Surgically resected esophageal squamous cell carcinoma: patient survival and clinicopathological prognostic factors
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We aimed to report patients’ survival after surgical resection of eSCC and to ascertain the clinical, imaging, and pathological factors related to patient prognosis. This
retrospective study included 435 patients with eSCC of <stage T2 (median follow-up period, 49.3 months). A total of 103 (23.7%) patients died, and 89 (20.5%) experienced recurrence during
follow-up. The maximum standardized uptake value (SUVmax) on positron emission tomography (PET)/computed tomography (CT) of the primary tumor was significantly correlated with tumor length,
nodal metastasis, and pathologic T stage in a positive linear fashion. In the multivariate analysis, higher SUVmax on PET/CT was a negative prognostic factor for both disease-free survival
(DFS) and overall survival (OS). Contrarily, the presence of nodal metastasis was a prognostic factor only for DFS, and pathologic T stage only for OS. By applying SUVmax cut-off, both DFS
and OS were significantly different among three groups when divided by cut-off values (A: SUVmax ≤ 3.05, B: SUVmax 3.06 - 5.64, C: SUVmax ≥ 5.65). In patients with a surgically resectable
eSCC, measuring the SUVmax of the primary tumor during PET/CT can help predict patient survival. Additionally, PET/CT renders triage criterion for endoscopic submucosal dissection (ESD; T1a
cancer and SUVmax, ≤3.05). SIMILAR CONTENT BEING VIEWED BY OTHERS RISK STRATIFICATION OF OESOPHAGEAL SQUAMOUS CELL CARCINOMA USING CHANGE IN TOTAL LESION GLYCOLYSIS AND NUMBER OF
PET-POSITIVE LYMPH NODES Article Open access 25 February 2023 18F-FLUORODEOXYGLUCOSE POSITRON EMISSION TOMOGRAPHY/COMPUTED TOMOGRAPHY PARAMETERS ARE ASSOCIATED WITH HISTOLOGICAL OUTCOMES IN
SUPERFICIAL ESOPHAGEAL SQUAMOUS CELL CARCINOMA Article Open access 30 July 2024 COMPARISON OF THREE LYMPH NODE STAGING SYSTEMS IN EVALUATING THE PROGNOSIS OF PATIENTS WITH PT3 ESOPHAGEAL
SQUAMOUS CELL CARCINOMA Article Open access 13 October 2020 INTRODUCTION Esophageal cancer (ECA) is the eighth most prevalent cancer, accounting for 4% of all cancers. It is also the sixth
leading cause of cancer-related deaths. Although the prevalence of esophageal adenocarcinoma is rapidly increasing in Western countries, including the United States of America, the United
Kingdom, and Australia, the most common histopathologic subtype worldwide is esophageal squamous cell carcinoma (eSCC); it accounts for 85% of all esophageal cancers1. The incidence of ECA
is rapidly increasing. The overall 5-year survival rate ranges from 15% to 25%, with the best outcomes expected in ECAs diagnosed early2. In patients with an early-stage eSCC less than
clinical T2 stage, treatment of choice is esophagectomy3,4. However, esophagectomy brings high risks of surgical complication and hospital mortality with incidences varying between 17–74%
and 7–9%, respectively5. In patients with a T1a eSCC, endoscopic resection such as endoscopic submucosal dissection (ESD) can be considered as an alternative treatment option to surgery with
cure rates similar to those in esophagectomy and with low complication rate3,4,6. According to recent Japanese Esophageal Society Guidelines, relative indication of ESD is extended to some
portion of T1b eSCC involving the muscularis mucosa or <200-μm invasion of the submucosa7,8. Thus, adequate and accurate staging should be performed to provide adequate treatment options
to patients, especially in elderly patient who cannot tolerate surgical procedure. Most clinical staging work-ups of ECAs are conducted using endoscopic ultrasonography (EUS), computed
tomography (CT), and 18F-fluorodeoxyglucose positron emission tomography (FDG) PET/CT. In our previous study, we showed that the depth of tumor invasion (T stage) and the presence of LN
metastasis (N stage) could be identified with FDG PET/CT even in early esophageal SCCs. However, the results are slightly inferior to those of EUS9. With the measurement of maximum
standardized uptake value (SUVmax) of the primary tumor at FDG PET/CT, the depth of tumor invasion (T stage) could be readily predicted. Therefore, we hypothesized that measuring the SUVmax
of the primary tumor in early-stage eSCCs could help predict patient prognosis. The purpose of this study was to report patients’ survival after surgical resection of eSCCs, including T1a-,
T1b-, and T2-stage cancers, and to ascertain the clinical, imaging, and pathological factors related to patient prognosis. RESULTS PATIENT CHARACTERISTICS AND CLINICAL OUTCOMES Of the 435
patients, 403 were men, and 32 were women. Their ages ranged from 31 to 90 years (mean, 64 years). One-hundred and thirty-one patients had less than T1a stage disease, 234 patients had T1b
stage disease, and 70 patients had T2 stage disease. Primary tumors presented pathologically with N0 disease in 298 (68.5%) patients. The presence of nodal metastasis was confirmed with
surgical specimens in 137 (31.5%) patients. N1 disease was identified in 99 (22.8%) patients, N2 in 32 (7.4%) patients, and N3 in six (1.4%) patients. Other patient characteristics are
detailed in Table 1. During surgical resection, four patients underwent R1 resection indicating the presence of microscopically cancer-cell-positive tumor margins. Seventy-seven patients
underwent adjuvant therapy, of which 74 underwent chemotherapy, two underwent radiotherapy, and one underwent concurrent chemoradiation. The median follow-up duration was 49.3 months (range;
0.8 to 105 months). One-hundred and three patients died, and 89 patients experienced recurrence during the follow-up period. Details of the relationship between death or recurrence and T
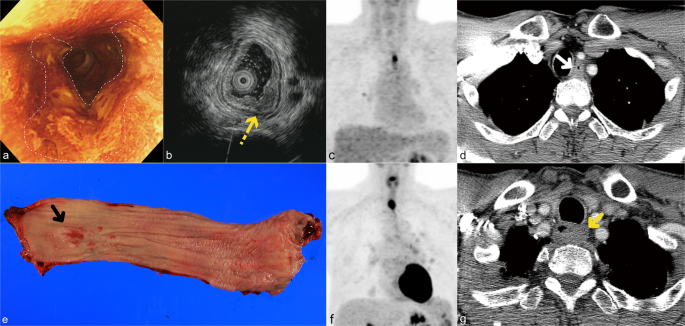
stages are summarized in Table 1. Among patients who experienced recurrence, there were three patients with an isolated local tumor recurrence (Fig. 1), 48 patients with isolated regional
lymph node metastasis, eight patients with both local recurrence and regional lymph node recurrence, 14 patients with regional lymph node recurrence and distant metastasis, 12 patients with
distant metastasis, three patients with local tumor recurrence, regional lymph node metastasis and distant metastasis, and one patient with local tumor recurrence and distant metastasis.
Among the 45 metastases in the 30 patients with distant metastasis, the most common sites of metastasis were the lungs (n = 13), liver (n = 12), bone (n = 9), pleura (n = 4), thyroid glands
(n = 2), adrenal glands (n = 2), spleen (n = 1), ureter (n = 1), and soft tissue (n = 1). RELATIONSHIPS AMONG SUVMAX AND TUMOR CHARACTERISTICS, T, OR N STAGE SUVmax was significantly
correlated with both pathologic T stage and the presence of nodal metastasis in a linearly positive fashion (r = 0.536, _p_ < 0.001; r = 0.282, _p_ < 0.001; respectively). However,
there was no significant correlation between SUVmax and tumor differentiation (_p_ = 0.520). SUVmax was also significantly correlated with pathologic N stage in a linearly positive fashion
(r = 0.313, _p_ < 0.001). Tumor size, defined as the maximum diameter of the tumor in a pathological specimen, was also significantly linearly correlated with SUVmax (r = 0.342, _p_ <
0.001). VALIDATION OF THE DIAGNOSTIC PERFORMANCE OF PREOPERATIVE T STAGING WITH PET/CT There were no significant differences in terms of the demographics or tumor characteristics of patients
in the validation group and the original experimental group. Details of patients’ characteristics are described in Table 2. For differentiating ≤T1a from other eSCCs, the sensitivity,
specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of PET/CT in the validation group were 51.7% (16/29, 95% confidence interval [CI]; 32.5–70.6%),
82.4% (71/85, 95% CI; 73.9–90.75%), 75.4% (86/114, 95% CI; 66.5–83.0%), 51.7% (15/29) and 83.5% (71/85), respectively, whereas those in the experimental study group were 74.8% (98/131, 95%
CI; 66.5–82.0%), 70.1% (213/304, 95% CI; 64.6–75.2%), 71.5% (311/435, 95% CI; 67.0–75.7%), 51.9% (98/189) and 86.6% (213/246), respectively. PROGNOSTIC SIGNIFICANCE OF SUVMAX WITH SURVIVAL
AND RECURRENCE In the receiver operating characteristic (ROC) curve analysis, a cut-off value of SUVmax 3.05 and 5.65, respectively, was the most useful for differentiating <T1a eSCCs
from other cancers and for differentiating T1 (<T1b) eSCCs from T2 eSCCs9. By applying these SUVmax cut-off values, we statistically proved that both DFS and OS were significantly
different among the three groups when divided by the cut-off values (A: SUVmax ≤ 3.05, B: SUVmax 3.06–5.64, C: SUVmax ≥ 5.65), except the OSs between groups A and B (DFS, _p_ < 0.001; A
vs. B, _p_ = 0.005; A vs. C, _p_ < 0.001; B vs. C, _p_ = 0.010; OS, _p_ < 0.001; A vs. B, _p_ = 0.167; A vs. C, _p_ < 0.001; B vs. C, _p_ = 0.009; Figs. 2 and 3). The five-year DFS
was 86.5% in group A, 78.4% in group B, and 59.5% in group C (Table 3). The five-year OS was 80.6% in group A, 78.7% in group B, and 59.5% in group C (Table 4). However, clinical T stage,
determined by EUS, was not statistically different between the groups in terms of DFS or OS (DFS: p = 0.324, OS: _p_ = 0.753) (Figs. 2 and 3). In the univariate analysis, sex, adjuvant
therapy, nodal metastasis, SUVmax, and pathological T stages were significant prognostic factors for tumor recurrence. SUVmax, the presence of nodal metastasis, and pathological T stage were
significant prognostic factors for survival. As the cut-off values of SUVmax were extracted by pathological T stage groups, we assumed two different models for the multivariate analysis
using pathological T stage and hypothetical stages using SUVmax (PET stage groups, Tables 3 and 4). This was despite the severity of multicollinearity not being significant between those
with pathological T stage and hypothetical stage as determined by the SUVmax (variance inflation factor [VIF] = 1.594). In the multivariate analysis, SUVmax and the presence of nodal
metastasis were significant factors for disease recurrence. SUVmax and pathological T stage were significant factors for patient survival (Tables 3 and 4; Figs. 2 and 3). COMPARISON OF THE
PROGNOSTICATION PERFORMANCE OF EACH STAGING SYSTEM For predicting DFS, the iAUC value of hypothetical staging using SUVmax was 0.645. This was greater than those of other staging systems at
all time points (pathological T staging, 0.598; clinical T staging using EUS, 0.533). For predicting the OS, the iAUC value of hypothetical staging using SUVmax was 0.602. This was also
greater than those of other staging systems at all time points (pathological T staging, 0.580; clinical T staging using EUS, 0.525). DISCUSSION It is known that with surgically resected
eSCCs, the pathological stage is an independent risk factor for recurrence within the first year after surgery and that the presence of lymph node metastasis is the most common relapse
pattern after an esophagectomy10. In our study, the SUVmax on PET/CT was also observed as an independent factor for predicting both future recurrences and patient survival, along with the
pathologic T stage (for predicting survival) and nodal metastasis presence (for disease recurrence). Additionally, the SUVmax was positively correlated with both pathological T and N stages
and tumor length. There have been a few studies published recently regarding the value of measuring SUVmax on PET/CT as a prognostic factor in eSCCs. These studies showed similar results to
those seen in this study. According to Jeon _et al_.11, venous invasion and high SUVmax could be important prognostic factors for disease recurrence in T1N0M0 eSCCs. Song _et al_. also
reported similar results from their cohort in which all stages of eSCCs were included12. Our results were derived from a patient cohort, included a larger study population. And our study had
a longer follow-up period than other studies. Moreover, we performed a more comprehensive analysis to look at predicting pathological T stage and also survival using the SUVmax of primary
tumors. In the Kaplan-Meier curve analyses for both DFS and OS based on the SUVmax cut-off, two survival curves of groups B and C crossed over each other at the time point of 65 months after
surgery (Figs. 2b and 3b). The same phenomenon was also seen in the Kaplan-Meier curve for OS based on the pathologic T staging (Fig. 3a). This may have resulted from a small number of
samples whose follow-up periods were greater than 70 months. The 95% CIs of group B for both DFS and OS widened rapidly 70 months after surgery (Supplemental Fig. 2). The usefulness of FDG
PET/CT for tumor staging or prognostication in esophageal cancer has been unclear, particularly in patients with early-stage (T1) cancers and in esophageal adenocarcinomas. Cuellar _et
al_.13 asserted that FDG PET/CT is not useful in evaluating adenocarcinoma of the esophagus when endoscopic biopsy discloses Tis and T1 in tumor stage. They believed that because regional
nodal metastases are uncommon and distant metastases are rare in patients with T1-stage esophageal cancers as well as because FDG PET/CT can lead to inappropriate management, FDG PET/CT
should not be used in evaluating patients with clinical Tis and T1 esophageal adenocarcinomas. Contrastingly, in our study, in which eSCCs of T1a, T1b, and T2 were included, measuring SUVmax
appeared to help differentiate the T stages and in predicting oncoming recurrent disease (DFS) and patient survival (OS) in patients who had a surgically resected eSCC. Reduced spatial and
contrast resolutions of PET/CT were considered one of the interpretative downsides of using PET/CT for T-descriptor14. However, by using SUVmax of the primary tumor rather than the
visualized gross tumor volume, PET/CT can help differentiate pathologic T stages. In our previous study, measuring the SUVmax of primary cancer was shown to assist in differentiating <T1a
from T1b or T2 cancers and between <T1 and T2 cancers9. In our study, the cut-off value of SUVmax 3.05 was effective in discriminating T1a-stage from T1b- or T2-stage eSCCs. Similarly,
Furukawa _et al_.15 showed that FDG-PET helped to diagnose tumors in 40 consecutive cT1N0M0 eSCC patients involving the submucosa but not beyond the middle one-third of the submucosa (SM2)
and beyond, or tumors having occult lymph node metastasis. Their proposed optimal cut-off SUVmax value of 2.7 can be used distinguishing ESD candidate patients from advanced stage eSCC
patients with an SM2 involvement and beyond (21, 52.5%) or lymph node metastasis (6, 15%). Our optimized cut-off value SUVmax 3.05 was slightly higher, partly because we tried to
discriminate eSCCs of T1a or less from higher-stage cancers. It could also be because we used a larger study population (435 eSCC patients). The pathologic T1b stage includes any tumors
involving the submucosa (SM1, SM2, and SM3). In a prospective validation study using different patient cohorts, PET/CT, and the SUVmax of the tumor showed its efficaciousness in triaging
patients with various T stages. Furthermore, in the current study, hypothetical T stages determined with the measured SUVmax regarding the primary tumor appear to be more practical and
accurate in predicting patients’ prognoses than those determined with EUS. When a time-dependent AUC metric was used, the prediction model adopting the hypothetical T sage on disease
recurrence and survival excelled the other models adopting pathologic T stage and clinical T stage by using EUS. The SUVmax of the tumor positively correlated with the depth of tumor
invasion (pathologic T stage) and also with the tumor length and nodal metastasis (pathologic N stage). The pathologic T stage or clinical T stage (determined by the use of EUS) might not
have reflected the relationship with nodal metastasis. It is known that the length of eSCC has a positive correlation with the SUVmax of the primary tumor16,17. In a study by Xu _et al_.18,
the tumor length demonstrated a positive correlation with tumor recurrence. However, in the present study, the tumor length was not an independent prognostic factor in surgically resectable
eSCCs. Our study had several limitations. First, it was conducted in a single tertiary referral hospital. Second, our study population was retrospectively recruited from our surgical
registry, and the patients had a resectable early-stage (only T1 and T2-stage) eSCC. This may have contributed to selection bias. Third, we included patients who underwent both CT and PET/CT
at our institution. Thus the measurement methods of SUVmax and CT and PET/CT study protocols were uniformly standardized. Therefore, the results of our study may not be generalized for
patients with eSCC worldwide. However, we tried to include a large number of early-stage eSCC patients. Further studies with a multi-centered prospective design and with a larger number of
patients may be needed to validate our study results. Fourth, we excluded patients who underwent neoadjuvant chemotherapy. Future studies including patients undergoing neoadjuvant chemo- or
radiation therapy may be needed to assess the effectiveness of measuring SUVmax on PET/CT in eSCC patients. Lastly, we did not consider the possible effect of the angiolymphatic or
perineural invasion of the primary tumor in the survival analysis. This was because there were only a few identified angiolymphatic or perineural invasion cases in our patient cohort. In
conclusion, of the 435 patients undergoing surgical resection with a T1- or T2-stage eSCC, 103 (23.7%) patients died, and 89 (20.5%) patients experienced tumor recurrence during the median
follow-up period of 49.3 months (range; 0.8 to 105 months). In the multivariate analysis, the SUVmax of the primary tumor (eSCC) on PET/CT was an independent factor for predicting future
recurrences and patient survival. The pathologic T stage was a predictor for survival, and nodal metastasis was a predictor for recurrence. Furthermore, the SUVmax was significantly
correlated with both pathological T and N stages in a linearly positive manner. Therefore, in patients with a surgically resectable eSCC, measuring the SUVmax of the primary tumor could help
predict patient survival. MATERIALS AND METHODS STUDY POPULATION AND DATA COLLECTION Using the Esophageal Cancer Surgery Registry at the Samsung Medical Center (a 1,979-bed tertiary
referral hospital in Seoul, South Korea), 1498 patients who received esophagectomy and lymph node dissection were identified between January 2010 and December 2016. Of them, 732 patients had
<T1a, T1b, or T2 stage eSCCs. Among them, 297 patients were excluded for the following reasons: 24 patients were excluded due to having undergone neoadjuvant chemoradiation therapy (_n_
= 7) or concurrent chemoradiation therapy (_n_ = 17), 10 patients did not undergo either enhanced chest CT or PET/CT, 165 patients had their PET/CT in outside hospital (difficulty in
measuring SUVmax), and 98 patients had their chest CT in an outside hospital (with incomplete or different CT parameters for evaluating ECA and its staging). The remaining 435 patients who
underwent both PET/CT and chest CT at our institution were included in this study9 (Fig. 4). For the validation study of FDG-PET/CT as a possible T descriptor, we also found 224 patients who
received an esophagectomy and lymph node dissection between January 2017 and December 2018. Of these, 110 patients were excluded for the following reasons: 50 were more than T3 stage, 38
had undergone their PET/CT in an outside hospital, and 22 had undergone surgery for recurrent ECA. Thus, 114 patients were included in the validation study, where we prospectively performed
T staging using the SUVmax criteria acquired with an experimental study. The diagnostic performance of FDG-PET/CT for a T descriptor was compared with that of the experimental study.
Patient-related (age, sex, adjuvant chemotherapy, and survival), surgery-related (type of surgery, and surgical resection margin)-, and tumor-related (length, location, histology, and
pathologic stage) factors were collected from the database. Details of patients’ surgeries and their pathologic specimen analyses are described in our previously published article9. The
Institutional Review Board (IRB) of the Samsung Medical Center approved this retrospective study (IRB no. 2017–04–093). Informed consent for reviewing patients’ electronic medical records
was waived by the IRB. Our study design, data collection, and analysis were performed as per the relevant guidelines and regulations. PREOPERATIVE PET/CT SCANNING AND INTERPRETATION All
patients fasted for at least 6 hours before their PET examination. Blood glucose levels were measured before the injection of FDG and were required to be <200 mg/dL in all patients.
Whole-body PET and unenhanced CT images were acquired using two types of PET/CT scanners (Discovery LS, GE Healthcare, Milwaukee, WI, USA; Discovery STe, GE Healthcare, Milwaukee, WI, USA),
60 minutes after the injection of FDG (5.5 MBq/Kg). When the Discovery STe scanner was used, a whole-body CT was performed using a continuous spiral technique with a 16-slice helical CT (140
keV; 30–170 mA; section width, 3.75 mm). After the CT scan, an emission scan was obtained from the head to middle thigh for 2.5 min per frame in a 3-dimensional mode. Attenuation-corrected
PET images (3.9 × 3.9 × 3.3 mm) were reconstructed from the CT data using an ordered-subset expectation maximization (OSEM) algorithm (20 subsets, 2 iterations). When the Discovery LS
scanner was used, a whole-body CT was performed using a continuous spiral technique with an 8-slice helical CT (140 keV; 40–120 mA; section width, 5 mm). After the CT scan, an emission scan
was obtained from the head to middle thigh for 4 min per frame in a 2-dimensional mode. Attenuation-corrected PET images (4.3 × 4.3 × 3.9 mm) were reconstructed from the CT data using an
OSEM algorithm (28 subsets, 2 iterations). The standardized uptake value (SUV) was derived from the injected dose of FDG, and the patient’s body weight9. One of the two nuclear medicine
physicians (16 years and 10 years of experience in PET/CT interpretation, respectively) and one chest radiologist (26 years of chest CT interpretation and 10 years of PET/CT interpretation),
who were blind to the clinical and pathologic results, evaluated the PET/CT in consideration of the chest CT results. When identifiable esophageal lesions were present, the location was
recorded with four anatomic landmarks used for categorization: thoracic inlet, azygos arch, inferior pulmonary veins, and the esophagogastric junction. As for T staging, the SUVmax was
measured at the tumor sites. When the primary cancer was not visualized or could not be distinguished from the background (_n_ = 70), the SUVmax was assigned an assumed default value of 1.0,
similar to the background uptake. In our previous study, the ROC curves were constructed and depicted to obtain the most appropriate cut-off values in terms of differentiating <T1a from
T1b or T2 and differentiating <T1 and T29. We divided patients into three hypothetical PET staging groups A, B and C using two cut-off values of SUVmax 3.05 and 5.65: A = SUVmax <
3.05, SUVmax 3.05 < B < SUVmax 5.65, and C = SUVmax ≥ 5.65, respectively. PATIENT SURVEILLANCE Patients in this retrospective cohort were followed up regularly following the specific
surveillance protocols at our institution after surgery. Specifically, out-patient based clinic appointments were arranged every 6 months at our institution for 5 years. After 5 years,
patients were usually referred to a secondary referral hospital in their hometown. Follow-up imaging studies were also performed at specific intervals: a chest x-ray every month for the
first 2 months to check for complications after surgery, a contrast-enhanced chest CT scan every 6 months for 5 years, a PET/CT and esophagogastroduodenoscopy every year, or at any time when
symptoms indicated a recurrence had occurred. Overall survival (OS) was defined as the length of time from either the date of diagnosis or the start of treatment to death. Disease-free
survival (DFS) was defined as the diagnosis of a locoregional recurrence, including lymph node metastasis or distant metastasis at any site. The closing date for survival data collection was
December 31, 2018, which was 2 years after the surgery of the most recently enrolled patients in our cohort. STATISTICAL ANALYSIS Pearson’s product-moment correlation coefficient was
calculated for analyzing the relationship between the SUVmax of the primary eSCC and the pathologic T and N stages, the presence of nodal metastasis, or primary tumor histologic
differentiation. Five-year OS and DFS were calculated and plotted using the Kaplan-Meier method. Differences in survival among the three (T1a, T1b, and T2 stages) groups were assessed by the
three staging methods (pathologic, clinical [EUS], and hypothetical PET by using the SUVmax) were compared using the log-rank test. Multivariate Cox proportional hazard models for OS and
DFS were built for those prognostic factors with a _p_-value of <0.1 in the univariate analysis. For detecting multi-co-linearity among the prognostic factors used for the multivariate
analysis, VIFs were calculated. To evaluate the predictive value for survival (prognostication performance) among the three staging systems, including pathologic T staging, clinical staging
using EUS, and hypothetical PET staging, we used an integrated time-dependent AUC (iAUC) calculated from time 0 to 60 months after surgery19. For the validation of SUVmax as a T descriptor,
we calculated the sensitivity, specificity, accuracy, PPV, and NPV, and compared the results with those of the experimental study. To see whether there were demographic differences between
the experimental and validation groups, the data were analyzed using the chi-square test. All statistical analyses were done using SPSS (SPSS for Windows, version 22.0; SPSS, Chicago, IL)
and the statistical computing language R (version 3.4.3, R Foundation). A _p-_value of <0.05 was considered statistically significant. DATA AVAILABILITY The datasets generated during
and/or analyzed in the current study are available from the corresponding author on reasonable request. REFERENCES * Ferlay, J. _et al_. Cancer incidence and mortality worldwide: sources,
methods and major patterns in GLOBOCAN 2012. _International journal of cancer_ 136 (2015). * Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. _The
Lancet_ 381, 400–412 (2013). Article Google Scholar * Ajani, J. A. _et al_. Esophageal and esophagogastric junction cancers. _Journal of the National Comprehensive Cancer Network_ 9,
830–887 (2011). Article Google Scholar * Lordick, F., Mariette, C., Haustermans, K., Obermannová, R. & Arnold, D. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. _Annals of Oncology_ 27, v50–v57 (2016). Article CAS Google Scholar * Low, D. E. _et al_. International consensus on standardization of data collection for
complications associated with esophagectomy. _Annals of surgery_ 262, 286–294 (2015). Article Google Scholar * Sun, F., Yuan, P., Chen, T. & Hu, J. Efficacy and complication of
endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. _Journal of cardiothoracic surgery_ 9, 78 (2014). Article CAS Google Scholar
* Kuwano, H. _et al_. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. _Esophagus_ 12, 1–30 (2015). Article Google
Scholar * Bhatt, A., Abe, S., Kumaravel, A., Vargo, J. & Saito, Y. Indications and techniques for endoscopic submucosal dissection. _The American journal of gastroenterology_ 110, 784
(2015). Article Google Scholar * Jeong, D. Y. _et al_. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and N staging performance of EUS and PET/CT. _Cancer Med_
7, 3561–3570 (2018). Article CAS Google Scholar * Xie, T., Xiang, R., Yang, X., Cui, Y. & Li, Q. Analysis of characteristics and factors affecting the recurrence of esophageal cancer
within the first year after surgery. _Zhonghua zhong liu za zhi [Chinese journal of oncology]_ 38, 146–149 (2016). CAS Google Scholar * Jeon, J. H. _et al_. Prognostic significance of
venous invasion and maximum standardized uptake value of 18F-FDG PET/CT in surgically resected T1N0 esophageal squamous cell carcinoma. _European Journal of Surgical Oncology (EJSO)_ 43,
471–477 (2017). Article CAS Google Scholar * Song, I.-H., Cho, S., Yang, H. C. & Jheon, S. The Role of Maximum Standardized Uptake Value in PET/CT as a Prognostic Factor in Patients
with Resected Esophageal Squamous Cell Carcinoma. _Thorac cardiovasc Surg_ 63, 335–340 (2015). Article Google Scholar * Cuellar, S. L. B. _et al_. Clinical staging of patients with early
esophageal adenocarcinoma: does FDG-PET/CT have a role? _Journal of Thoracic Oncology_ 9, 1202–1206 (2014). Article CAS Google Scholar * Bruzzi, J. F. _et al_. PET/CT of esophageal
cancer: its role in clinical management. _Radiographics_ 27, 1635–1652 (2007). Article Google Scholar * Furukawa, T. _et al_. Clinical significance of FDG-PET to predict pathologic tumor
invasion and lymph node metastasis of superficial esophageal squamous cell carcinoma. _Annals of surgical oncology_ 23, 4086–4092 (2016). Article Google Scholar * Mamede, M. _et al_.
Pre-operative estimation of esophageal tumor metabolic length in FDG-PET images with surgical pathology confirmation. _Annals of nuclear medicine_ 21, 553–562 (2007). Article Google Scholar
* Yu, W. _et al_. GTV spatial conformity between different delineation methods by 18FDG PET/CT and pathology in esophageal cancer. _Radiotherapy and oncology_ 93, 441–446 (2009). Article
Google Scholar * Xu, Y. _et al_. Factors influencing the risk of recurrence in patients with esophageal carcinoma treated with surgery: A single institution analysis consisting of 1002
cases. _Oncology letters_ 5, 185–190 (2013). Article Google Scholar * Heagerty, P. J. & Zheng, Y. Survival Model Predictive Accuracy and ROC Curves. _Biometrics_ 61, 92–105 (2005).
Article MathSciNet Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National R & D Program for Cancer Control, Ministry of Health & Welfare of
Korea [1720180]. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, South Korea Dong
Young Jeong, Kyung Soo Lee & Myung Jin Chung * Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, South Korea Joon
Young Choi * Division of Gastroenterology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, South Korea Yang Won Min * Department
of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, South Korea Hong Kwan Kim, Jae Ill Zo & Young Mog Shim * Division of
Hemato‐oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, South Korea Jong-Mu Sun Authors * Dong Young Jeong View author
publications You can also search for this author inPubMed Google Scholar * Kyung Soo Lee View author publications You can also search for this author inPubMed Google Scholar * Joon Young
Choi View author publications You can also search for this author inPubMed Google Scholar * Myung Jin Chung View author publications You can also search for this author inPubMed Google
Scholar * Yang Won Min View author publications You can also search for this author inPubMed Google Scholar * Hong Kwan Kim View author publications You can also search for this author
inPubMed Google Scholar * Jae Ill Zo View author publications You can also search for this author inPubMed Google Scholar * Young Mog Shim View author publications You can also search for
this author inPubMed Google Scholar * Jong-Mu Sun View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Study conception and design: D.Y.J. and
K.S.L. Data acquisition and analysis: D.Y.J., J.Y.C., M.J.C. and Y.W.M. Data interpretation and manuscript writing: D.Y.J. and K.S.L. Revision of manuscript and contribution of intellectual
content: D.Y.J., J.Y.C., M.J.C., Y.W.M., H.K.K., J.I.Z., Y.M.S., J.S. and K.S.L. CORRESPONDING AUTHOR Correspondence to Kyung Soo Lee. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jeong, D.Y., Lee, K.S., Choi, J.Y. _et al._ Surgically Resected Esophageal Squamous Cell Carcinoma: Patient Survival and
Clinicopathological Prognostic Factors. _Sci Rep_ 10, 5077 (2020). https://doi.org/10.1038/s41598-020-62028-5 Download citation * Received: 05 June 2019 * Accepted: 06 March 2020 *
Published: 19 March 2020 * DOI: https://doi.org/10.1038/s41598-020-62028-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative