Polyurethane-functionalized starch nanocrystals as anti-tuberculosis drug carrier
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Studies related to loading ability and delivery of clinically used first-line anti-tuberculosis drugs (ATDs) such as isoniazid, rifampicin, pyrazinamide and streptomycin on the
surface of starch-derived bulk and nanopolyurethanes (SBPUs and SNPUs) as drug delivery systems (DDS) have been focused to minimise or remove the drug-associated adverse effects. The
efficiencies of nanopolyurethanes obtained from the differently substituted cyclic aliphatic and aromatic isocyanates have been studied for drug loading and release purposes. Different
advanced instrumental techniques analysed the structural and morphological properties, thermal stability and crystallinity of the starch nanopolyurethans. Average particle sizes ranging from
27.35–42.38 nm to 126.89–218.60 nm for starch nanopolyurethans, SNPU3I and SNPU4I, respectively, were determined by high-resolution transmission electron microscopy. Similarly, the loading
efficiency of ATDs to the surfaces of SNPUs and SBPUs was observed in the range of 60–97% while ATDs-loaded SNPUs showed a sustainable release profile for all ATDs except for streptomycin.
However, most SBPUs provided burst-release for all the above-mentioned ATDs in pH-dependent studies. The anti-tuberculosis assay against the _Mycobacterium tuberculosis_ H37Rv strain
revealed that streptomycin-loaded SNPU4I and isoniazid-loaded SNPU7I are approximately 42 and 7 times more active than the native streptomycin and isoniazid, respectively. SIMILAR CONTENT
BEING VIEWED BY OTHERS FORMULATION AND CHARACTERIZATION OF BBR LOADED NIOSOMES USING SAPONIN AS A NONIONIC BIOSURFACTANT INVESTIGATING SYNERGISTIC EFFECTS TO ENHANCE ANTIBACTERIAL ACTIVITY
Article Open access 12 February 2025 DEVELOPMENT OF A NOVEL ANTI-TUBERCULOSIS NANODELIVERY FORMULATION USING MAGNESIUM LAYERED HYDROXIDE AS THE NANOCARRIER AND PYRAZINAMIDE AS A MODEL DRUG
Article Open access 18 August 2022 SYNTHESIS OF SILVER NANOPARTICLES EMBEDDED INTO MELAMINE POLYAMINAL NETWORKS AS ANTIBACTERIAL AND ANTICANCER ACTIVE AGENTS Article Open access 28 August
2024 INTRODUCTION All over the world, community-associated tuberculosis caused by _Mycobacterium tuberculosis_ is one of the most infectious diseases1,2,3. The clinically used
anti-tuberculosis drugs requires prolonged and intensive treatment time leading to the generation of either non-compliance with an increased possibility of relapse of tuberculosis (TB) or
even more severe multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB to a patient due to genetic and molecular structural changes4,5,6. The process of developing new and
improved drugs for the treatment of drug-resistant tuberculosis is exorbitant and time-consuming. Rather, the inclusion of new drug delivery devices or improving the bioavailability,
stability, target-oriented modification, drug distribution, metabolism, availability of drugs at the infected site, therapy time, drug resistance, and so on for the existing TB drugs would
be more economical and beneficial. In this direction, polyurethanes-based versatile materials, which are one of the most ubiquitously applied polymers in different fields, may be explored as
a drug delivery system (DDS)7 for anti-tuberculosis drug delivery. Different polyurethanes with a wide range of physicochemical properties are synthesized by varying either the reactant
partners or their stoichiometric ratios. Polyurethane-based drug delivery systems may be applied for sustained release of the clinically used TB drugs. Thus, for this purpose,
urethane-functionalised starch may be explored as the said modification may improve the properties of the native starch such as pore size, binding sites and volume; generally, these
properties are very much vital for the inclusion of water, drug molecules and foreign bodies. Nowadays, the semi-synthetic nanostructured polymeric materials as drug vehicles required for
many targeted therapies have been emerging as a frontier research area in pharmaceutical and biopharmaceutical field. For a drug to act in the body, it is supposed to be protected from the
degradation (enzymatic or chemical) before reaching the targeted site in physiological condition. In this context, polymeric nanoparticle-based vehicles might be appropriate to act as a
safeguard of the drug molecules. Not only that, the nanotechnology-based system improved higher payload capacity and ability to incorporate both lipophilic and hydrophilic drug substances
according to the administration routes, such as oral, topical, parenteral, pulmonary and so forth8,9,10. Generally, the natural improvement of the pharmacokinetics of nanoparticles to
increase the clearance time from blood has been achieved by tuning their sizes, shapes and surface modification etc_._ resulting the facilitation in penetration of the drugs into the
cellular level. These nanoparticles as a drug carrier can control the sustained release, which indirectly improves the drug bioavailability enabling a reduction in the dose as well as in
dosing frequency in addition to an improvement in patient compliance11. A controlled-release system constructed with biocompatible nanoparticles is the essential features for the innovative
drug delivery approach for the existing ATDs to fight against extensive infection of TB disease. In this scenario, carbohydrate-based nanoparticles12,13,14 are advantageous being
biodegradable and ornamented with a large number of hydroxy or other hydrophilic groups, such as carboxyl groups, which may find useful for post-translational surface modification.
Carbohydrate-derived nanoparticles particularly, starch-based nanoparticles (SNCs) find enormous applications in the drug delivery research. The SNCs-based drug vehicles can be designed for
the delivery of the covalently and/or physically attached drug molecules to the infected sites15,16,17,18,19. The urethane-functionalized starch in bulk and nano state acted as potential
drug delivery devices in the field of sustained-release of drug molecules20,21,22,23. Not only that, these covalently cross-linked polyurethane-based starch nanoparticles are capable to show
the high load-carrying capacity of the drug in the physiological medium with improved stability and biodegradability24,25,26,27,28. Usually, in industries, cross-linking methods have been
commonly used and ubiquitously applied for modifications of starch. As the drug loading and releasing efficiency depend on adsorption phenomena of the delivery systems, thus it varies based
on surface morphologies and backbone moieties through hydrophilic and hydrophobic interactions. Herein it is relevant to mention that starch nanopolyurethans are found as a better adsorbent
for biodiesel purification than that of simple SNCs as per or earlier studies29,30. Herein, we have performed the chemical modifications in the structural framework of starch and starch
nanocrystal for the formation of cross-linking using urethane functionalization to improve their stability, sorptivity, resilience and resistance against transformation, acid/base
hydrolysis, enzymatic hydrolysis, reduction, oxidation and many other chemical processes. The different polyurethanes were synthesized using differently substituted aromatic, and cyclic
aliphatic isocyanates and diisocyanates to introduce both hydrophobic and hydrophilic functionalities in the backbone of starch nanocrystals. Finally, the several challenges associated with
traditional anti-tuberculosis drugs were addressed by utilizing the cross-linked starch polyurethanes as a nano and bulk carrier for the loading of first-line anti-tuberculosis drugs (ATDs),
rifampicin (RIF), isoniazid (INH), pyrazinamide (PZA) and streptomycin (SM). Besides, the polyurethane-functionalized starch nanocrystals were also established as sustainable materials for
improved drug delivery and their release kinetics. Overall, syntheses, loading capacity (LC), loading efficiency (LE), release behavior of the polyurethane-functionalized starch
nanoparticles loaded with first-line ATDs and their biological efficacies against _M. smegmatis_ and _M. tuberculosis_ H37Rv strain have been studied. RESULTS AND DISCUSSION NATIVE
STARCH-BASED BULK POLYURETHANES (SBPUS) The synthesis of starch-derived bulk polyurethanes (SBPUs) was completed by the reaction between native waxy maize starch (1I) and six different
cyclic aliphatic/aromatic isocyanates such as 4,4′-methylene bis(phenyl isocyanate) (2I), 4,4′-methylene bis-(cyclohexyl isocyanate) (3I), 1,4-phenylene diisocyanate (4I), 1-naphthyl
isocyanate (5I), 1,3-bis(isocyanatomethyl) cyclohexane (6I), and isophorone diisocyanate (7I) according to our previous report29. In brief, the waxy maize starch was first dried overnight in
a high vacuum oven at 80 °C and then heated in DMSO at 55–60 °C for 24 h under constant stirring. After that, the isocyanate and an organometallic catalyst, stannous octoate was added and
the mixture was stirred further at 70–120 °C, over 4–8 h (Scheme 1) depending on the nature of isocyanates. After completion of the reaction, cold methanol was added with constant stirring
until the resulting polyurethane is precipitated. The solid residue was recovered through centrifugation at 8000 rpm and washed with acetone and water. Finally, the products (SBPU2I-7I) were
dried in a vacuum oven at 80 °C for 24 h and stored in vacuum desiccator for further use. The yields of the reactions are reported in between 30 and 40%. Chemical structures of the SBPUs
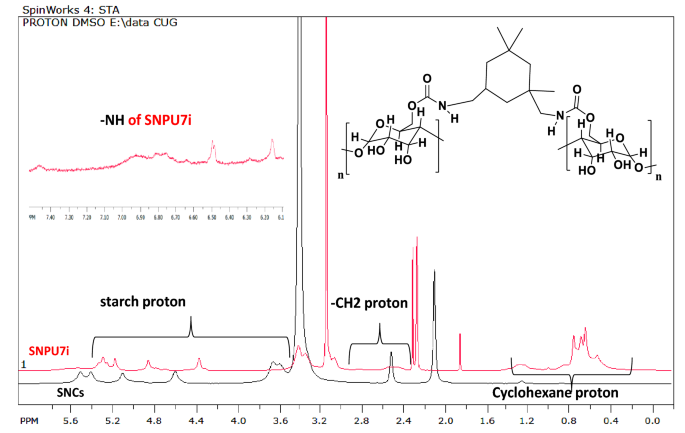
were corroborated by 1H NMR and FT-IR. 1H NMR signals corresponding to –NH group of the urethane linkages [–NH–(CO)–O–] and starch backbone of SBPU5I-7I were observed in the range of δ
7.1–8.5 and δ 3.0–5.8 ppm, respectively (Figs. S13 to S15). Whereas, for the case of SBPU6I-7I obtained from cyclic aliphatic isocyanates, the methylene protons of the cyclic backbone were
observed in the range of ~ δ 2.63–0.73 ppm (Figs. S14 and S15). The amide bond [–(CO)–NH–] and urethane carbonyl corresponding to the urethane linkages are also confirmed by the FTIR
spectroscopy; the sharp signals corresponding to amide bond and urethane carbonyl were detected at 1549 to 1559 cm−1 and 1655 to 1712 cm−1, respectively (Fig. S19). Chemical structures of
the other polyurethane derivatives (SBPU2I-4I) have been reported in our previous work29. Starch nanocrystal (SNCs) were first prepared according to our previous method29 from native waxy
maize starch by acid hydrolysis with H2SO4 and purified by washing with distilled water for the synthesis of starch-derived nano polyurethanes (SNPUs). Furthermore, the resulting product was
lyophilized for 36 h to render fine SNC powder in a fluffy white state. In order to stop their normal agglomeration, the SNCs were then suspended in chloroform and kept at 4° C. The average
particle size and polydispersity index (PDI) of SNCs were found to be 312.44 nm and 0.425, respectively, in which due to its intrinsic agglomeration/aggregation characteristics the
formation of the larger size of the SNC was realised. Using various mono and diisocyanates, the carbohydrate backbone of SNCs was cross-linked by urethane formation. In short, the SNCs were
dispersed for 4 h at 55–60 °C in DMSO and then a similar reaction was carried out following the process previously used for bulk polyurethane synthesis (Scheme 2). For the formation of
nanopolyurethanes, solvent displacements and precipitation methods have been used. The solids were recovered by centrifugation and washing with water/acetone mixture to produce SNPU2I-7I,
which were dried in a vacuum oven and stored in vacuum desiccators for further use. The structures of all cross-linked starch nanopolyurethanes (SNPU2I to SNPU7I) have been confirmed by NMR,
FTIR, DLS, HR-TEM, P-XRD, TGA and other spectroscopic techniques. The peak between δ3.03–5.86 ppm corresponded to starch protons in the 1H NMR of SNCs and the relevant 13C signals are seen
between δ78.4–71.2 ppm. Starch-derived nanopolyurethanes (SNPUs) contain urethanic bonds [–NH–(CO)–O–], confirmed by the NH peak in the range of δ 7.04–8.52 ppm in 1HNMR29 spectrum (Fig. S16
to S18); in Fig. 1, one of the examples (SNPU2I) is illustrated. In FTIR spectroscopy, the broad peak of hydroxy functionalities in starch at around 3200–3500 cm−1 was reduced upon
isocyanate reaction, and peaks appeared at 1639.2 cm−1 and 1700 cm−1 corresponding to –C–NH and –C=O stretching vibrations of urethane linkages [-NH-(CO)-O-] along with other peaks of nano
polyurethanes. A comparative study showed that the disappearance or the low intensity of the -NCO peak at ~ 2200 cm−1 suggested the use of isocyanates in the reaction (Fig. S20). The average
particle size of SNCs-based nanopolyurethanes (SNPU2I-7I) was measured by dynamic light scattering (DLS) and found in the range of 61–482 nm. The polydispersity index (PDI) of the polymer
ranges from 0.292 to 1.313, suggesting a narrow distribution of particle sizes (Fig. S3 and Table S1). The size and morphology of the SNPU3I-4I polymers were characterised by TEM, which
showed that the size of the SNPU3I and SNPU4I polymers was in the range of 27.35–42.38 nm and 126.89–218.60 nm, respectively. From the HRTEM image of SNPU2I (Fig. 2), it is evident that the
particle is spherical in shape and the mean size is ~ 132.18 nm (standard deviation ~ 49 nm), while the mean size of the spherical-shaped nanopolyrethanes (SNPU5I-7I) is in the range of 160
to 170 nm (standard deviation ~ 42 nm). Thermal properties of the bulk and nanopolyurethanes (SBPU2I-7I and SNPU2I-7I)31,32,33,34 studied by TGA thermograms have shown that the thermal
decomposition of starch nanocrystals varies from the starch-based bulk and nanopolyurethanes due to structural changes such as crystallinity and thus confirmed the grafting of the urethane
linkages to native structure of SNCs (Fig. 3). The changes in crystallinity of polyurethanes as detected by TGA were also corroborated from the crystallinity pattern of SNCs-based
polyurethanes investigated by P-XRD. The crystallinity pattern of SNC indicated four intense X-ray diffraction peaks at 15, 17, 18 and 23° 2θ. The cross-linking of SNC using urethane
linkages reduces the crystallinity in polysaccharides by the changes in intensity and shifting of peaks. In the case of starch-based nanopolyurethanes, 2 theta values from 18 to 20° of pure
SNCs was disappeared due to the formation of urethane linkages between starch and diisocyanates. From the literature precedents, it is found that the crystallinity is directly proportional
to the cross-linking of diisocyanates with the polysaccharides of SNCs, and hence in case of modified SNCs-based PUs, less amount of crystalline domains are developed (Fig. 4). Among all
SNPU2I-7I, the new crystalline domain was observed for SNPU2I and SNPU4I and for others; the changes in intensity and shifting of 2θ values indicated the non-crystalline or amorphous
molecular arrangement. Since the urethane linkages are symmetrical in both of the cases, the presence of aromatic moiety might assist in the π-stacking interaction for the formation of
crystalline nature. LOADING OF ATDS ON STARCH-DERIVED BULK AND NANOPOLYURETHANES (SBPU2I-7I AND SNPU2I-7I) The widespread use of nanoparticle-based carriers for the delivery of
anti-tubercular drugs has become on priority due to the unique and advantageous physicochemical and pharmacokinetic profiles. Four first-line anti-tuberculosis drugs, such as rifampicin
(RIF), isoniazid (INH), pyrazinamide (PZA), and streptomycin (SM) were considered for loading through physical adsorption on the surface of the starch-derived nano and bulk-polyurethanes
(SNPU2I-7I and SBPU2I-7I); the drug-loaded polyurethanes were used for the study of their efficacy in drug delivery (Fig. 5). The loading capacities of SNPU2I-7I and SBPU2I-7I for ATDs were
determined using a specific amount (10.0 mg) of isoniazid, pyrazinamide, and rifampicin in tetrahydrofuran (1.5 mL) and streptomycin in mixture of methanol and water (1:1). 100 mg of
SNPU2I-7I and SBPU2I-7I was suspended individually in 13.5 mL THF in a vial containing 1.5 mL of ATDs solution in THF. The resulting mixture was stirred at a moderate speed in dark condition
at room temperature for overnight. Then ATDs-loaded polymer was centrifuged and filtered, followed by two times washing with THF to remove free drugs. The drug loading efficiency (amount of
drug encapsulated for the initial amount of drug taken) and drug loading capacity (amount of drug encapsulated concerning the amount of polymer) was investigated. The ATDs-loaded
nanopolyurethanes were dried at room temperature for 24 h incubation period and stored in a desiccator; the drug loading capacity was measured by absorption spectroscopy. The loading
percentage of each drug was calculated from the observed λmax of streptomycin, isoniazid, pyrazinamide and rifampicin at 280, 270, 330 and 475 nm, respectively (Figs. S1, S2). These findings
also assisted in determination or calculation of the concentration of the unloaded drugs. After 24 h of stirring, the UV–Vis absorbance of the supernatant containing unloaded drugs was
measured from the linear plot of the respective ATDs drug. Calibration curves were plotted using 1 to 10% (0.0067 to 0.067 mg/mL) ATDs (isoniazid, pyrazinamide and rifampicin) added for the
loading in the polymer composites while due to low absorbance, the 10 to 100% (0.067 to 0.67 mg/mL) streptomycin was added for loading in methanol/water (1:1, v/v). Unloaded and loaded drug
concentrations were calculated using absorption spectroscopy. The loading capacities (%) of SNPU2I-7I and SBPU2I-7I are found almost similar for pyrazinamide, rifampicin and streptomycin in
the range of ~ 7.0 to 9.0%. In contrast, the same for isoniazid is found to be in the range of ~ 5.5 to 6.7% and the loading efficiency of SNPU4I for streptomycin is found as highest.
However, the loading efficiency of ATDs to SNPUs and SBPUs was found almost similar ranging from 60 to 97% (Fig. 6). The loading of ATDs to SBPU2I-7I (Figs. S21–S24) and SNPU2I-7I (Figs.
S25–S28) was also supported by the FTIR spectroscopic data. After loading of ATDs on starch nanopolyurethanes, the changes in the FTIR spectra were observed for nanopolyurethanes, such as
broadening, shifting and intensity differences of peaks due to formation of several hydrogen bonds and other molecular interactions with functional groups present in ATDs (such as –OH and
–NH2, C=O, aromatic C=C, CH etc_._). Besides that, the loading was also confirmed by FESEM images, which showed changes in polyurethane morphology before and after drug loading. FESEM images
(Fig. 7) of nano polyurethanes loaded with isoniazid (INH) and pyrazinamide (PZA) showed that SNPU2I nanoparticles appeared as smooth and homogeneous spherical shapes prior to loading with
pyrazinamide (Fig. 7A), but after loading they appeared to be irregular in shape (Fig. 7B). The changes in morphology before and after loading of isoniazid on SNPU4I are represented in Fig.
7C,D respectively. On loading of isoniazid, Fig. 7C showed the multi-branching chains and Fig. 7D displayed the spherical-shaped particle image embedded in the polymeric chain and branch. IN
VITRO RELEASE STUDIES OF ATDS-LOADED NANO AND BULK-POLYURETHANES The in vitro release experiment of streptomycin, isoniazid, pyrazinamide and rifampicin using the ATDs-loaded SNPU2I-7I were
performed by absorption spectroscopy at pH 2, 5, 7 and 8 in the Tris buffer. It was found that most of the drugs are released at pH 2 and pH 8 in the Tris buffer upon optimization. To
assess the quantity of ATDs released from SNPU2I-7I, 25.0 mg of SNPU2I-7I loaded with ATDs were separately dispersed into various sample vials containing 10.0 mL of Tris buffer at pH 2 and
pH 8 at 37 °C and stirred at 100 RPM. The 2.0 mL mixture solution of the individual release samples was applied to an equal volume of the corresponding Tris buffer at specific time intervals
and the supernatants were analysed in triplicate by absorption spectroscopy. The percentage of cumulative drugs released at pH 2 and pH 8 in the Tris buffer was calculated at the respective
λmax of streptomycin, isoniazid, pyrazinamide and rifampicin (280, 270, 270 and 475 nm), respectively. For rifampicin-loaded SNPU2I at pH 2 and pH 8, the cumulative drug release percentage
(CR%) was ~ 37 and 50%, respectively, within 24 h, while a maximum of 15 to 22% released was observed for SNPU3I and SNPU6I (Fig. 8A,B). Similarly, the CR% at pH 2 was found up to ~ 67%
within 36 h in the case of isoniazid-loaded SNPU2I-4I, while SNPU5I and SNPU7I contributed up to 90% to burst release (Fig. 8C) at 8 h. CR % of isoniazid-loaded SNPU3I-4i provided a better
result at pH 8 (CR % ~ 77%) compared to the result at pH 2 (Fig. 8C,D). The cumulative release percentage of pyrazinamide was found to be highest in case of pyrazinamide-loaded SNPU5I at pH
2 (CR% ~ 90%) while for pyrazinamide-loaded SNPU5I and SNPU6I at pH 8, CR% was ~ 70% and 54%, respectively, within 24 h. CR% of pyrazinamide for other pyrazinamide-loaded starch
nanopolyurethanes (SNPU3I,4I,6I,7I) contributed in the range of 30–40% at pH 2 and pH 8 in Tris buffer (Fig. 8E,F). For streptomycin-loaded nanopolyurethanes, no sustained release was
observed; most of them developed burst release within a short time, suggesting that the drugs are mainly present in the nanosphere's outer side. Individually, streptomycin-loaded SNPU3I
reported a cumulative streptomycin release of ~ 75% at pH 8 over 24 h (Fig. 8G). In Fig. 9, a mechanistic model was shown for burst release and sustained release. In general, the drugs are
present outside the nanoparticle for burst release and the drugs find stability into the nanoparticle core through hydrogen bonding, van der Walls types of forces and/or electrostatic
interactions for sustain release. Similarly, the release analysis of ATDs-loaded bulk-polyurethanes (SBPU2I-7I) at pH 2 and pH 8 in the Tris buffer showed that some of them have strong
releasing properties other than pyrazinamide-loaded bulk-polyurethanes (Fig. S12A-H). The pyrazinamide-loaded SBPU2I, SBP5I, SBPU7I released the drug at pH 2 and pH 8 with moderate CR % in
the range of ~ 30–45 percent. Isoniazid-loaded SBPU3I and SBPU7I, showed CR % up to ~ 69% for 30 h at pH 2, while other isoniazid-loaded SBPU2I and SBPU4I-6I, showed burst release at pH 2.
CR % of isoniazid was observed up to 65% at pH 8 for SBPU6I-7I, while other bulk-polyurethanes SBPU2I-5I have shown burst release within a short period of time at pH 8. At pH 2 and 8 buffer,
the cumulative release (%) of RIF-loaded bulk polyurethanes (SBPU2I-7I) contributed to a good rifampicin release profile. Rifampicin-loaded SBPU4I offered sustained release with CR% of
rifampicin up to ~ 92% at pH 8 over 30 h duration, while sustained release of rifampicin was not found for other bulk polyurethanes in both media. Cumulative release (%) of streptomycin for
streptomycin-loaded SBPU2I and SBPU4I showed better results with ~ 90% and 40% over 30 h’ time periods, respectively, while streptomycin-loaded other bulk polyurethanes provided burst
release at pH 2 and 8 buffers. The mathematical models for dissolution kinetic behavior of drug nanoparticles were studied to express the kinetics and release mechanism. Mathematical models
were used to the solid dosage form for conducting drug release study and the results in Table 1 presented the comparative correlation coefficient (R2) of different kinetic models. The
correlation coefficient (R2) is the prime factor in predicting the drug release mechanism through the best-fitted theoretical model. Table 1 shows the highest determination coefficient (R2)
best fitted for zero-order, first-order, Higuchi, Hixson-Crowell and Korsmeyer-Peppas models for SNPU/SBPU2I-7I as the correlation coefficient value towards 1.0 indicates a perfect fit, and
highly reliable for future forecasts using model, while a value of 0.0 would indicate that the calculation fails to accurately model the data at all. The zero-order model is best fitted for
RIF-SNPU2I-pH8, RIF-SNPU3I-pH8, PZA-SNPU2I-pH8, RIF-SBPU6I-pH2, SM-SBPU7I-pH8 and this model shows the release mechanism through diffusion. The first-order modal is best fitted for
RIF-SNPU2I-pH8, RIF-SNPU3I-pH8, INH-SNPU4I-pH2, INH-SNPU4I-pH8, PZA-SNPU5I-pH2, PZA-SNPU2I-pH8, PZA-SNPU5I-pH8, RIF-SBPU6I-pH2 and the release kinetic is explained by Fick’s first law of
diffusion mechanism. While, Higuchi model is best fitted for INH-SNPU2I-pH2, INH-SNPU3I-pH2, INH-SNPU4I-pH2, INH-SNPU3I-pH8, INH-SNPU4I-pH8, PZA-SNPU5I-pH8, RIF-SBPU4I-pH8, and follows the
diffusion medium-based mechanism according to Fick's first law. However, Hixson-Crowell model is the suitable for RIF-SNPU3i-pH8, INH-SNPU2I-pH2, INH-SNPU4I-pH2, INH-SNPU3I-pH8,
INH-SNPU4I-pH8, PZA-SNPU5I-pH2, PZA-SNPU2I-pH8, RIF-SBPU6I-pH2, which explains the erosion release mechanism of drug release kinetics (Figs. S29–S43). In Table 1, various drug loaded
carriers followed mixed mechanism of drug release such as diffusion, erosion, leaching, and Fick’s first law of diffusion mechanism etc35,36,37. ANTI-MYCOBACTERIAL ASSAY AGAINST
_MYCOBACTERIUM SMEGMATIS_ Prior to anti-tuberculosis assay, a rapid screening of the selected SBPUs was applied for detection of anti-mycobacterial activity against _Mycobacterium smegmatis_
by disk diffusion assay. The streptomycin, isoniazid, and rifampicin-loaded SBPUs produced a positive result against _M. smegmatis_ as illustrated in Table 2. The streptomycin-loaded SBPUs
produced promising activity result against _M. smegmatis_ where rifampicin was used as control. ANTI-TUBERCULOSIS ASSAY Minimum inhibition concentration (MIC) was calculated to determine the
anti-tuberculosis activity against _M. tuberculosis_ strain H37Rv using the Lowenstein-Jensen (LJ) slope method, which is a non-automated in vitro bacterial susceptibility procedure. The
protocol offers a quantitative outcome for the number of antimicrobial agents, required to inhibit the progression of a targeted microscopic organism. As a primary target, ATDs-loaded
SNPU2I-7I were studied for the anti-tuberculosis efficacy against _M. tuberculosis_ strain H37Rv. Surprisingly, nanopolyurethanes loaded with ATDs are 2 to 40 times more effective than
standard ATDs. MIC of standard isoniazid and streptomycin are 0.20 and 0.90 μg/mL, respectively, against H37Rv strains. In the case of streptomycin-loaded SNPU2I (SM-SNPU2I), the MIC is 0.10
μg/mL, which is 9.0 times more powerful than the standard streptomycin having concentration 0.90 μg/mL with 99% inhibition. It is worthful to conclude that, streptomycin-loaded SNPU4I
(SM-SNPU4I) is 42 times more effective than the native drug, which has proven to be one of the promising drug delivery systems (DDS) for streptomycin. Similarly, in contrast to the parent
ATDs, isoniazid-loaded SNPUs (isoniazid-SNPU3I, 5I-7I) revealed better performance. Actually, isoniazid-loaded SNPU6I, 3I, and 7I worked 2, 6 and 7 times more effectively against H37Rv
tuberculosis strains compared to that of native ATDs (Table 3). ATDs-loaded SNPUs have therefore acted as a powerful way to deliver systems for controlled and sustained drug release, which
is an intriguing and promising feature that cannot be achieved with unloaded/free drugs. MATERIALS AND METHODS Commercially available, analytical-grade starting materials for the synthesis
of carbohydrate-based nanopolyurethanes were purchased from Sigma-Aldrich, TCI, SRL, Rankem and used as received, unless otherwise noted. The FTIR was recorded on KBr pellets with a Perkin
Elmer Model 1600 infrared spectrometer. The structure of the synthesised compound was confirmed by 1H NMR spectroscopy and reported in dimethyl sulfoxide-d6 (DMSO-d6) at room temperature on
a Bruker Avance 500 MHz FTNMR spectrophotometer. 1H NMR data in the presence of internal standard (TMS, 0.0 ppm), chemical shift with multiplicity, coupling constants in Hz, and integration
were recorded in ppm (δ). Measurement of the average particle size of the polymeric nanoparticle was performed by dynamic light scattering (DLS), model no Microtrac Zetatrac, U2771 at 25 °C.
The morphological changes and size of the polyurethane nanoparticles were examined by the High Resolution Transmission Electron Microscope (HRTEM)/TEM, model no JEOL JEM 2100F at an
accelerating voltage of 200 kV. Samples are prepared on a copper grid of carbon-coated wire mesh, with an optimal sample thickness of less than 100 nm. The morphology of the SNPUs and
ATDs-loaded SNPUs was analyzed by field emission scanning electron microscopy (FESEM: ULTRA-55 system, India). Powder X-ray diffraction (P-XRD) measurements were carried out in a Rigaku-made
Smart Lab 9 kW rotating anode X-ray diffractometer (Kyoto, Japan) using Cu-Kα radiation (λ = 1.5418 e´) at 40 kV and 100 mA at an ambient temperature of 2θ (2° min−1). Thermal decomposition
of SNPUs was studied with by TG/DTA 73,000 (EXSTAR) (TGA). Drug loading and release of ATDs-loaded polymeric polyurethanes were studied by UV − vis absorption on a Spectro 2060 + UV −
Visible spectrophotometer. GENERAL PROCEDURE FOR THE PREPARATION OF NANOPOLYURETHANES (SNPU2I-7I) The dried SNCs (200 mg, 0.3 mmol, 1.0 equiv.) in 4.0 mL DMSO was heated at 55 °C for 4 h.
Then, diisocyanate (2I-7I) (2.3 equiv.) was added in the mixture followed by stannous octoate (8I) (0.03 equiv.) was added dropwise as a catalyst, and the resulting solution was further
stirred at 70–85 °C for 5–8 h. The reaction was monitored primarily by the presence of diisocyanates on a TLC plate. Then the cold methanol was added in excess amount until the resulting PU
was precipitated. The solids were recovered by centrifugation and washed extensively with water followed by acetone. Finally, the product was dried in a vacuum oven for 24 h and stored in
vacuum desiccators for further use. THE CHARACTERIZATION DATA FOR SBPU2I, SBPU3I, SBPU4I AND SNPU2I, SNPU3I, SNPU4I29 SBPU5I: 1H NMR (500 MHz, DMSO-d6): δ 5.46–2.06 (starch protons), δ
2.06–5.46 (starch protons), 7.50–8.24 (naphthalene ring Ar protons), 9.18 (NH) ppm; FTIR: ν cm−1 3280 (O–H str., amide N–H), 2933 (C-H str.), 1719 (urethanic C=O str.), 1646 (CNH str.), 1504
(Ar. C=C str.), 1424 (C-N str.), 1152 (C–OH str.). SBPU6I: 1H NMR (500 MHz, DMSO-d6 δ 0.73–0.95 (exocyclic methylene protons), 1.13–2.63 (protons of the cyclic ring), 2.68–5.76 (starch
protons) ppm; FTIR: ν cm−1 3381 (O–H str., amide N–H), 2930, 2852 (C-H str.), 1715 (urethanic C=O str.), 1568 (CNH str.), 1568 (Ar. C=C str.), 1434 (C-N str.), 1156 (C–OH str.). SBPU7I: 1H
NMR (500 MHz, DMSO-d6): δ 0.78–0.91 (exocyclic methylene protons), 1.23–2.07 (cyclic protons), 2.72–5.86 (starch protons), 7.03–7.15 (NH proton) ppm; FTIR: ν cm−1 3288 (O–H str., amide N–H),
2953, 2915 (C-H str.), 1713 (urethanic C=O str.), 1559 (CNH str.), 1568 (Ar. C=C str.), 1446 (C-N str.), 1155 (C–OH str.). SNPU5I: 1H NMR (500 MHz, DMSO-d6 δ 2.15–5.60 (starch protons),
7.65–8.31(naphthalene ring Ar protons), 9.26 (NH). ppm; FTIR: ν cm−1 3397 (O–H str., amide N–H), 2956, 2925, 2836 (C-H str.), 1742 (urethanic C=O str.), 1646 (CNH str.), 1506 (Ar. C=C str.),
1430 (C-N str.), 1157 (C–OH str.). SNPU6I: 1H NMR (500 MHz, DMSO-d6 δ 0.83–0.95 (exocyclic methylene protons), 1.35–2.22 (cyclic protons), 3.05–5.50 (starch protons), 6.85 (NH proton) ppm;
FTIR: ν cm−1 3420 (O–H str., amide N–H), 2956, 2925, 2846 (C-H str.), 1711 (urethanic C=O str.), 1639 (CNH str.), 1564 (Ar. C=C str.), 1455 (C-N str.), 1157 (C–OH str.). SNPU7I: 1H NMR (500
MHz, DMSO-d6 δ 0.75–0.91 (exocyclic methylene protons), 1.25–2.08 (cyclic protons), 2.73–5.83 (starch protons), 7.01–7.15 (NH proton) ppm; FTIR: ν cm−1 3366 (O–H str., amide N–H), 2956,
2925, 2846 (C-H str.), 1702 (urethanic C=O str.), 1649 (CNH str.), 1560 (Ar. C=C str.), 1467 (C-N str.), 1154 (C–OH str.). EXPERIMENTAL PROCEDURE FOR PREPARATION OF ATDS-LOADED STARCH
NANOPOLYURETHANES (SNPU2I-7I) For the preparation of ATDs-loaded SNPU2I-7I, isoniazid, pyrazinamide and rifampicin (10.0 mg) was dissolved in 1.5 mL THF except streptomycin (10.0 mg), which
was dissolved in 1.5 mL water/methanol mixture (1:1, v/v). Then it was added in 13.5 mL THF containing 100.0 mg of nano-PUs and stirred for 24 h at moderate speed. Then, loading efficiency
was determined; a supernatant solution containing the amount of the free drug was taken out and UV–vis absorbance of all samples was measured to examine the unloaded drug concentration.
Further, the loading capacity and loading efficiency were calculated. SOLUTION PREPARATION OF ATDS-LOADED STARCH NANOPOLYURETHANES (SNPU2I-7I) FOR UV–VIS ABSORBANCE SPECTROSCOPY Loading
capacity and loading efficiency of SNPUs for ATDs were calculated using UV–vis titration. A solution of 10.0 mg of isoniazid, pyrazinamide and rifampicin were dissolved separately in 15.0 mL
THF and assumed as 100% (0.67 mg/mL) solutions and diluted it with THF to prepare 1 to 10% solution (0.0067 to 0.067 mg/mL) for INH and PZA, while for RIF, it was converted to10 to 100%
(0.067 mg/mL to 0.67 mg/mL). In case of streptomycin (SM), 10.0 mg was dissolved in 15.0 mL water: methanol (1:1, v/v) mixture and considered as 100% solutions and diluted it with the
mixture of methanol and water to prepare 10 to 100% solution (0.067 to 0.67 mg/mL). The supernatant of ATDs-loaded solutions was diluted to get absorbance data. EXPERIMENTAL PROCEDURE FOR
RELEASE OF ATDS-LOADED STARCH-DERIVED NANO POLYURETHANES (SNPU2I-7I) The release of ATDs-loaded nanopolyurethanes (SNPU2I-7I) was studied in Tris buffer at pH 2 and 8. An amount of 25.0 mg
ATDs-loaded PUs was taken in a vial containing 10.0 mL Tris buffer solution and the mixture was stirred at room temperature at moderate speed. To determine the releasing efficiency, a
specific amount of supernatant was withdrawn and analyzed using absorption spectroscopy, and an equal volume of fresh Tris buffer was added to the sample vial. The collected sample was
analyzed by absorption spectroscopy for determination of release percentage of the drug. SOLUTION PREPARATION OF ATDS-LOADED STARCH NANOPOLYURETHANES (SNPU2I-7I) FOR DRUG RELEASE STUDY BY
UV–VIS ABSORBANCE SPECTROSCOPY Spectroscopic titration curve of the drug shows a plot of absorbance as a function of the different amount of ATDs. UV–vis absorbance spectra of ATDs were
recorded using the same releasing medium with known concentration. UV–vis absorbance spectra were recorded with 1 to 10% (0.02 to 0.20 mg/mL) pyrazinamide, 1 to 10% (0.02 to 0.20 mg/mL)
isoniazid, 1 to 50% (0.02 to 1.0 mg/mL) rifampicin, and 10 to 100% (0.2 to 2.0 mg/mL) streptomycin at pH 2 and 8 in Tris buffer. UV–vis spectra of supernatant were documented using the same
medium as blank. All the data were measured at room temperature. ANTI-MYCOBACTERIAL ACTIVITY AGAINST _MYCOBACTERIUM SMEGMATIS_ The fast-growing, acid-fast bacilli _Mycobacterium smegmatis_
are used for the screening of potential samples for the assessment of anti-tuberculosis compounds38,39. The use of this rapidly growing acid-fast bacillus was advantageous over _M.
tuberculosis_ because anti-mycobacterial testing method of compounds against this organism is simple, economic and less tedious. Their rapid growth rate within 2–3 days and nonpathogenic
nature made the handling safe and time-saving. METHOD FOR IN VITRO ANTI-TUBERCULOSIS EFFICACY: MINIMUM INHIBITION CONCENTRATION BY LOWENSTEIN–JENSEN (LJ) SLOPE METHOD The ATDs-loaded SNPUs
were used for anti-tuberculosis assay. Different types of controls like drugs, vehicles, agar, organisms, and known antibacterial drugs were applied for the study. Experimental samples and
standard drugs were tested against the _M. tuberculosis_ H37Rv cultures [Acid Fast Bacilli] and MDR strains (resistant to isoniazid and rifampicin). The LJ nutrient medium was used to grow
and dilute the drug suspension, and the McFarland standard was followed for inoculum size on bacterial growth to test strain. Herein, the DMSO acted as diluents/vehicle to find the desired
concentration of the synthesized anti-tuberculosis agents and standard drugs to test upon standard microbial strains. STATISTICAL ANALYSIS All acquired data were reproduced and reported as
means ± standard deviation (SD). Origin was used to evaluate any significant differences in the experiments. CONCLUSIONS We have successfully developed starch-derived bulk and
nanopolyurethanes for utilizing as promising and biodegradable materials for delivery devices to improve the anti-tuberculosis therapeutic efficacy of commonly used first-line
anti-tuberculosis drugs such as isoniazid, rifampicin, pyrazinamide and streptomycin. The structural and morphological features, thermal stability and crystalline quality of the experimental
compounds were unraveled by various advanced instrumental techniques, such as NMR, FTIR, UV–Vis, DLS, TEM, HRTEM, FESEM, TGA and XRD. The SNPU3I and SNPU4I were synthesised with an average
particle size ranging from 27.35–42.38 nm to 126.89–218.60 nm, respectively, from the six starch-derived nanopolyurethanes (SNPU2I-7I). The loading efficiency of anti-tuberculosis drugs on
starch-derived nanopolyurethanes and native starch was moderate to good and was found in the 60–97%. For this analysis, the pH-dependent drug-releasing study showed that starch-derived
nanopolyurethanes had a sustained release profile for all drug-loaded carriers except streptomycin; however, mostly burst-release was reported by starch-derived bulk polyurethanes. For
pyrazinamide-loaded SNPU5I, the cumulative release of pyrazinamide (%) was up to ~ 93% at pH 2 and ~ 70% at pH 8; the cumulative drug release percentage for isoniazid at pH 8 was up to ~
80%, while SNPU2I and SNPU6I exhibited ~ 90% and 54% cumulative release for isoniazid, respectively, over 24 h at pH 8. Apart from SNPU3I, which showed ~ 75% cumulative release of
streptomycin at pH 8 over 24 h, most streptomycin-loaded nanopolyurethanes showed burst release within a short period of time. Primarily, the selected samples of SBPUs against _M.
smegmatis_, with rifampicin as control, presented good results for streptomycin. Streptomycin-loaded nanopolyurethane (0.021 μg/mL) was found to be ~ 40 times more active than free
streptomycin (0.90 μg/mL) itself in the anti-tuberculosis assay against the _Mycobacterium tuberculosis_ H37Rv strain. In comparison, activity enhancement was demonstrated by
isoniazid-loaded SNPUs and pyrazinamide-loaded SNPUs. INH-loaded SNPU6I, 5I, 3I, and 7I served 2, 5, 6, and 7 times more effective against H37Rv tuberculosis strains and found as better
anti-tuberculosis agents as compared to pure INH. REFERENCES * Daniel, T. M. The history of tuberculosis. _Respir. Med._ 100, 1862–1870 (2006). Article Google Scholar * Hunter, R. &
Actor, J. The pathogenesis of post-primary tuberculosis A game-changer for vaccine development. _Tuberculosis_ 116, S114–S117 (2019). Article CAS Google Scholar * Fogel, N. Tuberculosis:
A disease without boundaries. _Tuberculosis_ 95, 527–531 (2015). Article Google Scholar * Singh, S., Mariappan, T., Shankar, R., Sarda, N. & Singh, B. A critical review of the probable
reasons for the poor variable bioavailability of rifampicin from anti-tubercular fixed-dose combination (FDC) products, and the likely solutions to the problem. _Int. J Pharm._ 228, 5–17
(2001). Article CAS Google Scholar * Panchagnula, R. _et al._ Fixed dose combinations for tuberculosis: Lessons learned from clinical, formulation and regulatory perspective. _Methods
Find Exp. Clin. Pharmacol._ 26, 703–721 (2004). Article CAS Google Scholar * Addington, W. W. Patient compliance: the most serious remaining problem in the control of tuberculosis in the
United States. _Chest_ 76, 741–743 (1979). Article CAS Google Scholar * Desai, S., Bera, S. & Mondal. D. Multifaceted synthesis, properties and applications of polyurethanes and its
composites. _Curr. Org. Chem._ 23, 1–29, (2019). and reference therein. * Garg, T., Rath, G. & Murthy, R. Current nanotechnological approaches for an effective delivery of bioactive drug
molecules to overcome drug resistance tuberculosis. _Curr. Pharm. Des._ 21, 3076–3089 (2015). Article CAS Google Scholar * Garg, T., Bhandari, S., Rath, G. & Goyal, A. K. Current
strategies for targeted delivery of bio-active drug molecules in the treatment of brain tumor. _J. Drug Target._ 23, 865–887 (2015). Article CAS Google Scholar * Malik, R., Garg, T.,
Goyal, A. K. & Rath, G. Diacerein-Loaded novel gastroretentive nanofiber system using PLLA: Development and in vitro characterization. _Artif. Cells Nanomed. Biotechnol._ 44, 928–936
(2016). CAS PubMed Google Scholar * Navalakhe, R. & Nandedkar, T. Application of nanotechnology in biomedicine. _Indian J. Exp. Biol._ 45, 160–165 (2007). CAS PubMed Google Scholar
* Bera, S. & Mondal, D. Insights of synthetic analogues of anti-leprosy. _Bioorg. Med. Chem._ 27, 2689 (2019). Article CAS Google Scholar * Bera, S. & Mondal, D. A role for
ultrasound in agents the fabrication of carbohydrate-supported nanomaterials. _J. Ultrasound_ 22, 131 (2019). Article Google Scholar * Bera, S., Mondal, D. _Stimuli-Sensitive Nanomaterials
for Antimicrobial Drug Delivery_ (Ed. Prof. A. M. Grumezescu) 271–302 (Elsevier Inc., 2018) * Aboutaleb, E. _et al._ Improved antimycobacterial activity of rifampin using solid lipid
nanoparticles. _Int. Nano Lett._ 2, 33 (2012). Article Google Scholar * Leitzke, S. _et al._ Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin
in experimental Mycobacterium avium infection. _Agents Chemother._ 42, 459–461 (1998). Article CAS Google Scholar * Pandey, R., Ahmed, Z., Sharma, S. & Khuller, G. Nanoparticle
encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. _Tuberculosis_ 83, 373–378 (2003). Article Google Scholar * Dube, A. _et al._
Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. _Nanomedicine_ 10, 831–838 (2013). Article Google Scholar * Burkeev, M. Z.,
Tazhbaev, E. M., Zhaparova, L. Z., Zhappar, N. K. & Zhumagalieva, T. S. Synthesis and characterization of poly (DL-lactic acid) nanoparticles loaded with the antituberculosis drug
isoniazid. _Pharm. Chem. J._ 50, 38–42 (2016). Article Google Scholar * Xu, R., Manias, E., Snyder, A. J. & Runt, L. Low permeability biomedical polyurethane nanocomposites. _J.
Biomater. Res._ 64, 114–119 (2002). Google Scholar * Xu, R., Manias, E., Snyder, A. J. & Runt, L. New biomedical poly (urethane urea)−layered silicate nanocomposites. _Macromolecules_
34, 337–339 (2001). Article ADS CAS Google Scholar * Anderson, J. M. _et al._ Recent advances in biomedical polyurethane biostability and biodegradation. _Polym. Int._ 46, 163–171
(1998). Article CAS Google Scholar * Gorna, K. & Gogolewski, S. Biodegradable polyurethanes for implants. II. In vitro degradation and calcification of materials from poly
(ε‐caprolactone)–poly (ethylene oxide) diols and various chain extenders. _J. Biomed. Mater. Res._ 60, 592–606 (2002). * Cao, Y., Baiyisaiti, A., Wong, C.-W., Hsu, S.-H. & Qi, R.
Polyurethane nanoparticle-loaded fenofibrate exerts inhibitory effects on nonalcoholic fatty liver disease in mice. _Mol. Pharm._ 15, 4550−4557 (2018). * Muttil, P. _et al._ Inhalable
microparticles containing large payload of anti-tuberculosis drugs. _Eur. J. Pharm. Sci._ 32, 140–150 (2007). Article CAS Google Scholar * Bhosle, G. S., Nawale, L., Yeware, A. M.,
Sarkar, D. & Fernandes, M. Antibacterial and anti-TB tat-peptidomimetics with improved efficacy and half-life. _Eur. J. Med. Chem._ 152, 358–369 (2018). * Solanki, A., Das, M. &
Thakore, S. A review on carbohydrate embedded polyurethanes: An emerging area in the scope of biomedical applications. _Carbohydr. Polym._ 181, 1003–1016 (2018). Article CAS Google Scholar
* Ou, C. W., Su, C. H., Jeng, U. S. & Hsu, S. H. Characterization of biodegradable polyurethane nanoparticles and thermally induced self assembly in water dispersion. _ACS Appl. Mater.
Interfaces_ 6, 5685–5694 (2014). Article CAS Google Scholar * Desai, S. Bera, S., Mondal, D., Singh. M. Polyurethane-functionalized starch nanoparticles for the purification of
biodiesel. _J. Appl. Polym. Sci. 134_, 44463 (2017). * Desai, S., Bera, S., Mondal, D. & Singh, M. Better biodiesel: A nano-cleansing solution. _Curr. Sci._ 112, 442 (2017). Google
Scholar * Da Róz, A., Curvelo, A. & Gandini, A. Preparation and characterization of cross-linked starch polyurethanes. _Carbohydr. Polym._ 77, 526–529 (2009). Article Google Scholar *
Liang, R.-C. Gemini quaternary ammonium-incorporated biodegradable multiblock polyurethane micelles for brain drug delivery. _RSC Adv._ 5, 6160–6171 (2015). Article ADS CAS Google
Scholar * Freichels, H. mannose functionalized hydroxyethyl starch nanocapsules: En route to drug delivery systems with targeting properties. _J. Mater. Chem. B_ 1, 4338–4348 (2013).
Article CAS Google Scholar * Kubota, T. Synthesis of new carbohydrate-based polyurethanes and their application in the purification of methyl esters (biodiesel). _J. Polym. Res._ 20, 48
(2013). Article Google Scholar * Main mechanisms to control the drug release, Editor(s): Bruschi, M. L. _Strategies to Modify the Drug Release from Pharmaceutical Systems_ 37–62 (Woodhead
Publishing, 2015). ISBN 9780081000922. * Mathematical models of drug release, Editor(s): Bruschi, M. L., _Strategies to Modify the Drug Release from Pharmaceutical Systems_ 63–86 (Woodhead
Publishing, 2015). ISBN 9780081000922. * Fu, Y. & Kao, W. J. Drug release kinetics and transport mechanisms of nondegradable and degradable polymeric delivery systems. _Expert Opin. Drug
Deliv._ 7, 429–444 (2010). Article CAS Google Scholar * Jimenez-Arellanes, A., Meckes, M., Ramirez, R., Torres, J. & Luna-Herrera, J. Activity against multidrugresistant
_Mycobacterium tuberculosis_ in Mexican plants used to treat respiratory diseases. _Phytother. Res._ 17, 903–908 (2003). Article Google Scholar * Gupta, V. K. Anti-mycobacterial activity
of lichens. _Pharm. Biol._ 45, 200–204 (2007). Article Google Scholar Download references ACKNOWLEDGEMENTS SD is grateful for the non-NET fellowship to UGC, India and we all are indebted
to the Central University of Gujarat, India, for the infrastructural facilities. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Chemical Sciences, Central University of Gujarat,
Gandhinagar, 382030, India Shivang K. Desai, Dhananjoy Mondal & Smritilekha Bera Authors * Shivang K. Desai View author publications You can also search for this author inPubMed Google
Scholar * Dhananjoy Mondal View author publications You can also search for this author inPubMed Google Scholar * Smritilekha Bera View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS S.B. and D.M. wrote the main manuscript and S.D. prepared all the figures. All authors reviewed the manuscript. CORRESPONDING AUTHORS
Correspondence to Dhananjoy Mondal or Smritilekha Bera. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is
not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Desai,
S.K., Mondal, D. & Bera, S. Polyurethane-functionalized starch nanocrystals as anti-tuberculosis drug carrier. _Sci Rep_ 11, 8331 (2021). https://doi.org/10.1038/s41598-021-86767-1
Download citation * Received: 19 February 2020 * Accepted: 16 March 2021 * Published: 15 April 2021 * DOI: https://doi.org/10.1038/s41598-021-86767-1 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative