3′-Sialyllactose prebiotics prevents skin inflammation via regulatory T cell differentiation in atopic dermatitis mouse models
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
3′-Sialyllactose (3′-SL), a natural prebiotic, maintains immune homeostasis and exerts anti-inflammatory and anti-arthritic effects. Although regulatory T cells (Tregs) prevent excessive
inflammation and maintain immune tolerance, the effect of 3′-SL on Treg regulation is unclear. This study aimed to investigate the effect of 3′-SL on Treg responses in atopic dermatitis (AD)
pathogenesis. Oral administration of 3′-SL reduced AD-like symptoms such as ear, epidermal, and dermal thickness in repeated topical application of house dust mites (HDM) and
2,4-dinitrochlorobenzene (DNCB). 3′-SL inhibited IgE, IL-1β, IL-6, and TNF-α secretion and markedly downregulated AD-related cytokines including IL-4, IL-5, IL-6, IL-13, IL-17, IFN-γ, TNF-α,
and Tslp through regulation of NF-κB in ear tissue. Additionally, in vitro assessment of Treg differentiation revealed that 3′-SL directly induced TGF-β-mediated Treg differentiation.
Furthermore, 3′-SL administration also ameliorated sensitization and elicitation of AD pathogenesis by suppressing mast cell infiltration and production of IgE and pro-inflammatory cytokines
in mouse serum by mediating the Treg response. Furthermore, Bifidobacterium population was also increased by 3′-SL administration as prebiotics. Our data collectively show that 3′-SL has
therapeutic effects against AD progression by inducing Treg differentiation, downregulating AD-related cytokines, and increasing the Bifidobacterium population.
Prebiotics are substances that improve the intestinal environment as a nutrient source for probiotics that help the growth of intestinal microbiota1. Prebiotics are often composed of
carbohydrates such as oligosaccharides, most of which are in the form of dietary fibre2. Probiotics have a beneficial effect on the intestinal environment, prevent the growth of harmful
bacteria in the intestine, improve immunity, ameliorate skin diseases such as atopic dermatitis (AD) and psoriasis, and inhibit metabolic syndrome3. There are hundreds of beneficial bacteria
that live in the intestine, but very few strains can be cultured outside the gut. Therefore, the only way to increase the number of desired strains without external supplementation is to
use prebiotics. Notably, these beneficial microbiotas are known to be closely related to many autoimmune diseases such as AD.
AD is a chronic inflammatory skin disease common among infants and children and is characterized by itching erythema and thick skin caused by immune disruption, genetic defects, and
environmental factors4. In AD, Th2 cell-mediated immune responses are more predominant than Th1-mediated immune responses and they play an important role in the pathogenesis5. When foreign
antigens penetrate the damaged skin barrier, dendritic cells recognize the antigen and activate Th2 cells. In activated Th2 cells, IL-4, IL-5, IL-13, and atopic-related cytokines including
IL-17, and Tslp are secreted, and B cells are activated by IL-4 to secrete IgE5,6,7.
Mast cells are the key effector cells causing allergic reactions and are typically activated by IgE receptors, and undergo degranulation, wherein various inflammatory substances are
released, e.g., histamine, serotonin, and tumor necrosis factor-alpha (TNF-α)8,9. Furthermore, mast cells synthesize and release various cytokines, including IL-4. These cytokines promote
inflammation and facilitate intradermal penetration of more inflammatory cells8,10.
Regulatory T cells (Tregs) regulate allergic reactions and significantly contribute to immunosuppression and immune tolerance11. Tregs markedly express the forkhead box p3 transcription
factor (Foxp3), encoded by X-chromosomal gene FOXP312. A dysfunctional Treg leads to autoimmune diseases, including atopic dermatitis, systemic lupus, and asthma12,13,14. Numerous studies
have shown that the depletion of Tregs results in an autoimmune phenotype, increasing the production of Th2-related cytokines, and elevating serum IgE levels in mice13,14,15. Both an
imbalance in Th1 and Th2 cells and aberrant immune regulation caused by Tregs constitute an important mechanism underlying the pathogenesis of AD16. Indeed, it has been suggested that Tregs
reside in the skin, contributing to immune surveillance17,18. Rapamycin and metformin regulate Tregs through Treg expansion in tissues; hence, these compounds are used as therapeutic agents
for autoimmune diseases19,20. However, because some of the side effects of these drugs include lactic acidosis, accelerated cataract formation, gastrointestinal intolerance, and erythema,
new substances are required21,22. There has been growing interest in therapy for autoimmune diseases, including AD, based on probiotics and prebiotics through the induction of Treg
differentiation. Prebiotics increase immunological tolerance via expansion of the intestinal microbiota, which induces the differentiation of Tregs and promotes suppressive activities
through cell surface receptors such as GPCR (GPR45 and GPR109A).
The prebiotic 3′-SL is abundant in human milk and consists of N-acetylneuraminic acid linked to the galactosyl subunit of lactose23. Numerous studies have reported on the beneficial effects
of 3′-SL for inflammation and immune homeostasis via changing the intestinal microbiota profiling24,25. Moreover, 3′-SL ameliorates the progression of rheumatoid arthritis by downregulating
chemokines and cytokines and alleviates osteoarthritis by stimulating cartilage regeneration and protecting cartilage from destruction26,27. However, the effect of 3′-SL on AD pathogenesis
and its regulation of Treg responses are largely unknown. Accordingly, this study aimed to investigate the effect of 3′-SL on Treg responses in AD pathogenesis.
Major triggering allergens are those of the Dermatophagoides farinae and D. pteronyssinus house dust mites (HDM), and 95 percent of human patients with AD display serum levels of
HDM-specific IgE28. To determine the effect of 3′-SL on AD progression, experimental AD was induced via treatment of mouse ears with HDM at 2-day intervals (Fig. 1, Supplementary Fig. 1b,c),
or by treatment with 1% 2,4,-dinitrochlorobenzene (DNCB) at 7-day intervals (Fig. 2, Supplementary Fig. 1d,e). Furthermore, we checked whether 3′-SL prevents allergic sensitization,
elicitation, or both. Firstly, 3′-SL was orally administered after elicitation phase (Fig. 1, Supplementary Fig. 1b) or sensitization phase (Supplementary Fig. 1d, 2) in HDM-induced AD mice
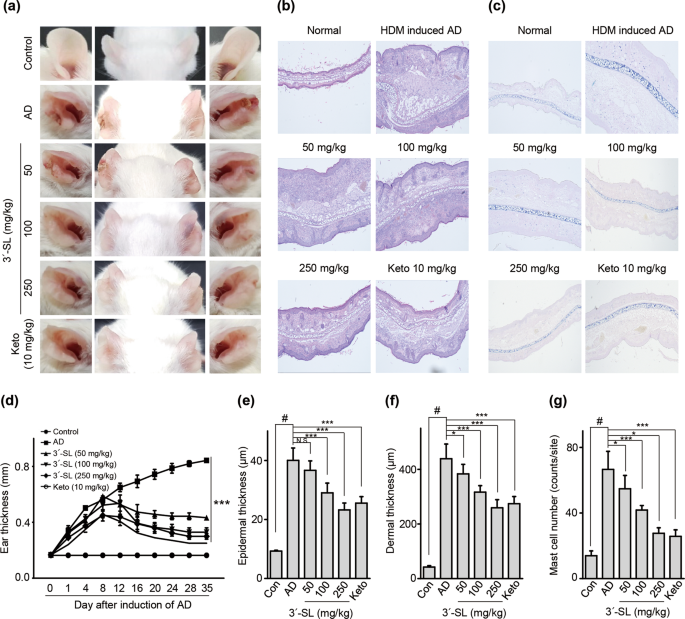
model. Although mouse-ear thickness increased upon treatment with HDM, 3′-SL significantly decreased the ear thickness in both elicitation phase (Figs. 1a, 2d) and sensitization
(Supplementary Fig. 2a,d). Furthermore, after elicitation (Fig. 1) or sensitization stage, macroscopic analysis revealed that HDM induced AD lesions, including erythema, oedema, scaling, and
bleeding, which were suppressed upon administration of 3′-SL (Fig. 1a, Supplementary Fig. 2d). Histological changes were analysed by haematoxylin-eosin, and Toluidine Blue staining, which
indicated that epidermal and dermal thickness increased upon HDM treatment, and intradermal mast cell infiltration significantly increased in comparison with the control. However, these
HMD-mediated changes were markedly decreased by 3′-SL oral administration (Fig. 1c,e–g Supplementary Fig. 2c,e–g).
Therapeutic effect of 3′-sialyllactose (3′-SL) after elicitation in HDM-induced atopic dermatitis (AD) mouse model. Oral administration of 3′-SL and or Ketotifen as positive control done at
2-day intervals for 4 weeks after elicitation stage. (a) Variation in ear thickness during the course of HDM-induced AD. Atopic episodes during the experiment are shown as photographs. (d)
Variation in ear thickness from 35 d before the experiment to the end of the experiment. Microphotographs of sections of the left ear 35 d after the onset of AD. The sections were stained
with haematoxylin and eosin (H&E) (b) and Toluidine Blue (c). Original magnification was 100×. (e) Epidermal and (f) dermal thickness was quantified from H&E-stained microphotographs. (g)
The number of infiltrating mast cells in the ear sections, as determined through Toluidine Blue staining. At least three randomly selected sites were analysed for each cell count experiment.
Data are presented as mean ± SD values for each group (n = 6). #P