasticity describes the ability of prey to respond to an increased predation risk by developing adaptive phenotypes. Upon the perception of chemical
predator cues, the freshwater crustacean _Daphnia longicephala_ develops defensive crests against its predator _Notonecta_ spec. (Heteroptera). Chemical predator perception initiates a
cascade of biological reactions that leads to the development of these morphological features. Neuronal signaling is a central component in this series, however how the nervous system
perceives and integrates environmental signals is not well understood. As neuronal activity is often accompanied by functional and structural plasticity of the nervous system, we
hypothesized that predator perception is associated with structural and functional changes of nervous tissues. We observe structural plasticity as a volume increase of the central brain,
which is independent of the total number of brain cells. In addition, we find functional plasticity in form of an increased number of inhibitory post-synaptic sites during the initial stage
of defense development. Our results indicate a structural rewiring of nerve-cell connections upon predator perception and provide important insights into how the nervous system of prey
species interprets predator cues and develops cost–benefit optimized defenses. SIMILAR CONTENT BEING VIEWED BY OTHERS BEHAVIOURAL AND NEURAL RESPONSES OF CRABS SHOW EVIDENCE FOR SELECTIVE
ATTENTION IN PREDATOR AVOIDANCE Article Open access 15 June 2022 EVOLUTION OF SCHOOLING DRIVES CHANGES IN NEUROANATOMY AND MOTION CHARACTERISTICS ACROSS PREDATION CONTEXTS IN GUPPIES Article
Open access 27 September 2023 PREDATION THREATS FOR A 24-H PERIOD ACTIVATED THE EXTENSION OF AXONS IN THE BRAINS OF _XENOPUS_ TADPOLES Article Open access 16 July 2020 INTRODUCTION
Phenotypic plasticity describes the ability of an organism with a given genotype to adapt its phenotype in response to changing environmental conditions1. One well-known example of
phenotypic plasticity is inducible defenses. Here, the presence of predators, indicated by chemical cues (kairomones), induces defensive features in the prey, which make them less
susceptible to predation. The freshwater crustacean _Daphnia_ is a prime example of predator-induced defenses2, which range from morphological features such as helmets in _D. cucullata_3,
neck teeth in _D. pulex_4 or crests in _D. longicephala_5 to changes in life history6,7 or adaptive behavior8. Interestingly, morphological defenses are not only expressed in a
predator-density specific manner, but rather they are fine-tuned to the predation risk, so that also the conspecific density is decisional for the degree of defense expression9. Such
condition-dependent adjustments require a nervous system capable to decipher different chemical cues from the heterogeneity of many environmental factors and transfer this into a
cost–benefit optimized phenotype. _Daphnia_ perceive chemical cues via the antennules, upon which a signaling cascade involving cholinergic, dopaminergic, glutamatergic and GABAergic
components is initiated, which ultimately leads to the transformation of the undefended into the defended phenotype10,11,12,13,14,15. It is still unknown how the nervous system detects and
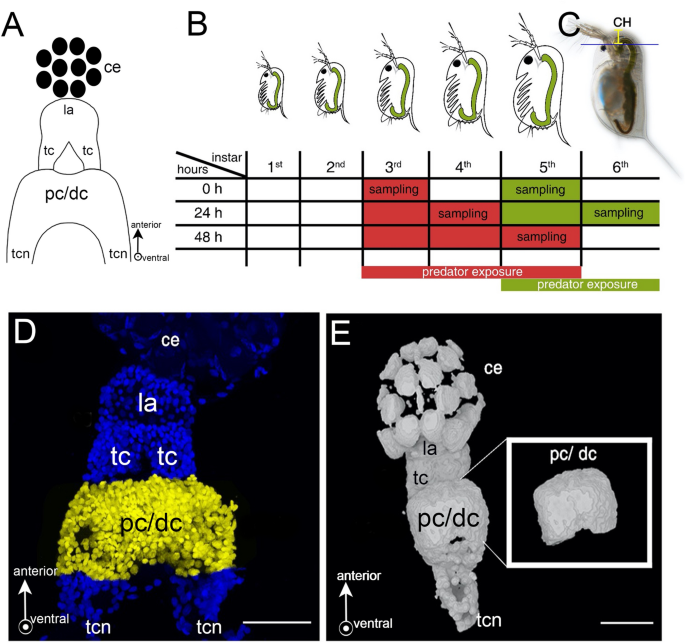
integrates environmental information when the _Daphnia_ brain morphologically appears rather simple (Fig. 1A). It is subdivided into three main neuropils: protocerebrum, deutocerebrum and
tritocerebrum16. The protocerebrum receives direct visual input from the compound eye via the optic ganglia (lamina and tecta) and chemosensory input from the antennules via the
deutocerebrum16. Via the circumoral connectives, the protocerebrum is connected to the tritocerebrum. This consists of two ganglia inferior to the esophagus that are transversally connected
by the tritocerebral commissures. These two neuropils connect with the mandibular ganglia and extend into the rope-ladder-like nerve cord innervating the body and appendages16. Between these
three neuropils, the protocerebrum comprises the largest part of the brain and is discussed to be centrally involved in the analysis and interpretation of environmental sensory signals17.
Such environmental signal integration is often associated with changes in form and function of nervous systems. While changes in form are referred to as structural brain plasticity, changes
in function are referred to as functional plasticity18. Structural plasticity has been reported in vertebrates and invertebrates, e.g. rats living in complex environments show larger brains
with larger cells and more dendritic fields than rats raised in homogeneous, uncomplex environments19. Tadpoles of the leopard frog develop smaller and narrower brains upon an emerging
predator threat20. Similarly, different insect species have been found to undergo structural brain plasticity associated with learning, food search and novel tasks21,22,23,24. In honeybees
certain brain regions, i.e. the mushroom bodies in the protocerebrum, experience anatomical reorganization associated with labor division due to differences in foraging experience25.
_Drosophila_ develop larger brain structures in diversified environments than in isolation26. All these structural changes are discussed to be cost–benefit optimized so that the neuronal
structures become as large as needed in order to be highly effective for the given task. At the same time brain size is limited as nervous tissues are energetically demanding27. In contrast
to structural brain plasticity, functional brain plasticity affects the nervous system´s function by altering the abundance and reactive strength of neuronal connections28. Neuronal
connections are e.g. made at chemical synapses. These consist of a presynaptic bouton with the active zone (AZ), responsible for the regulated release of neurotransmitters, and the
postsynaptic density (PSD), representing the neurotransmitter reception apparatus. These synaptic sites are intermitted by the synaptic cleft into which neurotransmitters are released.
Neuronal activity can either trigger neurotransmitter release at excitatory synapses, increasing the activity of the postsynaptic neuron, or at inhibitory synapses, reducing neuronal
activity29. Functional plasticity by means of differences in postsynaptic receptor abundance has been shown in the avian brain during the filial imprinting process30 and in mammals
experiencing enriched environments19,31. As space is the factor limiting the number of synaptic connections in one neuron32, only an enlargement of the neuronal surface area can increase
synaptic connections. The surface area can increase through an enlarged cell volume and when many cells enlarge their volume, the overall neuropil mass will increase and is therefore a
component of structural plasticity18. Thus, structural and functional brain plasticity are closely related as the formation of new neuronal connections requires space19 and may therefore be
able to shape neuropil form and size. Especially inhibitory synapses are important with regard to the control and regulation of overall neuronal excitability within neuronal networks of
cognitive processes33,34. The scaffolding protein gephyrin is a central component of the inhibitory nervous system, as it anchors GABAA and glycine receptors at the PSD35. In addition, this
protein is involved in post-Golgi transport of the respective receptors to the PSD36. Taken together, gephyrin is a good indicator of functional plasticity, as it indicates activity changes
of the inhibitory PSD by receptor shuffling. Here, we investigated structural and functional brain plasticity underlying predator perception in _Daphnia longicephala._ As cognition is a
central task of the protocerebrum37, we focused on the proto- and deutocerebrum (pc/dc-complex), which are indistinct in _Daphnia_. We determined pc/dc-complex volume together with a
reorganization of neuronal circuitry by determination of the number of neuronal cells. To exclude that alterations in pc/dc-complex volume are a result of the predator induced overall body
growth and the crest, we also determined the volume of the optic ganglia (OG). Additionally, we investigated cells expressing the anchoring protein gephyrin in pc/dc-complexes of
predator-exposed and control _D. longicephala_. In order to exclude an age-dependent effect, we conducted the experiment at two different developmental points in time. We demonstrate that
there is structural and functional plasticity independent of developmental age associated with predator perception. MATERIAL AND METHODS _DAPHNIA LONGICEPHALA_ CULTURE _D. longicephala_
clone LP1 (Lara-Pond, Australia) was cultured in artificial _Daphnia_ medium (ADaM (Klüttgen et al. 1994)) in 1 L beakers (Weck®, Germany) containing 20–25 age-synchronized individuals under
constant day:night conditions (16 h:8 h) at 20 ± 1 °C. Animals were fed ad libitum with the algae _Acutodesmus obliquus_. The beakers were cleaned every 48 h. Half of the medium was
exchanged weekly. PREDATOR CULTURE Backswimmers (_Notonecta_ spec.) were collected from the ponds of the Ruhr-University Botanical Gardens. Animals were maintained in 1 L beakers (WECK®,
Germany) filled with charcoal-filtered tap water under standardized conditions (20 ± 1 °C with 16 h:8 h light:dark cycle). They were fed regularly ad libitum with _Daphnia_ spec.
EXPERIMENTAL CONDITIONS All animals of the culture and the experiments were maintained in ADaM and reared at 20 °C ± 0.1 °C in incubators at constant light with a 16 h:8 h day:night cycle.
EXPERIMENTAL SETUP We collected 14 h (± 1 h) old _D._ _longicephala_ from age-synchronized cultures and reared 30 animals per 1 L beaker. Once the animals reached the 3rd instar i.e., after
the successful completion of two molting cycles, they were transferred into predator exposure or control conditions. For the predator exposure, net cages (mesh size 100 µm) containing one
_Notonecta_ spec. were placed into 1 L beakers. Notonectids were fed with five _D._ _longicephala_ per day to ensure continuous kairomone exposure. The experimental _D. longicephala_ were
located outside of the net cage, which prevented predators from feeding on the test specimens. Predator exposure was conducted for 48 h until _D. longicephala_ reached the 5th instar.
Control treatments were cultivated accordingly, but without predators. Control and predator-exposed specimens were collected at explicit points in time and treatment from the moment of
predator exposure i.e. 0 h, 24 h, and 48 h. Per point in time and treatment, we collected 20 to 30 _D._ _longicephala_ (Fig. 1B, red). The complete experimental set-up was repeated 9 times.
Since _D. longicephala_ is responsive to predator cues also at later stages15, we also conducted experiments where predator exposure started in the 5th instar (Fig. 1B, green) focusing on
the relevant points in time i.e. 0 h and 24 h and replicated this 3 times. MEASUREMENT OF DEFENSIVE FEATURES We measured defense expression at above-described points in time using a SZX16
stereomicroscope (Olympus, Japan) equipped with a TSO digital-camera (TSO-KST 6000849) controlled by the software Vidmess Version 3.0 (TSO Thalheim Spezialoptik GmbH, Germany). We measured
the crest height from the upper margin of the compound eye to the distal height of the crest (Fig. 1C). IMMUNOHISTOCHEMISTRY Animals were fixed using 4% PFA (formaldehyde (J.T. Baker)
diluted in phosphate-buffered saline (PBS) 0.1 M; pH 7.4) for 5 min. Then, we dissected the pc/dc-complex, still attached to the optic ganglia and compound eye (prospectively referred to as
‘brain mounts’) using fine forceps. We used the dark pigmented compound eye as a visual guide to facilitate working with the small and unpigmented neuronal tissue. Brain mounts were
transferred to poly-L-lysine coated object slides (VWR, Germany). All subsequent incubation steps were performed in a humidified chamber and on an orbital shaker (neoLab®, Germany) at 15 rpm
and 4 °C. Brain mounts were post-fixed for 10 min in 4% PFA and rinsed with PBS 3*5 min. Fixed brain mounts were blocked with 5% goat normal serum (Dianova, Germany) diluted in PBS-TX 0.1%
(PBS, 0.1 M; pH 7.4 with 0.05% Triton-X 100; Sigma Aldrich, Germany) for 3 h. Subsequently, we applied the primary antibody anti-gephyrin raised in mouse (Cat. No. 147011; Synaptic Systems,
Germany; RRID:AB_887717; 1:100 in PBS-TX 0.1%). Samples were incubated overnight and then washed with PBS-TX 0.1% 3*10 min. The secondary antibody anti-mouse Alexa488 (Dianova, Germany;
RRID:AB_2338845) was applied (1:250 diluted in PBS-TX 0.1%) and incubated for 2 h. Brain mounts were rinsed 3*10 min with PBS-TX 0.1%, mounted in DAPI-Vectashield (Vector Laboratories, USA)
for nuclei staining and preservation. Slides treated with secondary antibody only and DAPI-Vectashield served as negative controls (Fig. S1A, B). In order to confirm that gephyrin is
associated with inhibitory glycinergic neurons in _Daphnia_, we performed a double staining using the anti-gephyrin antibody and an anti-glycine receptor (GlyR) antibody raised in rabbit
(Cat. No. 146008; Synaptic Systems, Germany; RRID:AB_2636914) (1:50 diluted in PBS-TX 0.1%). This antibody binds to the glycine receptor α1-subunit. Detection was performed with the
secondary antibody anti-rabbit594 (1:250) (Dianova, Germany; RRID:AB_2307325) on brain mounts of adult _D. longicephala_. Every step was performed as described above. Antibody validation is
described in the supplement (Fig. S2). CONFOCAL IMAGES We acquired image stacks of the _D. longicephala_ brain mounts using a confocal laser scanning microscope (SP5II, Leica Microsystems,
Wetzlar, Germany) and Leica cLSM (SP8, Leica Microsystems, Germany). Image stacks were acquired with a 40× water immersion objective, a 63× and a 100× oil immersion objective. Step intervals
were chosen between 0.5 and 0.8 µm, according to nuclear size ensuring the recording of all cells in one stack. Figures were composed and labelled in Photoshop CS 4 (Adobe Systems, San
Jose, CA). Brightness, saturation, and contrast were optimized; no further image editing was conducted. CELL COUNTING AND VOLUMETRIC MEASUREMENT IN PC/DC-COMPLEXES AND OG 3D projections of
captured image stacks were composed in ImageJ 1.52n (Fiji)38. The total number of nerve cells (identified with the nuclear marker 2-[4-(Aminoiminomethyl)phenyl]-1H-Indole-6-carboximidamide
hydrochloride (DAPI) and the number of gephyrin-labelled cells in the pc/dc-complex were counted manually in 3D projections using the ‘multipoint tool’ (Fig. 1D). To determine the
pc/dc-complex and OG volume, we used cLSM image stacks of the brain mount DAPI staining and created tif-stacks with Fiji for MorphoGraphX import39 (Fig. 1E). Here, we used the ´Gaussian
Blur` with a rate of 1 µm in each direction. Using ´Marching Cubes Surface` we created a mesh surface with a cube size of 1 µm. Since we aimed to measure only the pc/dc-complex or OG volume
respectively, we removed the surrounding parts of the brain mount using the ´pixel edit` tool (Fig. 1E insert). Each stack was saved as ply-file before conversion into stl-files using
MeshLab40. These files were required for the analysis in Blender, an open-source 3D graphics and animation package. The stl-files were opened in Blender and the volume was calculated with
the ‘3D printing’ and ‘volume’ function as described in Horstmann et al.41 (Fig. 1E). OG volume was only measured in individuals that were exposed from the 3rd to the 5th instar. STATISTICAL
ANALYSES We tested for normal distribution using a Shapiro–Wilk test. Only crest height, and brain volume followed a normal distribution, so that all other data was log-transformed. To
analyze crest expression in line with structural and functional plasticity across three instars starting from the third to the fifth instar we used a one-way analysis of variance (ANOVA)
with instar as factor. By this we determined age-dependence of the measured parameters (i.e. crest height, brain volume, OG volume, log(#gephyrin-labelled cells), log(#DAPI-labelled cells),
log(#gephyrin/#DAPI-labelled cells) individually per treatment (control; induced), as the interaction of stage and instar was irrelevant to our hypothesis. Post hoc comparisons to determine
differences between individual instars were performed using a Tukey Test. We then determined differences between control and predator-induced groups using a Student’s t-test for each
developmental instar and adjusted the significance threshold for multiple testing using the alpha-correction. The analysis of the control experiment using a later instar only comprised two
instars, so that we investigated for differences between instar and treatment using a Student’s t-test. Levels of significance were again adjusted for multiple testing. Data is displayed
untransformed. Statistics were performed in STATISTICA 14 (Statsoft inc.) plots were made in R using the ggplot2 package42,43. RESULTS INDUCIBLE DEFENSES Crests are constant across instars
in the control group but grow significantly larger in the predator-induced treatment. Across instars, defenses grow significantly, so that we find a significantly increased crest height in
predator-exposed animals after 48 h (5th instar) compared to 0 h (3rd instar, Fig. 2A; Tables S1, S2). Within this time, crests increased 1.2-fold in the predator exposed treatment and
remain constant in size in the control treatment (1.0×). We find a tendency towards an increased crest expression in predator-exposed animals compared to controls after 24 h (4th instar;
(_P_ = 0.061; t = − 1.91; df = 57) and a significantly increased crest height after 48 h (5th instar; _P_ = 0.01; t = − 2.64; df = 28, Table S3). Individuals that were exposed to predator
cues in the 5th instar also showed an increased crest expression after 24 h predator exposure (_P_ ≤ 0.001; t = 4.18; df = 44; Fig. 2B; Tables S4, S5). STRUCTURAL PLASTICITY IN THE
PC/DC-COMPLEX VOLUMETRIC CHANGES OF THE PC/DC-COMPLEX We observed increasing pc/dc-complex volumes in both treatments with age. Naïve, control individuals showed a mean volume of 249,348.70
µm3 ± 70,955.12 in the 3rd instar and a mean volume of 353,098.40 ± 142,285.70 µm3 in the 5th instar (Fig. 3A, Tables S1, S2). In predator-exposed animals pc/dc-complex volume increased
significantly from 247,623.70 µm3 ± 97,067.05 µm3 in the 3rd instar to 451,761.40 µm3 ± 146,165.9 µm3 in the 4th instar and to 565,640.5 µm3 ± 340,501.1 µm3 in the 5th instar (Fig. 3A;
Tables S1, S2). We did not observe volumetric changes in pc/dc-complexes of naïve individuals from the 5th to the 6th instar (Fig. 3B, Table S4). Predator-exposed _D. longicephala_ showed an
increase in pc/dc-volumes after 24 h (mean 5th instar: 310,848.2 µm3 ± 122,657 µm3; mean 6th instar: 424,134.2 µm3 ± 43,543.65 µm3; Fig. 3B, Table S5). When animals were exposed to the
predator in the 3rd instar, no significant difference between control and predator-exposed _D. longicephala_ pc/dc-complex volumes was observed at 0 h (Table S3). Twenty-four hours later,
pc/dc-complex volume in predator-exposed individuals was significantly larger than in 24 h controls with a mean of 451,761.40 ± 146,165.90 µm3 (Fig. 3A, Table S3). In 48 h predator-exposed
_D. longicephala_, the volume was also significantly increased compared to the control (mean induced: 565,640.50 µm3 ± 340,501.1; Fig. 3A, Table S3). During this time the pc/dc volume
increases 1.4-fold in control animals, and 2.2-fold in predator exposed animals. Accordingly, among individuals exposed in the 5th instar, we detected a significant increase in pc/dc-complex
volumes of predator-exposed animals compared to the control after 24 h (mean control: 350,290.4 µm3 ± 81,781.9 µm3, mean induced: 424,134.0 µm3 ± 43,543.70; Fig. 3B; Tables S4, S5).
VOLUMETRIC CHANGES OF THE OG Control and predator-exposed _D. longicephala_ show an increase in OG volume with increasing age so that animals in the 5th instar have significantly larger OG
than animals in the 3rd instar (mean control 3rd instar: 75,837.76 µm3 ± 21,080.22 µm3, mean control 5th instar: 226,191.2 µm3 ± 63,729.89 µm3; mean induced 3rd instar: 89,783.36 µm3 ±
20,623.36 µm3, mean induced 5th instar: 259,253.9 µm3 ± 81,789.8 µm3; Fig. 3A; Tables S1, S2). We observed no significant differences in OG volume between control and predator-exposed
individuals at any point in time (Fig. 3A; Tables S3, S4, S5). Optic ganglia of control specimens increase by 2.9-fold and of predator induced specimens by 2.88-fold over the developmental
stages. NERVE CELL NUMBERS IN THE PC/DC-COMPLEX We detected a tendency towards an increased number of cells in the pc/dc-complex of naïve _D._ _longicephala_ from 734.86 ± 180.13 cells in
the 3rd to 903.18 ± 319.73 cells in the 5th instar (Fig. 3C; Tables S1, S2). Across developmental stages the number of cells increase 1.23-fold irrespective of the treatment. Between control
and predator-exposed specimens, we did not detect differences in nerve cell numbers at any point in time (Fig. 3C, D; Tables S3, S4). FUNCTIONAL PLASTICITY IN THE PC/DC-COMPLEX
CHARACTERIZATION OF SYNAPTIC COMPOSITION Cells labelled with anti-gephyrin and anti-GlyR antibodies were found throughout the whole pc/dc-complex tissue (Fig. S3, Fig. 4A–C). We found
gephyrin in the cytoplasm and on the surface of the cell soma (Fig. 4D–F). GlyRs are limited to the cell surface in colocalization with gephyrin (Fig. 4G–I). REORGANIZATION OF INHIBITORY
SYNAPTIC SITES In control animals the number of gephyrin-labelled cells did not change over time (Fig. 5A; Tables S1, S2). In individuals that we exposed to predators from the 3rd instar
onwards, the number of gephyrin-labelled cells increased significantly from a mean of 352.62 ± 105.41 cells (0 h) to 440.62 ± 138.76 cells within 24 h (Fig. 5A; Tables S1, S2). In 48 h
predator exposed specimens the number of gephyrin-labelled cells remained constant in comparison to 24 h exposed specimens (Fig. 5A; Tables S1, S2). At 0 h no significant difference in the
number of gephyrin-labelled cells was detected between the control and predator-exposed group (Fig. 5A; Table S3). After 24 h predator exposure individuals showed a significantly increased
number of gephyrin-labelled cells compared to the control (Fig. 5A; Table S1). After 48 h predator exposure no significant difference in the number of gephyrin-labelled cells was detected
between the two treatments (Fig. 5A; Table S1). In individuals that we exposed from the 5th instar onwards the number of gephyrin-labelled cells in predator-exposed _D. longicephala_ was
significantly increased in comparison to the control after 24 h predator exposure (Fig. 5B, Table S4). At the beginning of the experiment and prior to predator exposure (0 h), we detected no
significant differences between the gephyrin-labelled cells per absolute cell number in the two treatments (Fig. 5C; Table S3). After 24 h predator-exposed individuals showed a
significantly higher ratio than controls (Fig. 5C; Table S3). 48 h after initial predator exposure no significant differences between the treatments were detected (Fig. 5C; Table S3). The
ratio of gephyrin-labelled cells/neurons in individuals that we exposed from the 5th instar onwards, was significantly increased in predator-exposed individuals compared to controls after 24
h predator exposure as well (Fig. 5D; Table S5). DISCUSSION INDUCIBLE DEFENSE EXPRESSION When we expose _D. longicephala_ to predators in the 3rd instar, we observe a tendency towards
larger crests in the 4th instar and significant crest expression in the 5th instar. We observe a similar result when we expose the animals in the 5th instar, upon which crests are also
significantly expressed 24 h later. This indicates that predator exposure gradually induced crest expression within a short time frame of 24 h, so that defenses become effective in a fast
manner as reported previously15. STRUCTURAL PLASTICITY IN THE PC/DC-COMPLEX VOLUMETRIC CHANGES OF THE PC/DC-COMPLEX AND THE OG As the main cognitive functions are performed by the
protocerebrum, we focused on the pc/dc-complex. The mean volume of naïve _D. longicephala_ pc/dc-complexes increases 1.4-fold over the observed instars showing the highest volume in the 6th
instar with ~ 350,000 µm3. Larvae of the fruit fly _Drosophila_ show a total brain volume of ~ 800,000 µm3 44 and adult flies even ~ 80 Mio µm3 45, with a body size similar to that of adult
_D. longicephala_. When exposed to predators for 24 h and 48 h, _D. longicephala_ showed significantly larger pc/dc-complex volumes than the respective controls. We detected this
predator-induced volumetric increase independent of the _Daphnias’_ age. Therefore, the growth of the morphological defense is accompanied with the growth of the pc/dc-complex. Our results
show that the volume of predator-induced animals’ pc/dc-complexes more than doubles (2.2-fold) within the first 48 h of predator exposure from ~ 250,000 µm3 to a volume of ~ 565,000 µm3.
This means that predator-exposed individuals show a 60% larger pc/dc-complex compared to controls after 48 h. In _Drosophila,_ different brain structures have been observed to increase in
response to diversified environments that are e.g. enriched with food vials, odor sources and conspecifics. The _Drosophila_ central brain is probably best comparable to the pc/dc- complex
in _Daphnia,_ and this structure increased its volume by 7.5% when encountering complex environments for 19 days46. The largest volumetric increase of 21% was observed in the calyx, a part
of the mushroom bodies26. The volumetric increase of the _Daphnia_ pc/dc-complex is three times as high as the largest volumetric increase in _Drosophila_ brains. Furthermore, the increase
in the _Daphnia_ pc/dc-complex appears much faster and reflects a strong structural plasticity in a very short time. This may be an important factor when coping with predators, as the
defenses need to be expressed in due time so that strong and rapid structural plasticity is probably selected for. Mechanistically, such a high degree of volumetric expansion can only be
achieved if either the absolute number of cells, the cell size or intercellular distances increase. Larger intercellular distances provide additional space for cell- cell connections in the
form of dendrites that expand the cell surface. It is reasonable to suggest that the basis of such novel cell–cell connections is established during the first 24 h of predator perception.
Since we detected no volumetric changes in the OG between the observed treatments, the functional plasticity observed in the pc/dc-complex has to be a result of predator-exposure. This
supports the hypothesis that the pc/dc-complex plays a central role in inducible defense expression. NERVE CELL NUMBERS IN THE PC/DC-COMPLEX The 1.4-fold pc/dc-complex volume increase that
we observe with age and the 2.2 fold increase that we observe with predator exposure must originate from some kind of gain in neuronal tissue. Therefore, we determined the overall number of
cells, irrespective of their type, in the pc/dc-complex. With increasing instars, we detected a 1.2-fold increase in cell numbers within the pc/dc-complex. Cell numbers increased from ~ 750
cells in the 3rd instar to ~ 900 cells in the 6th instar. So, with age the animals obtain more cells in this central complex. As the animals grow continuously throughout life, it is possible
that the number of cells in the brain continues to increase. In fact, a life-long addition of new neurons, e.g. in the olfactory pathways of insects47 and crustaceans48,49,50, has already
been reported. Similarly, the continuous increase in body size requires the addition of new receptor neurons to cope with the increased surface area of the body, the length of the appendages
or the size of the compound eye51,52,53. This probably also leads to the observed volumetric increase in the OG with increasing age (i.e. ~ 2.9-fold increase in control and predator exposed
_D. longicephala_). The 1.23-fold increase in the number of brain cells in the pc/dc-complex lies within the range of the volumetric changes across instars (1.4-fold), so that there may be
a direct correlation between volume and cell number. However, this correlation only holds true when comparing successive instars. Between control and predator-exposed animals, we did not
observe that the 2.2-fold increase in brain volume is due to an increase in the cell number (i.e. only 1.23-fold). Therefore, the predator-induced increase in pc/dc-complex volume must rely
on other factors, e.g. less dense brain tissues and/or larger neurons in predator-exposed _D._ _longicephala_. In fact, in rats it has already been reported that an enriched and demanding
environment leads to increased brain volume based on reduced cell density and increased cell size but the number of neurons in the brain tissue was not affected (reviewed in32). It can be
speculated that larger intercellular clefts provide more space for structures like dendrites and synapses, while bigger cell bodies provide a larger surface area on which more synapses can
attach. This has been observed in the visual cortex of rats19. Individuals exposed to a complex environment had larger neurons, allowing the attachment of 20% more synapses per neuron than
rats from less complex environments. It was hypothesized that a larger neuron size may improve cellular stability and efficiency. Likewise, larger cells may also be required to ensure the
metabolic support of more extensive dendritic fields19. As synaptic density remains unaltered in plastic brain tissue32, an increased neuron size is necessary for the attachment of
additional synapses. Therefore, the here observed increase in pc/dc-complex volume is not correlated with an increase of cell numbers but must depend on an increase in inter-neuronal
connections. We hypothesize that there is a predator-induced rewiring of inter-neuronal connections i.e. through the development of new dendrites, increasing intercellular distances and thus
the overall volume. Likewise, we anticipate that neuron size is increased, which enables the attachment of more synapses and enhances cell–cell stability. This may contribute to the
formation of a dedicated neuronal circuitry that is associated with predator perception. FUNCTIONAL PLASTICITY IN THE PC/DC-COMPLEX REORGANIZATION OF INHIBITORY SYNAPTIC SITES In order to
investigate potential remodeling and/or emergence of a dedicated cell circuitry, we focused on the number and distribution of inhibitory cell synapses indicated by the scaffolding protein
gephyrin. The staining pattern of gephyrin shows intensive markings in the cytoplasm and on the surface of the cell soma, which is typical for this antibody and explained by the fact that
gephyrin is involved in post-Golgi transport and cell surface delivery of GlyRs36. In contrast, GlyRs are observed with a typical dot-like staining pattern on the cell membranes. Gephyrin
was found in colocalization with GlyRs, and all cells that stained with the anti-GlyR antibody showed a colocalization with the gephyrin antibody. This colocalization clearly demonstrates
that also in _Daphnia_, gephyrin is the anchoring protein for receptors like GlyRs at inhibitory PSDs. The fact that we observe gephyrin-labelled cells that do not co-localize with GlyRs
indicates that gephyrin also anchors other inhibitory receptors like the GABAA receptor known from other species33. Unfortunately, we so far did not find a specific GABAAR antibody to test
for this co-localization and therefore this awaits further investigation. Both, gephyrin as well as GlyRs are homogeneously distributed throughout the pc/dc-complex. We did not observe any
kind of neuropil concentric localization of gephyrin that would point to a specialized brain area. This pattern maintained also throughout predator exposure. However, after 24 h of predator
exposure, the number of gephyrin-labelled cells in the pc/dc-complex is significantly increased compared to controls. As the number of neurons is stable between treatments, the ratio of
gephyrin-labelled cells per neuron is also significantly higher in predator-exposed individuals after 24 h. This coincides with the onset of crest development and is again age independent.
An increased number of cells expressing gephyrin indicates the presence of more inhibitory synaptic connections, as gephyrin anchors and clusters GABAARs and GlyRs at the postsynaptic
site35. Our findings show that the number of cells possessing inhibitory synapses in pc/dc-complexes of predator-exposed _D._ _longicephala_ increases in line with the morphological defense
expression. It is possible that glycinergic signals play a role in the pathway underlying predator perception. Controlling the excitability of nerve cells is central to ensuring normal brain
function54, and increased inhibition could thus lead to a more directional excitability that focuses on crucial functions. Hence, adapted inhibition patterns in the _Daphnia_ brain might be
a central element of predator perception. While the involvement of glycinergic responses is still unknown, the involvement of GABAergic signaling in predator perception has been
demonstrated in _D. pulex_ responding to fish and _Chaoborus_ predation13_._ It is very likely that the emergence of additional GABAARs occurs upon the perception of predator cues.
Functionally, GABAARs are a central component of synaptic plasticity by ensuring that glutamatergic N-methyl-D-aspartate receptors (NMDARs) are predominantly activated during high frequency
synaptic transmission only55. Especially, NMDARs play a fundamental role in long-term potentiation (LTP) and long-term depression (LTD), two common forms of synaptic plasticity responsible
for fostering cell–cell connections56,57. It is therefore possible that such mechanisms play a role in deciphering and determining the predation risk amongst the diversity of environmental
cues. We only observe this increase in gephyrin-labelled cells in the instar that coincides with the onset of defense expression i.e. 24 h of predator exposure. In the later stage, we do not
see differences in the number of gephyrin-labelled cells in comparison to the control group. However, although defense expression persists in the following instar i.e. after 48 h predator
exposure, we detect no further changes in gephyrin abundance between control and predator-exposed individuals, which is an effect of a slight and not significant increase of the total number
of nerve cells in the controls. With the increase in the total number of cells, also the proportion of gephyrin-labelled cells increases. Even if this increase is not significant, it
diminishes the differences between the predator-induced and control groups in 5th instar specimens and is a result of the gradual growth of the animals. Nevertheless, the number of
gephyrin-labelled cells remains at a constant level, so that there are no differences between 4th and 5th instar predator exposed specimens. We hypothesize that there is a dedicated rewiring
event during the initial phase of predator exposure. Once this rewiring is accomplished, this new neuronal circuitry is manifested, indicated by the unchanged number of gephyrin-labelled
cells of 48 h exposed specimens. It remains to be determined whether this pattern will persist at later stages and will require a longer observation period in future experiments. Taken
together, the increase in gephyrin expression supports our hypothesis that putatively larger cells enable the enrichment of synaptic sites. Thus, the increased gephyrin abundance might not
only indicate a higher number of cells possessing inhibitory synapses but also more inhibitory synapses per neuron within pc/dc-complexes of predator-induced individuals. By this, the
directional excitability might be further enhanced as the activation of inhibitory receptors has also been found to interfere with glutamatergic inputs if the respective synapses are located
on the same dendritic branch58,59. The increase or reduction of activity at synapses is achieved by adjustments of the postsynaptic response, like modulation of receptor availability at the
post synaptic site60,61. Highly mobile GABAAR forms allow trafficking of inhibitory receptors, which has been postulated to be important for the modulation and expression of various forms
of synaptic plasticity56,62. Additionally, GABAAR mediated transmission itself can be modified as a result of plasticity in inhibitory synapses63. In this regard, evidence for the potential
modification of GABAARs has been found in form of increasing receptor numbers56,64. Although we focused on the rewiring of the inhibitory nervous system, inhibition and excitation act in
concert to form directed and dedicated neuronal signal patterns. It is thus very likely to suggest that there is also a rewiring of excitatory synapses accompanying the signal integration of
predator perception. In conclusion, our findings suggest three mechanisms that are involved in signal integration of predator-specific chemical cues in the _Daphnia_ pc/dc-complex: 1.) An
increase in the pc/dc-complex volume provides space for more cell–cell connections 2.) The formation of more inhibitory synaptic connections that control cellular activity, increasing
directionality of neuronal signaling 3.) The rewiring of a neuronal circuitry that is associated with predator perception. Taken together, our study provides new insights into how organisms
interpret environmental challenges and transform these into phenotypic adaptations. DATA AVAILABILITY Data associated with this manuscript is given in the supplements. REFERENCES * Bradshaw,
A. D. Evolutionary significance of phenotypic plasticity in plants. _Adv. Genet._ 13, 115–155 (1965). Article Google Scholar * Weiss, L. C. & Tollrian, R. Predator induced defenses in
Crustacea. in _The Natural History of Crustacea: Life Histories, Volume 5_ (eds. Welborn, G. & Thiel, M.) 303–321 (Oxford University Press, 2018). Chapter Google Scholar * Tollrian,
R. Predator-induced helmet formation in _Daphnia cucullata_ (Sars). _Arch. für Hydrobiol._ 119, 191–196 (1990). Article Google Scholar * Krueger, D. A. & Dodson, S. I. Embryological
induction and predation ecology in Daphnia pulex. _Limnol. Oceanogr._ https://doi.org/10.4319/lo.1981.26.2.0219 (1981). Article Google Scholar * Grant, J. W. G. & Bayly, I. A. E.
Predator induction of crests in morphs of the _Daphnia carinata_ King complex. _Limnol. Oceanogr._ https://doi.org/10.4319/lo.1981.26.2.0201 (1981). Article Google Scholar * Macháček, J.
Indirect effect of planktivorous fish on the growth and reproduction of _Daphnia galeata_. _Hydrobiologia_ https://doi.org/10.1007/BF00028397 (1991). Article Google Scholar * Stibor, H.
& Luning, J. Predator-induced phenotypic variation in the pattern of growth and reproduction in _Daphnia hyalina_ (Crustacea: Cladocera). _Funct. Ecol._ https://doi.org/10.2307/2390117
(1994). Article Google Scholar * Dodson, S. I., Tollrian, R. & Lampert, W. _Daphnia_ swimming behaviour during vertical migration. _J. Plankton Res._ 19, 969–978 (1997). Article
Google Scholar * Tollrian, R., Duggen, S., Weiss, L. C., Laforsch, C. & Kopp, M. Density-dependent adjustment of inducible defenses. _Sci. Rep._ 5, 12736 (2015). Article ADS CAS
Google Scholar * Miyakawa, H. _et al._ Gene up-regulation in response to predator kairomones in the water flea Daphnia pulex. _BMC Dev. Biol._ https://doi.org/10.1186/1471-213X-10-45
(2010). Article PubMed PubMed Central Google Scholar * Oda, S., Kato, Y., Watanabe, H., Tatarazako, N. & Iguchi, T. Morphological changes in _Daphnia galeata_ induced by a crustacean
terpenoid hormone and its analog. _Environ. Toxicol. Chem._ https://doi.org/10.1002/etc.378 (2011). Article PubMed Google Scholar * Miyakawa, H., Sato, M., Colbourne, J. K. & Iguchi,
T. Ionotropic glutamate receptors mediate inducible defense in the water flea _Daphnia pulex_. _PLoS ONE_ 10, 1–12 (2015). Article Google Scholar * Weiss, L. C., Kruppert, S., Laforsch,
C. & Tollrian, R. _Chaoborus_ and _Gasterosteus_ anti-predator responses in _Daphnia pulex_ are mediated by independent cholinergic and gabaergic neuronal signals. _PLoS ONE_ 7, e36879
(2012). Article ADS CAS Google Scholar * Weiss, L. C., Leese, F., Laforsch, C. & Tollrian, R. Dopamine is a key regulator in the signalling pathway underlying predatorinduced
defences in _Daphnia_. _Proc. R. Soc. B Biol. Sci._ https://doi.org/10.1098/rspb.2015.1440 (2015). Article Google Scholar * Weiss, L. C., Leimann, J. & Tollrian, R. Predator-induced
defences in _Daphnia longicephala_: location of kairomone receptors and timeline of sensitive phases to trait formation. _J. Exp. Biol._ 218, 2918–2926 (2015). Article Google Scholar *
Bullock, T. & Horridge, G. A. Structure and function in the nervous systems of invertebrates. (San Francisco, 1965). * Fritsch, M., Kaji, T., Olesen, J. & Richter, S. The development
of the nervous system in Laevicaudata (Crustacea, Branchiopoda): insights into the evolution and homologies of branchiopod limbs and ‘frontal organs’. _Zoomorphology_ 132, 163–181 (2013).
Article Google Scholar * Kolb, B. & Whishaw, I. Q. Brain plasticity and behavior. _Annu. Rev. Psychol._ https://doi.org/10.1146/annurev.psych.49.1.43 (1998). Article PubMed Google
Scholar * Turner, A. M. & Greenough, W. T. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. _Brain Res._
https://doi.org/10.1016/0006-8993(85)90525-6 (1985). Article PubMed Google Scholar * Woodley, S. K., Mattes, B. M., Yates, E. K. & Relyea, R. A. Exposure to sublethal concentrations
of a pesticide or predator cues induces changes in brain architecture in larval amphibians. _Oecologia_ 179, 655–665 (2015). Article ADS Google Scholar * Gronenberg, W., Heeren, S. &
Hölldobler, B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. _J. Exp. Biol._ 199, 2011–2019 (1996). Article CAS
Google Scholar * Barth, M. & Heisenberg, M. Vision affects mushroom bodies and central complex in Drosophila melanogaster. _Learn. Mem._ https://doi.org/10.1101/lm.4.2.219 (1997).
Article PubMed Google Scholar * Barth, M., Hirsch, H. V. B., Meinertzhagen, I. A. & Heisenberg, M. Experience-dependent developmental plasticity in the optic lobe of Drosophila
melanogaster. _J. Neurosci._ https://doi.org/10.1523/jneurosci.17-04-01493.1997 (1997). Article PubMed PubMed Central Google Scholar * van Dijk, L. J. A., Janz, N., Schäpers, A.,
Gamberale-Stille, G. & Carlsson, M. A. Experience-dependent mushroom body plasticity in butterflies: Consequences of search complexity and host range. _Proc. R. Soc. B Biol. Sci._ 284,
0–7 (2017). Google Scholar * Withers, G. S., Fahrbach, S. E. & Robinson, G. E. Selective neuroanatomical plasticity and division of labour in the honeybee. _Nature_
https://doi.org/10.1038/364238a0 (1993). Article PubMed Google Scholar * Heisenberg, M., Heusipp, M. & Wanke, C. Structural plasticity in the _Drosophila_ brain. _J. Neurosci._
https://doi.org/10.1523/jneurosci.15-03-01951.1995 (1995). Article PubMed PubMed Central Google Scholar * Niven, J. E. & Laughlin, S. B. Energy limitation as a selective pressure on
the evolution of sensory systems. _J. Exp. Biol._ https://doi.org/10.1242/jeb.017574 (2008). Article PubMed Google Scholar * Berlucchi, G. & Buchtel, H. A. Neuronal plasticity:
historical roots and evolution of meaning. _Exp. Brain Res._ https://doi.org/10.1007/s00221-008-1611-6 (2009). Article PubMed Google Scholar * Zhai, R. G. & Bellen, H. J. The
architecture of the active zone in the presynaptic nerve terminal. _Physiology_ https://doi.org/10.1152/physiol.00014.2004 (2004). Article PubMed Google Scholar * Horn, G., Bradley, P.
& McCabe, B. J. Changes in the structure of synapses associated with learning. _J. Neurosci._ https://doi.org/10.1523/jneurosci.05-12-03161.1985 (1985). Article PubMed PubMed Central
Google Scholar * Beaulieu, C. & Colonnier, M. Richness of environment affects the number of contacts formed by boutons containing flat vesicles but does not alter the number of these
boutons per neuron. _J. Comp. Neurol._ https://doi.org/10.1002/cne.902740305 (1988). Article PubMed Google Scholar * Anderson, B. J. Plasticity of gray matter volume: The cellular and
synaptic plasticity that underlies volumetric change. _Dev. Psychobiol._ https://doi.org/10.1002/dev.20563 (2011). Article PubMed Google Scholar * Tyagarajan, S. K. & Fritschy, J.-M.
Gephyrin: a master regulator of neuronal function?. _Nat. Rev. Neurosci._ 15, 141–156 (2014). Article CAS Google Scholar * Dutertre, S., Becker, C. M. & Betz, H. Inhibitory glycine
receptors: an update. _J. Biol. Chem._ https://doi.org/10.1074/jbc.R112.408229 (2012). Article PubMed PubMed Central Google Scholar * Fritschy, J. M., Harvey, R. J. & Schwarz, G.
Gephyrin: where do we stand, where do we go?. _Trends Neurosci._ https://doi.org/10.1016/j.tins.2008.02.006 (2008). Article PubMed Google Scholar * Choii, G. & Ko, J. Gephyrin: a
central GABAergic synapse organizer. _Exp. Mol. Med._ https://doi.org/10.1038/emm.2015.5 (2015). Article PubMed Google Scholar * Phillips-Portillo, J. & Strausfeld, N. J.
Representation of the brain’s superior protocerebrum of the flesh fly, _Neobellieria bullata_, in the central body. _J. Comp. Neurol._ https://doi.org/10.1002/cne.23094 (2012). Article
PubMed PubMed Central Google Scholar * Schindelin, J. _et al._ Fiji: An open-source platform for biological-image analysis. _Nat. Methods_ https://doi.org/10.1038/nmeth.2019 (2012).
Article PubMed PubMed Central Google Scholar * de Reuille, P. B. _et al._ MorphoGraphX: a platform for quantifying morphogenesis in 4D. _Elife_ https://doi.org/10.7554/eLife.05864
(2015). Article Google Scholar * Cignoni, P. et al. MeshLab: An open-source 3D mesh processing tool. In _6th Eurographics Italian Chapter Conference 2008 - Proceedings_ (2008). Google
Scholar * Horstmann, M. _et al._ Scan, extract, wrap, compute—a 3D method to analyse morphological shape differences. _PeerJ_ 2018, 1–20 (2018). Google Scholar * R Development Core Team,
R. R: A Language and Environment for Statistical Computing. _R Foundation for Statistical Computing._ https://doi.org/10.1007/978-3-540-74686-7 (2011). Article Google Scholar * Wickham, H.
_et al._ ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag. _New York_ (2016). * Ohyama, T. _et al._ A multilevel multimodal circuit enhances action selection in Drosophila.
_Nature_ 520, 633–639 (2015). Article ADS CAS Google Scholar * Simpson, J. H. Chapter 3 Mapping and Manipulating Neural Circuits in the Fly Brain. _Advances in Genetics._
https://doi.org/10.1016/S0065-2660(09)65003-3 (2009). Article Google Scholar * Boyan, G., Williams, L. & Liu, Y. Conserved patterns of axogenesis in the panarthropod brain. _Arthropod
Struct. Dev._ https://doi.org/10.1016/j.asd.2014.11.003 (2015). Article PubMed Google Scholar * Cayre, M., Strambi, C. & Strambi, A. Neurogenesis in an adult insect brain and its
hormonal control. _Nature_ https://doi.org/10.1038/368057a0 (1994). Article Google Scholar * Harzsch, S. & Dawirs, R. R. Neurogenesis in the developing crab brain: Postembryonic
generation of neurons persists beyond metamorphosis. _J. Neurobiol._ https://doi.org/10.1002/(SICI)1097-4695(199603)29:3%3c384::AID-NEU9%3e3.0.CO;2-5 (1996). Article PubMed Google Scholar
* Sandeman, R., Clarke, D., Sandeman, D. & Manly, M. Growth-related and antennular amputation-induced changes in the olfactory centers of crayfish brain. _J. Neurosci._
https://doi.org/10.1523/jneurosci.18-16-06195.1998 (1998). Article PubMed PubMed Central Google Scholar * Harzsch, S., Miller, J., Benton, J. & Beltz, B. From embryo to adult:
Persistent neurogenesis and apoptotic cell death shape the lobster deutocerebrum. _J. Neurosci._ https://doi.org/10.1523/jneurosci.19-09-03472.1999 (1999). Article PubMed PubMed Central
Google Scholar * Letourneau, J. G. Addition of sensory structures and associated neurons to the crayfish telson during development. _J. Comp. Physiol. A_ https://doi.org/10.1007/BF00656778
(1976). Article Google Scholar * Sandeman, D. C. Organization of the central nervous system. in _The Biology of Crustacea. Vol. 3. Neurobiology: Structure and Function_ 1–61 (Academic
Press, 1982). Google Scholar * Laverack, M. S. The numbers of neurones in decapod Crustacea. _J. Crustac. Biol._ 8, 1–11 (1988). Article Google Scholar * Moss, S. J. & Smart, T. G.
Constructing inhibitory synapses. _Nat. Rev. Neurosci._ 2, 240–250 (2001). Article CAS Google Scholar * Bliss, T. V. P. & Collingridge, G. L. A synaptic model of memory: long-term
potentiation in the hippocampus. _Nature_ https://doi.org/10.1038/361031a0 (1993). Article PubMed Google Scholar * Collingridge, G. L., Isaac, J. T. R. & Yu, T. W. Receptor
trafficking and synaptic plasticity. _Nat. Rev. Neurosci._ https://doi.org/10.1038/nrn1556 (2004). Article PubMed Google Scholar * Atwood, H. L. & Wojtowicz, J. M. Short-term and
long-term plasticity and physiological differentiation of crustacean motor synapses. _Int. Rev. Neurobiol._ 28, 275–362 (1986). Article CAS Google Scholar * Liu, G. Local structural
balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. _Nat. Neurosci._ https://doi.org/10.1038/nn1206 (2004). Article PubMed PubMed Central
Google Scholar * Hao, J., Wang, X. D., Dan, Y., Poo, M. M. & Zhang, X. H. An arithmetic rule for spatial summation of excitatory and inhibitory inputs in pyramidal neurons. _Proc. Natl.
Acad. Sci. USA._ https://doi.org/10.1073/pnas.0912022106 (2009). Article PubMed Google Scholar * Fu, A. K. & Ip, N. Y. Regulation of postsynaptic signaling in structural synaptic
plasticity. _Curr. Opin. Neurobiol._ https://doi.org/10.1016/j.conb.2017.05.016 (2017). Article PubMed PubMed Central Google Scholar * Chater, T. E. & Goda, Y. The role of AMPA
receptors in postsynaptic mechanisms of synaptic plasticity. _Front. Cell. Neurosci._ https://doi.org/10.3389/fncel.2014.00401 (2014). Article PubMed PubMed Central Google Scholar *
Velazquez, J. L., Thompson, C. L., Barnes, E. M. & Angelides, K. J. Distribution and lateral mobility of GABA/benzodiazepine receptors on nerve cells. _J. Neurosci._ 9, 2163–2169 (1989).
Article CAS Google Scholar * Gaiarsa, J. L., Caillard, O. & Ben-Ari, Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. _Trends
Neurosci._ https://doi.org/10.1016/S0166-2236(02)02269-5 (2002). Article PubMed Google Scholar * Nusser, Z., Hájos, N., Somogyi, P. & Mody, I. Increased number of synaptic GABA(A)
receptors underlies potentiation at hippocampal inhibitory synapses. _Nature_ https://doi.org/10.1038/25999 (1998). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS We
thank Dr. Martin Horstmann for supporting 3D _Daphnia_ brain constructions and volume measurements. Additionally, we acknowledge Sabine Kindermann for excellent technical support. FUNDING
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Friedrich-Ebert-Foundation and the German Science Foundation WE 6019/2-1. AUTHOR INFORMATION Author
notes * These authors contributed equally: A Graeve and I Ioannidou. AUTHORS AND AFFILIATIONS * Department of Animal Ecology, Evolution and Biodiversity, Ruhr-University Bochum,
Universitätsstrasse 150, 44780, Bochum, Germany A Graeve, I Ioannidou, D. M. Görl & LC Weiss * Department of Cell Morphology and Molecular Neurobiology, Ruhr-University Bochum,
Universitätsstrasse 150, 44780, Bochum, Germany J Reinhard & A Faissner Authors * A Graeve View author publications You can also search for this author inPubMed Google Scholar * I
Ioannidou View author publications You can also search for this author inPubMed Google Scholar * J Reinhard View author publications You can also search for this author inPubMed Google
Scholar * D. M. Görl View author publications You can also search for this author inPubMed Google Scholar * A Faissner View author publications You can also search for this author inPubMed
Google Scholar * LC Weiss View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.C.W. conceived, and L.C.W., J.R., A.F. designed the experiment,
I.I., A.G., D.M.G. carried out the labwork, A.G., I.I., L.C.W. analyzed the data, A.G. and L.C.W. drafted the manuscript, L.C.W., A.G.; J.R., A.F. contributed to the final version. All
authors gave final approval for publication and agree to be held accountable for the work performed therein. CORRESPONDING AUTHOR Correspondence to LC Weiss. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's
Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not
permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Graeve, A., Ioannidou, I., Reinhard, J. _et al._ Brain volume increase and neuronal
plasticity underly predator-induced morphological defense expression in _Daphnia longicephala_. _Sci Rep_ 11, 12612 (2021). https://doi.org/10.1038/s41598-021-92052-y Download citation *
Received: 08 April 2021 * Accepted: 03 June 2021 * Published: 15 June 2021 * DOI: https://doi.org/10.1038/s41598-021-92052-y SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative