Rhizodegradation of petroleum oily sludge-contaminated soil using cajanus cajan increases the diversity of soil microbial community
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Most components of petroleum oily sludge (POS) are toxic, mutagenic and cancer-causing. Often bioremediation using microorganisms is hindered by the toxicity of POS. Under this
circumstance, phytoremediation is the main option as it can overcome the toxicity of POS. _Cajanus cajan_ a legume plant, was evaluated as a phyto-remediating agent for petroleum oily
sludge-spiked soil. Culture dependent and independent methods were used to determine the rhizosphere microorganisms’ composition. Degradation rates were estimated gravimetrically. The
population of total heterotrophic bacteria (THRB) was significantly higher in the uncontaminated soil compared to the contaminated rhizosphere soil with _C. cajan_, but the population of
hydrocarbon-utilizing bacteria (HUB) was higher in the contaminated rhizosphere soil. The results show that for 1 to 3% oily sludge concentrations, an increase in microbial counts for all
treatments from day 0 to 90 d was observed with the contaminated rhizosphere CR showing the highest significant increase (p < 0.05) in microbial counts compared to other treatments. The
metagenomic study focused on the POS of 3% (w/w) and based on the calculated bacterial community abundance indices showed an increase in the values for Ace, Cho, Shannon (Shannon-Weaver) and
the Simpson’s (measured as InvSimpson) indices in CR3 compared to CN3. Both the Simpson’s and the Shannon values for CR3 were higher than CN3 indicating an increase in diversity upon the
introduction of _C. cajan_ into the contaminated soil. The PCoA plot revealed community-level differences between the contaminated non-rhizosphere control and contaminated rhizosphere
microbiota. The PCoA differentiated the two treatments based on the presence or absence of plant. The composition and taxonomic analysis of microbiota-amplified sequences were categorized
into eight phyla for the contaminated non-rhizosphere and ten phyla for the contaminated rhizosphere. The overall bacterial composition of the two treatments varied, as the distribution
shows a similar variation between the two treatments in the phylum distribution. The percentage removal of total petroleum hydrocarbon (TPH) after 90 days of treatments with 1, 2, 3, 4, and
5% (w/w) of POS were 92, 90, 89, 68.3 and 47.3%, respectively, indicating removal inhibition at higher POS concentrations. As the search for more eco-friendly and sustainable remediating
green plant continues, _C. cajan_ shows great potential in reclaiming POS contaminated soil. Our findings will provide solutions to POS polluted soils and subsequent re-vegetation. SIMILAR
CONTENT BEING VIEWED BY OTHERS RHIZODEGRADATION OF PAHS DIFFERENTIALLY ALTERED BY C3 AND C4 PLANTS Article Open access 30 September 2020 SEWAGE SLUDGE FERTILIZATION AFFECTS MICROBIAL
COMMUNITY STRUCTURE AND ITS RESISTOME IN AGRICULTURAL SOILS Article Open access 09 September 2024 IMPACT OF ARTISANAL REFINING ACTIVITIES ON BACTERIAL DIVERSITY IN A NIGER DELTA FALLOW LAND
Article Open access 16 February 2024 INTRODUCTION The global over-dependent on fossil fuels as sources of energy increases the rate and extent of exploration, transportation, refining and
storage of crude oil by the petroleum industries1,2. Petroleum industries produce a large amount of leftover from petroleum oil as a residue, which is called petroleum oily sludge (POS). The
indiscriminate disposal of inadequately treated oily sludge to the environment is causing a lot of environmental and human health problems. All environmental protection agencies and the
experts have classified oily sludge as a hazardous organic waste. Oily sludge is a complex mixture of organic compounds and inorganic metalloids, of which the structure and bioavailability
of these compounds resist natural degradation. POS constitutes 50–80% hydrocarbon, 10–30% solid material, 20–30% water and 5–10% heavy metals3. Several physical and chemical methods are
being extensively used for the remediation of oily sludge from temporary deposit sites and contaminated environments. However, these methods tend to be expensive and environmentally
unfriendly. An alternative is biological methods which can provide an effective and eco-friendly approach. By making use of these kinds of technologies, the content of harmful components is
often decreased or eradicated, as well as its negative environmental and health influences can, therefore, be reduced4,5. Nevertheless, because of the recalcitrant character of POS6, the
quest for remediation strategies together with the requirement of fulfilling stringent environmental laws and lowering the cost of remediation is a continuing process3. The complexity of
oily sludge composition of hydrocarbon, heavy metals and others made it hardly bioavailable for microbial degradation5. In Malaysia, POS waste falls under the scheduled waste with code SW
311, and about 4,293 tons were generated in 2015 alone (DOE, 2012). Phytoremediation is one of the strategies of bioremediation, and it uses selected plants to cleanup polluted environment.
Plants are more tolerant in general to excessive concentrations of POS compared to microorganisms7. Consequently, phytoremediation is potentially a fresh possibility of a much more effective
and sustainable answer for the remediation of POS-contaminated soils8. Numerous plants have already been recognized to assist in the phytoremediation of areas polluted with petroleum
hydrocarbons, and great number of research have identified grasses and legumes due to their reportedly greater tolerance to hydrocarbon and also due to their other beneficial qualities9. The
plant- microorganisms connection has a tendency to boost the degradation of contaminants in the rhizosphere by way of a symbiotic connection. Plants can promote microbial activity anywhere
from 10 to 100 times greater in the rhizosphere through the release of exudates that contain compounds including amino acids, carbohydrates and flavonoids10. The secretion of these
nutrients-containing exudates offers nitrogen and carbon sources to soil microorganisms. In addition, this process generates an environment that is nutrient-rich in which the activity of
microorganisms can be activated. Along with the capability to secrete compounds that can facilitate the activity and growth of rhizospheric microorganisms, plants can furthermore discharge
specific enzymes that have the capability to degrade pollutants. The compounding effect results in a more efficient remediation of toxicants, including POS11. Legumes are known to have the
edge over non-leguminous plants in the process of phytoremediation due to their capacity for nitrogen fixation12 and therefore, do not need to contest with microorganisms as well as other
plants for the constrained resources of accessible soil nitrogen. Common desirable characteristics of these plants are the ability to fix nitrogen (a major limiting factor for effective
degradation of pollutants) which is strategic to establish nutrient-rich rhizosphere as it is known that areas contaminated with oily sludge have a deficit in the C:N ratio9. The
legume-bacterial interaction is a synergistic strategy of which leguminous plants provide the microorganisms in the rhizosphere with growing space and essential nutrients, as the
microorganisms use their degrading machinery to biodegrade hydrocarbon pollutant in their surroundings13. Additionally, rhizo- and endophytic microorganisms, in collaboration with the legume
improve the supply of nutrient and growth hormones that can promote plants’ growth, and at the same time increase hydrocarbon bioavailability14,15,16,17. _Cajanus cajan_ (pigeon pea) is
also known as Kacang dhal in Malay. It is a very common legume crop in tropical countries. The plant provides an important source of protein to the diet of human. It features a lengthy root
system which could endure in various soil types18. It can withstand the alterations in pH over a broad range. In addition, the plant is known to grow at a broad range of temperature from 10
to 35 °C19. In India, this plant is utilized as a multi-purpose plant in the agroforestry industry, in which its many functions include as sources of firewood, manure, food and fodder19.
Although the plant species utilized in this study has been documented to degrade polluted soil contaminated with spent engine20, no prior research has documented its use in the remediation
of POS-contaminated soil. In this study, we report for the first time, the phytoremediation of POS in soil using _Cajanus cajan_. MATERIALS AND METHODS EXPERIMENTAL SETUP The experiment was
carried out in the Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia. The petroleum oily sludge (POS) used in this study was obtained from a Shell refinery center
in Port Dickson, Negeri Sembilan Malaysia in 2014. The POS is in the form of clay mud and black in colour with a pH of 7.1221. The soil was collected from an agricultural farm in Universiti
Putra Malaysia. The seeds of _C. cajan_ (Pigeon pea) and plastic pots were obtained from a local grocery company. EXPERIMENTAL DESIGN POT PREPARATION Soil, which has been previously
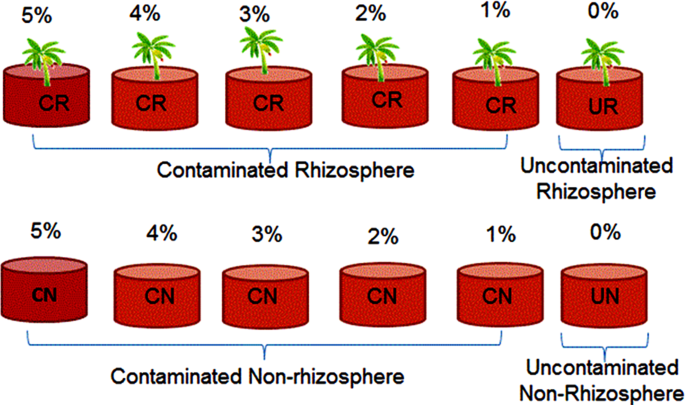
air-dried and sieved using a 2 mm sieve was placed in each corresponding pot (3 kg), and labelled as contaminated rhizosphere (CR), contaminated non-rhizosphere (CN), uncontaminated
rhizosphere (UR) and uncontaminated non-rhizosphere (UN) (Fig. 1). TREATMENT OF SOIL WITH POS POS was added into each pot to the final concentrations of 1%, 2%, 3%, 4%, and 5% (w/w) and
homogenously mixed. The soil was properly agitated and thoroughly mixed to ensure homogeneity and uniformity of soil to the POS in a fume hood. The pots were allowed to remain undisturbed
for one week for the volatilization processes. The soil sample was taken for physicochemical analysis before treatment. After one week, seeds of _C. cajan_ were planted accordingly. Three
independent experiments were carried out. PLANTING OF SEEDS SEED VIABILITY TEST AND BREAKING OF DORMANCY The viability of the seed of _C. cajan_ was tested using the floatation method. The
seeds were tested for viability by soaking it in distilled water for 5 min. After that, all floating seeds are sieved out as non-viable and the water was drained immediately from the seeds
that sank which are now considered viable seeds. The seeds were then surface sterilized with 10% hydrogen peroxide solution before planting. The seeds was sown to a depth recommended for _C.
cajan_, which is from 1.5 to 3 cm22. For an excellent establishment of the plants, five seeds were planted in a hole. Likewise, the respective pots were irrigated every day and emergence
was observed subsequently. The plants were moderately watered every two days with tap water. The appearance of the plants in response to the presence of POS in soil was monitored to
determine if there is any phytotoxicity of POS to the plants. MICROBIOLOGICAL ANALYSIS The media used were Nutrient Agar (NA); for the enumeration and isolation of bacteria, mineral salts
media and a modified Bushnell and Haas media the for isolation of hydrocarbon-utilizing bacteria referred to here as oil agar (OA) where diesel oil (0.1% w/v) was used as the carbon source
in the medium23. CULTURE DEPENDENT MICROBIAL ANALYSIS The bacterial population of the soil samples for 0, 30, 60, and 90 days was enumerated by serially diluting 1 g of soil sample collected
from the rhizosphere of _C. cajan_. Suitably diluted samples (10−5, 10−6 and 10−7) were transferred into the already prepared media using a dropper pipette, 500 µL of each dilution was
inoculated on NA and OA which contained 0.1% v/v diesel agar surface23. The plates were incubated at 30 °C for 24 h, for NA, and five days for OA, respectively23. The number of viable total
heterotrophic rhizobacteria and hydrocarbon-utilizing bacteria in the soil samples was estimated from the number of colonies formed using a colony counter. CULTURE-INDEPENDENT MICROBIAL
ANALYSIS Culture-independent bacterial community in _C. cajan_ rhizosphere after 90 days was determined using metagenomics analysis of the rhizospheric soil collected from the contaminated
rhizosphere CR of 3% oily sludge and contaminated non-rhizosphere CN of 3% oily sludge as control. SOIL EXTRACTION AND PCR AMPLIFICATION Microbial DNA was extracted from samples in
treatments CN3 and CR3 after 90 days using Power soil® DNA Isolation Kits (MOBIO, USA) following the manufacturer’s instruction. The marker region of the bacteria was amplified by PCR using
a PCR (BioRad Thermal Cycler, United Kingdom) with the following conditions; 95 °C for 2 min, followed by 25 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final
extension for 5 min using the primers sequences 5'- barcode 2 - (reverse primer sequence) −3'and 5'-barcode 1- (forward primer sequence) – 3', where the barcode consists
of an eight-base sequence that is sample-specific and distinctive to each sample. The PCR reaction was carried out in triplicate 20 µL-mixtures containing 2 µL of 2.5 mM dNTPs, 4 µL of 5 ×
FastPfu buffer, 0.4 µL of FastPfu Polymerase, 0.8 µL of each primer (5 µM) and 10 ng of template DNA. Extraction of amplicons was carried out from a 2% agarose gel. Purification of the
amplicons was carried out utilizing AxyPrep DNA Gel Extraction Kit (Axygen Bioscience, Union City, CA, USA) in accordance to the instruction furnished by the manufacturer and quantified
using QuantiFluorTM –ST (Promega, USA). Construction of the library was carried out utilizing Linked ‘Y’ adapter; with adapter dimer removed by utilizing beads and the library was
concentrated via PCR and hydroxides utilized in the generation of single-stranded DNA fragments. Sample libraries were then pooled in equimolar concentrations, and paired-end sequenced using
the Illumina MiSeq platform (2 × 250/300 bp) according to standard protocols. DATA ANALYSIS Several criteria were utilized for demultiplexing raw Fasta files and QIIME-based
quality-filtration (version 1.9.1). The criteria include overlapped relationship and merging of paired-reads into a single read. The merged reads were then utilized for the clustering of
operational taxonomic units (OUT), classification of taxonomy and assessment of community diversity. The software Trimmomatic was utilized for processing sequence reads24, and then the reads
were assembled utilizing the software Flash25 followed up by more analysis using the software MOTHUR v 1.33.026. Alignment of unique sequences was based to the SILVA database with the
settings put to default. In addition, removal of chimeric sequences was carried out. Obtained sequences that passed the screening process were then classified using the Ribosomal Database
Project naïve Bayesian rRNA classifier at a confidence of 80%. The proportion of sequence identities was calculated at each taxonomic level as the percentage of all sequences classified in
that particular sample. Classification of OTUs was based on the similarities of 97%. The software MOTHUR was utilized in the classification of the alpha-diversity indices, which include
observed OTUs (Sobs), Ace, Chao, Shannon and InvSimpson indices. PCoA or principal coordinates analysis conducted in R (Version 3.1.2) was utilized to find the bacterial community structure,
which is based on the OTU composition. MEASUREMENT OF BIODEGRADATION IN THE SOIL GRAVIMETRIC METHOD Five grams of soil from all treatments were collected and transferred into 100 mL conical
flasks. Then 50 mL of hexane was added and shaken on a Protech orbital shaker (USA) at 150 rpm for 24 h. The layer separating the solvent with oil and the soil was transferred to a
pre-weighted clean conical flask, the sample was left overnight in a fume hood for the evaporation of the solvent, and the amount of residual TPH was gravimetrically determined using the
formula. $$ \%
\,{\rm{Biodegradation}}=\frac{{\rm{Weight}}\,{\rm{of}}\,{\rm{oil}}\,({\rm{control}})\,-\,{\rm{Weight}}\,{\rm{of}}\,{\rm{oil}}\,({\rm{degraded}})}{{\rm{Weight}}\,{\rm{of}}\,{\rm{oil}}\,({\rm{control}})}\times
100$$ RESULTS AND DISCUSSION TOTAL HETEROTROPHIC RHIZOSPHERE BACTERIAL COUNT The total counts for the heterotrophic rhizospheric bacteria (THRB) were enumerated at 0, 30, 60 and 90 days of
treatments at various concentrations of POS. The result shows that for 1% POS, the THRB counts for contaminated rhizosphere or CR increased significantly (p < 0.05) but slightly from 125
× 107 to 148 × 107 CFU/g, while the count for contaminated non-rhizospheric CN decreased significantly (p < 0.05) from 112 × 107 to 77.3 × 107 CFU/g, the counts for the uncontaminated
rhizosphere UR stayed about the same from 243 × 107 to 241 × 107 CFU/g and the counts for the uncontaminated non-rhizosphere UN decreased significantly (p < 0.05) from 202 × 107 to 157
× 107 CFU/g (Fig. 2(a)). The THRB counts for the 2% POS concentration from 0 to 90 days show a significant (p < 0.05) increase in rhizospheric CR counts from 12.7 × 107 to 144.8 × 107
CFU/g, no significant change (p > 0.05) to the contaminated non-rhizospheric CN count (from 66.3 × 107 to 44.7 × 107 CFU/g), no significant change (p > 0.05) to the uncontaminated
rhizospheric UR count (243 × 107 to 232 × 107) CFU/g and also no significant change (p > 0.05) to the uncontaminated non-rhizospheric UN count (from 221 × 107 to 219 × 107) CFU/g (Fig.
2(b)). The THRB counts for the 3% POS concentration from 0 to 90 days show that the contaminated rhizospheric counts CR were increased but not significant (p > 0.05) (from 123 × 107 to
145.4 × 107 CFU/g), the contaminated non-rhizospheric CN counts were not increased significantly (p > 0.05) (from 35.8 × 107 to 31.3 × 107) CFU/g, the uncontaminated rhizospheric UR
counts were increased significantly (p < 0.05) from 152 × 107 to 232 × 107 CFU/g, and the THRB counts in the uncontaminated non-rhizosphere UN were also not increased significantly (p
> 0.05) (from 202 × 107 to 219 × 107) CFU/g (Fig. 2(c)). The THRB counts for the 4% POS concentration from 0 to 90 days shows that the contaminated rhizospheric count CR was not
significantly increased (from 96.7 × 107 to 112 × 107 CFU/g), the contaminated non-rhizospheric CN counts were decreased significantly (p < 0.05) (from 41.6 × 107 to 23.7 × 107 CFU/g),
the uncontaminated rhizospheric UR counts were not significantly (p > 0.05) increased (from 233 × 107 to 232 × 107 CFU/g) and similarly in the uncontaminated non-rhizospheric UN, the
bacterial counts were not significantly (p > 0.05) increased (from 200 × 107 to 219 × 107 CFU/g) (Fig. 2(d)). At the highest POS concentration tested (5%), there were a decreasing trend
or no change in overall for the THRB counts. For example, for the contaminated rhizospheric CR counts, a decrease was observed (from 57.7 × 107 to 45.4 × 107 CFU/g), but this decrease was
not significant (p > 0.05), in the contaminated non-rhizosphere CN counts, a reduction from 23 × 107 to 13.2 × 107 CFU/g was observed and this reduction was significant (p < 0.05). In
the uncontaminated rhizospheric UR bacterial count, a reduction from 243 × 107 to 223 × 107 CFU/g was observed, but similarly, this reduction was not significant (p < 0.05) and finally,
in the uncontaminated non-rhizospheric UN counts, there were no significant changes (p > 0.05) observed (from 202 × 107 to 200 × 107 CFU/g) (Fig. 2(e)). In general, the results show a
significant (p < 0.05) increase in the numbers of THRB counts in the uncontaminated rhizosphere (UR) compared to the uncontaminated non-rhizospheric (UN) bacterial counts. Although the
microbial community in the rhizosphere is about 10–100 times higher than that of the non-rhizosphere10, the increase in the THRB counts in UR compared to UN as found in this study is
marginal. As anticipated, the presence of the contaminant POS significantly reduces the number of THRB counts in both of the contaminated rhizosphere (CR) and non-rhizosphere (CN) treatments
over the uncontaminated treatments (UN and UR). The microbial counts were much more pronounced in the uncontaminated soil than the contaminated soil because of the inhibitory effects of POS
to the microorganism in general in the contaminated soil. However, there was a significant increase in the number of bacterial populations over time in the rhizosphere treatments (CR), as
there were higher bacterial counts after 90 days of treatment compared to that of 30 days while a decrease in THRB counts was generally observed in CN. This shows that the presence of _C.
cajan_ favours the growth of THRB bacteria in the soil as found in other studies22,27. The result is also supported by a previous finding28, who reported a total decrease in the number of
viable heterotrophic bacteria in soil contaminated with oily sludge. The hydrocarbon utilizing rhizosphere bacteria (HURB) at 1% POS from 0 to 90 days indicated a significant increase (p
< 0.05) in bacterial count in all of the treatments such as in the contaminated rhizosphere CR (from 31.3 × 107 to 131 × 107 CFU/g), in the contaminated non-rhizosphere CN (from 28 × 107
to 54 × 107 CFU/g), uncontaminated rhizosphere UR (from 30 × 107 to 86 × 107 CFU/g) and in the uncontaminated non-rhizosphere UN (from 25 × 107 to 58 × 107 CFU/g) (Fig. 3(a)). In the 2% POS
concentration, the HURB counts from 0 to 90 days show a similar increase in counts where the contaminated rhizosphere CR bacterial counts increased significantly (p < 0.05) from 43 × 107
to 136 × 107 CFU/g, no significant increase (p > 0.05) in the contaminated non-rhizosphere CN (from 28 × 107 to 35 × 107 CFU/g), a significant increase (p < 0.05) for the bacterial
count in the uncontaminated rhizosphere UR from 38 × 107 to 67 × 107 CFU/g), and a significant increase (p < 0.05) for the bacterial count in the uncontaminated non-rhizosphere UN as well
(from 23 × 107 to 46 × 107 CFU/g) (Fig. 3(b)). Similarly, for the 3% POS concentration, the HURB counts increased significantly (p < 0.05) from 0 to 90 days for all treatments such as in
the contaminated rhizosphere CR (from 32 × 107 to 140 × 107 CFU/g), in the contaminated non-rhizosphere CN (from 28 × 107 to 46 × 107 CFU/g), in the uncontaminated rhizosphere UR (from 32 ×
107 to 67 × 107 CFU/g) and finally in the uncontaminated non-rhizosphere UN (from 22 × 107 to 43 × 107 CFU/g) (Fig. 3(c)). For the 4% POS concentration, the counts for HURB for CR were
significantly increased from 22 × 107 to 115 × 107 CFU/g, while no significant increase (p > 0.05) in the contaminated non-rhizosphere CN was observed from 20 × 107 to 23 × 107 CFU/g. A
significant increase was observed for the uncontaminated rhizosphere UR from 17 × 107 to 60 × 107 CFU/g and also for the uncontaminated non-rhizosphere UN from 14 × 107 to 41 × 107 CFU/g
(Fig. 3(d)). At the highest concentrations of POS tested (5%) HURB counts increased significantly (p < 0.05) for all treatments. The bacterial counts in the contaminated rhizosphere CR
were increased from 11 × 107 to 111 × 107 CFU/g), while the contaminated non-rhizosphere CN counts were increased from 8 × 107 to 22 × 107 CFU/g. In the uncontaminated rhizosphere, the UR
counts were increased from 8 × 107 to 60 × 107 CFU/g) and in the uncontaminated non-rhizosphere, the UN counts were increased from 16 × 107 to 38.7 × 107 CFU/g (Fig. 3(e)).
Hydrocarbon-utilizing rhizospheric bacterial counts revealed higher numbers in the contaminated rhizosphere (CR) for all of the POS concentrations tested, although the density tends to
decrease as the POS concentration was increased. This result is similar to previous findings29,30,31,32, which include studies on the phytoremediation of soil amended with waste lubricating
oil with _Jatropha curcaa_31 and _Hibiscus cannabinus_29 where significant increase in hydrocarbon-utilizing bacteria was observed after 30 days of incubation. However, both of these studies
require the addition of organic wastes such as brewery spent grain (BSG) and spent mushroom compost (SMC) as additional carbon and nitrogen sources. The results obtained in this work
demonstrate the stimulatory effect of rhizosphere to the hydrocarbon-utilizing bacterial population without the need for additional carbon or nitrogen sources. Considering the complexity of
the rhizosphere, a considerable number of microbial communities tend to withstand the toxic effect of the contaminant and are capable of using hydrocarbon as the source of carbon and energy
compared to the community in the uncontaminated control. This is similarly reported in a study of epiphytic hydrocarbon-utilizing bacteria where the average number of bacterial density in a
given contaminated soil is significantly greater than in the corresponding control, directly indicating that the contaminant is being utilized by the soil bacteria33. The results suggest
that microbial enumeration is a direct indicative method to prove the response of microorganisms to hydrocarbons34,35. For a successful and effective phytoremediation process, the bacterial
community in the hydrocarbon-contaminated soil must be well connected to the plant’s ability to enhance microbial association in the rhizosphere, resulting in a higher number of
hydrocarbon-utilizing bacteria and enhancing their degradative capacity36. Apart from the presence of petroleum oil which serves as the carbon and energy source, the higher population of
hydrocarbon-degrading bacteria in the contaminated soil may also be attributed to the additive effect of the _C. cajan_ roots which release organic compounds to further stimulate the
degradation and bacterial growth. The increase in HURB counts in the presence of _C. cajans_ observed in this study are also observed in several similar studies where higher counts of
heterotrophic and oil-degrading bacteria were observed in contaminated rhizospheric soil than in the unplanted contaminated soil8,37. CULTURE-INDEPENDENT METAGENOMICS ANALYSIS A sum of
59,873 and 59,756 sequences for CN3 and CR3 was found after a sequence optimization method, with an average number of 442.56 and 435.39 sequences for CN3 and CR3, respectively. A sum of
50821 and 47999 reads on CN3 and CR3, respectively, were subsampled from each replicate for further analysis. The calculated bacterial community abundance indices showed an increase in the
values for Ace, Cho, Shannon (Shannon-Weaver) and the Simpson’s index in CR3 compared to CN3 (Table 1). In phylogeny, OTU is the most commonly applied microbial diversity unit where OTU is
clustered with a cutoff of 97% similarity for the investigation of the abundance of group or species in the microbial community. The difference between CN3 and CR3 was seen in the number of
OTU, which were 512 and 650 for CN3 and CR3, respectively, indicating an increase in richness upon the addition of _C. cajan_. A similar increase in OTU from 48 (control) to 62 (addition of
rhizobacteria) is reported during the rhizoremediation of hexachlorobenzene in constructed wetlands38. The coverage indices showed a significant difference in the two treatments. The
coverage did not change by much in this study. In general, a reduction in coverage rate indicates higher diversity. In a similar study of rhizoremediation of hexachlorobenzene using _T.
angustifolia_ rhizosphere and _P. australis_ rhizosphere in constructed wetlands38, little reduction in the coverage index was observed for _T. angustifolia_ rhizosphere treatment (from 50
to 47.5) while greater reduction in coverage was observed in _P. australis_ rhizosphere soil treatment (from 50 to 29) despite both treatments showing nearly equal efficacy in remediating
hexachlorobenzene. This may imply that coverage change alone may not be adequate in describing potential remediating ability of rhizodegraders. Of all the indices used in population
diversity studies, the robust Shannon and Simpson indices have been recommended in measuring microbial diversity39, and it was observed that both of the Simpson’s (measured as InvSimpson)
and the Shannon values for CR3 were higher than CN3. A change in both indicating an increase in diversity upon the introduction of _C. cajan_ into the contaminated soil, which is a common
theme seen in several studies involving phytoremediation using legumes11,38,40. Both measurements of population were lower in CN3, which may be due to the toxic effect of the contaminant on
the bacterial community. The shift of soil bacterial community organization is also seen in the metagenomics sequences, and the results showed (Fig. 4a) that the contaminated rhizosphere
(CR3) shows a diverse community of bacterial phyla, in comparison to the change of the microbial community structure seen in the contaminated non-rhizosphere (CN3). A lower diversity in CN3
may be the cause of a lower removal rate of petroleum hydrocarbon which has similarly been reported in previous studies40,41 where with an increase in the concentration of the contaminant,
this results in an increase in the toxicity which reduces the efficiency of microbial degradation. This shows the important role of plant-like _C. cajan_, which stabilizes the C:N:P ratio
for the effective degradation of hydrocarbon by the microbial community22. On the other hand, some study reported the inability of plant growth-promoting rhizobacteria to acquire nutrient
for growth in severely polluted environments41. The composition and taxonomic analysis of microbiota amplified sequences were categorized into eight phyla (CN3) and ten phyla in (CR3). The
overall bacterial composition of the two treatments varied, as the distribution shows a similar variation between the two treatments in the phylum, class, family and genus level
distributions, represented by Fig. 4a to d, respectively. This variation in microbial community is also shown by the PCoA plot, which revealed community-level differences between the
contaminated non-rhizosphere control (CN3) and contaminated rhizosphere (CR3) microbiota (Fig. 5). The contaminated non-rhizosphere (CN3) shows a trend of bacterial phylum such as
_Proteobacteria, Actinobacteria, Acidobacteria, Bacteroidetes, Firmicutes, Chloroflexi, Saccharibacteria_ and some uncategorized group. The contaminated rhizosphere (CR3) shows
_Actinobacteria, Proteobacteria, Bacteroidetes, Acidobacteria, Firmicutes, Gemmatimonadetes, Saccharibacteria, Chloroflexi_ and _Verrucomicrobia_ to be the dominant phyla, and an
unclassified group. In both of the treatments, the phylum _Proteobacteria_ constitute the phylum with higher relative abundance accounting for > 60% in CN3 and almost 42% in CR3. In
general, the phylum _Proteobacteria_ dominated the communities of CN3 and CR3 at 70% and 42%, respectively. In the CN3, the _Proteobacteria_ was dominated by the genus _Rhizobium,
Sphingomonas_, and _Herbaspirillum_. The two phyla were not observed in CN3, but the phyla observed were _Verrucomicrobia and Gemmatimonadetes_. The presence of _Verrucomicrobia_ is an
indicator to the rhizospheric effect created by the presence of _C. cajan_ as bacteria from this phylum are mostly found inhabiting grasslands and in subsurface soil horizons, where they
were habitually the prevailing bacterial phylum42. Similarly, _Gemmatimonadetes_ tend to dominant in soil with high rhizosphere activities, although their ecology remains poorly understood,
and appear to be the dominant phyla in many soil bacterial communities; with bacteria from the phylum _Gemmatimonadetes_ featuring nearly 2% of soil bacterial communities. Nevertheless, very
little is understood of their ecology as a result of an insufficient study on the occurrence and ecology of this bacterial group43. The degradation of petroleum oily sludge hydrocarbons, in
general, is accredited to indigenous microorganisms which are found in soil, but the presence of _C. cajan_ will stimulate the habitat for the formation of favourable conditions of
metabolisms to the microbial communities as demonstrated in this study where the culture-independent metagenomics technique to access a much more in-depth knowledge of the biological
processes of petroleum oily sludge degradation during rhizodegradation shows promising results that agrees in principal to what was observed in the experiments. An assessment at the phylum
level identifies the bacteria belonging to the _Proteobacteria_ as the richest community, and the richness was significantly increased. _Proteobacteria_ covers a group of Gram-negative
bacteria that have been widely reported to be able to degrade POS41. BIODEGRADATION OF PETROLEUM OILY SLUDGE IN SOIL GRAVIMETRIC ANALYSIS The result of oily sludge degradation in the soil
shows the effectiveness of _C. cajan_ in plant-microbe bioremediation process. The result of the gravimetric analysis shows that almost 50% biodegradation of the oily sludge was observed at
lower concentrations of oily sludge (CR1%, CR2% and CR3%) after 30 days of planting _C. cajan_ (Fig. 6). A low percentage of biodegradation (19%) was observed at the highest concentration of
oily sludge tested (CR5%) after 30 days of planting with _C. cajan_. This may be as a result of inhibition at high concentrations of POS. The biodegradation shows significant (p < 0.05)
increase after 60 days of planting of _C. cajan_ for all oily sludge concentrations with CR1%, CR2%, CR3% and CR4% showing 74, 65, 63 and 52% biodegradation of POS, respectively, while CR5%
was at 37% which also shows a significant increase albeit at a much lower percentage of degradation. A significant (p < 0.05) increase in biodegradation of POS was shown at the 90 days of
the planting of _C. cajan_ where CR1%, CR2%, CR3% and CR4% show 92, 90, 89 and 68% degradation, respectively. Likewise, CR5% had the lowest biodegradation at 47%. This result corresponds to
the pattern of many studies observed in different plants, and the biodegradation also varies. In a previous study31, they reported that the phytoremediation of soil contaminated with 2.5
and 1% of spent engine oil using _J. curcas_ at day 180 results in the 56.6% and 67.3% biodegradation of waste lubricating oil, respectively. Once the addition of organic waste to _J.
curcas_ was carried out, the remediation rapidly increases the removal of 2.5 and 1% spent engine oil by 89.6 and 96.6%, respectively. The variation is that the plant was stimulated with
organic waste to achieve 96% biodegradation at 1% spent engine oil concentration whereas in this study, _C. cajan_ being a legume plant, the addition of organic source is not necessary
making _C. cajan_ a better phytoremediating plant. Another study in the plant _Hibiscus cannabinus_ for soil contaminated with 2.5 and 1% used lubricating oil for 90 days show the same
pattern of biodegradation where the stimulation of the plant with organic waste resulted in the biodegradation of 86.4 and 91.8%, respectively, while in the unstimulated plant much lower
biodegradations were observed at 52.5 and 58.9%, respectively, indicating the need for the addition of organic waste in non-nitrogen fixing plants29. Legumes have been shown to independently
stimulate biodegradation of various forms of hydrocarbons including poly-aromatic hydrocarbons (PAHs) and their constituents44. In all of the phytoremediation studies, legumes are more
effective at remediation than other non-legume plant species tested15,17,20,44,45,46,47,48,49,50. In some studies, the biodegradation of legume was reported to be lower at the beginning of
the experiment but significantly increases as the experiments progresses44. DATA AVAILABILITY All data generated or analyzed during this study are included in this published article (and its
Supplementary Information files). REFERENCES * Sabullah, M. K. _et al_. Bioremediation of Hydrocarbon: A Mini Review. _J. Biochem. Microbiol. Biotechnol._ 6, 1–6 (2018). Google Scholar *
Zahaba, M. Luminescent bacterial testing for monitoring hydrocarbon bioremediation – a review. _J. Biochem. Microbiol. Biotechnol._ 3, 13–20 (2015). Google Scholar * Islam, B. Petroleum
sludge, its treatment and disposal: A review. _Int. J. Chem. Sci._ 13, 1584–1602 (2015). CAS Google Scholar * Prakash, V., Saxena, S., Sharma, A., Singh, S. & Singh, S. Treatment of
oil sludge contamination by composting. _J. Bioremediation Biodegrad_. 06, (2015). * Hu, G., Li, J. & Zeng, G. Recent development in the treatment of oily sludge from petroleum industry:
A review. _J. Hazard. Mater._ 261, 470–490 (2013). Article CAS PubMed Google Scholar * Ubani, O., Atagana, I. H. & Thantsha, S. M. Biological degradation of oil sludge: A review of
the current state of development. _Afr. J. Biotechnol._ 12, 6544–6567 (2013). Article CAS Google Scholar * Aisien, F. A., Aisien, E. T. & Oboh, I. O. Phytoremediation of petroleum-
polluted soils. in _Management of Environmental Contaminants_ (eds. Ansari, A. A., Gill, S. S., Gill, R., Lanza, G. R. & Newman, L.) vol. 1 243–252 (Springer International Publishing,
2015). * Muratova, A. Y., Dmitrieva, T. V., Panchenko, L. V. & Turkovskaya, O. V. Phytoremediation of oil-sludge–contaminated soil. _Int. J. Phytoremediation_ 10, 486–502 (2008). Article
CAS PubMed Google Scholar * Vázquez-Luna, D. Biological indices of toxicity in tropical legumes grown in oil-contaminated soil. _Ecol. Indic._ 53, 43–48 (2015). Article CAS Google
Scholar * Weller, D. M. & Thomashow, L. S. Current Challenges in Introducing Beneficial Microorganisms into the Rhizosphere. in _Molecular Ecology of Rhizosphere Microorganisms_ 1–18
(John Wiley & Sons, Ltd, 2007). https://doi.org/10.1002/9783527615810.ch1. * Liu, W. _et al_. Rhizobacteria (_Pseudomonas_ sp. SB) assist phytoremediation of oily-sludge-contaminated
soil by tall fescue (_Testuca arundinacea_ L.). _Plant Soil_ 371, 533–542 (2013). Article CAS Google Scholar * Ugrinovic, M. _et al_. Intercropped red beet and radish with green bean
affected microbial communities and nodulation by indigenous rhizobia. _Agric. Food Sci_. 173–185 (2014). Article Google Scholar * Bauddha, K., Singh, K., Singh, B. & Singh, R. Ricinus
communis: A robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. _Ecol. Eng. Jo_ 84, 640–652 (2015). Article Google Scholar * Alaru, M. _et al_. Crop
yields and supply of nitrogen compared in conventional and organic farming systems. _Agric. Food Sci_. 317–326 (2014). Article Google Scholar * Remigi, P., Zhu, J., Young, J. P. &
Masson-Boivin, C. Symbiosis within symbiosis: Evolving nitrogen-fixing legume symbionts. _Trends Microbiol._ 24, 63–75 (2016). Article CAS PubMed Google Scholar * Jerez, C. J. A. &
Romero, R. M. Evaluation of Cajanus cajan (pigeon pea) for phytoremediation of landfill leachate containing chromium and lead. _Int. J. Phytoremediation_ 6514, 00–00 (2016). Google Scholar
* Saadani, O. _et al_. _In situ_ phytostabilisation capacity of three legumes and their associated Plant Growth Promoting Bacteria (PGPBs) in mine tailings of northern Tunisia. _Ecotoxicol.
Environ. Saf._ 130, 263–269 (2016). Article CAS PubMed Google Scholar * Lim, T. K. Cajanus cajan. _Edible Medicinal And Non-Medicinal Plants: Volume 2_, Fruits (ed. Lim, T. K.) 549–568
(Springer Netherlands, 2012). https://doi.org/10.1007/978-94-007-1764-0_70. Google Scholar * Singh, N. K. _et al_. The first draft of the pigeonpea genome sequence. _J. Plant Biochem.
Biotechnol._ 21, 98–112 (2012). Article PubMed Google Scholar * Ismail, H. Y., Ijah, U. J. J., Riskuwa, M. L., Allamin, I. A. & Isah, M. A. Assessment of phytoremediation potentials
of legumes in spent engine oil contaminated soil. _Eur. J. Environ. Saf. Sci._ 2, 59–64 (2014). CAS Google Scholar * Shamsul Harumain, Z. A. Biodegradation of petroleum sludge by
Methylobacterium sp. (Universiti Putra Malaysia, 2012). * Sharma, R. _et al_. Survival, efficacy and rhizospheric effects of bacterial inoculants on Cajanus cajan. _Agric. Ecosyst. Environ._
240, 244–252 (2017). Article Google Scholar * Chikere, C. B., Okpokwasili, G. C. & Ichiakor, O. Characterization of hydrocarbon utilizing bacteria in tropical marine sediments. _J.
Biotechnol._ 8, 2541–2544 (2009). CAS Google Scholar * Bogler, A. M., Lohse, M. & Usadel, B. Trimmomatric: a flexible trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–2120
(2014). Article CAS Google Scholar * Magoc, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. _Bioinformatics_ 27, 2957–2963 (2011).
Article CAS PubMed PubMed Central Google Scholar * Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy
and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. _Appl. Environ. Microbiol._ 79, 5112–5120 (2013). Article CAS PubMed PubMed Central
Google Scholar * Sangeetha, J. & Thangadurai, D. Effect of biologically treated petroleum sludge on seed germination and seedling growth of vigna unguiculata (l.) walp. (fabaceae).
_Braz. Arch. Biol. Technol._ 57, 427–433 (2014). Article CAS Google Scholar * Mansur, A. A. _et al_. Assessing the hydrocarbon degrading potential of indigenous bacteria isolated from
crude oil tank bottom sludge and hydrocarbon-contaminated soil of Azzawiya oil refinery, Libya. _Environ. Sci. Pollut. Res._ 21, 10725–10735 (2014). Article CAS Google Scholar * Abioye,
O. P., Agamuthu, P. & Abdul Aziz, A. R. Phytotreatment of soil contaminated with used lubricating oil using Hibiscus cannabinus. _Biodegradation_ 23, 277–286 (2012). Article CAS PubMed
Google Scholar * Abioye, O. P., Agamuthu, P., Abdul Aziz, A. R., Afzal, M. & Khan, Q. M. Microbe assisted phytoremediation of oil sludge and role of amendments: a mesocosm study. _J.
Environ. Manage._ 12, 1–8 (2012). Google Scholar * Agamuthu, P., Abioye, O. P. & Aziz, A. A. Phytoremediation of soil contaminated with used lubricating oil using Jatropha curcas. _J.
Hazard. Mater._ 179, 891–894 (2010). Article CAS PubMed Google Scholar * Agnello, A. C., Huguenot, D., van Hullebusch, E. D. & Esposito, G. Citric acid- and Tween® 80-assisted
phytoremediation of a co-contaminated soil: alfalfa (Medicago sativa L.) performance and remediation potential. _Environ. Sci. Pollut. Res_. 9215–9226 (2016)
https://doi.org/10.1007/s11356-015-5972-7. Article CAS PubMed Google Scholar * Diab, A. & Badry, K. A. Biodegradation of PAH compounds in the rhizosphere of Tamarix nilotica: A salt
tolerant wild plant. _J. Am. Sci._ 7, 115–124 (2011). Google Scholar * Ukaegbu-Obi, K. M. & Mbakwem-Aniebo, C. C. Bioremediation potentials of bacteria isolated from rhizosphere of some
plants of oil contaminated soil of Niger Delta. _J. Appl. Environ. Microbiol._ 2, 194–197 (2014). Google Scholar * Ali, N., Sorkhoh, N., Salamah, S., Eliyas, M. & Radwan, S. The
potential of epiphytic hydrocarbon-utilizing bacteria on legume leaves for attenuation of atmospheric hydrocarbon pollutants. _J. Environ. Manage._ 93, 113–120 (2012). Article CAS PubMed
Google Scholar * Koolivand, A. _et al_. Degradation of petroleum hydrocarbons from bottom sludge of crude oil storage tanks using in-vessel composting followed by oxidation with hydrogen
peroxide and Fenton. _J. Mater. Cycles Waste Manag._ 15, 321–327 (2013). Article CAS Google Scholar * Basumatary, B., Saikia, R. & Bordoloi, S. Phytoremediation of crude oil
contaminated soil using nut grass, Cyperus rotundus. _J Env. Biol_ 33, 891–896 (2012). Google Scholar * Zhang, C. _et al_. Structure and function of the bacterial communities during
rhizoremediation of hexachlorobenzene in constructed wetlands. _Environ. Sci. Pollut. Res._ 24, 11483–11492 (2017). Article CAS Google Scholar * Kim, B.-R. _et al_. Deciphering diversity
indices for a better understanding of microbial communities. _J. Microbiol. Biotechnol._ 27, 2089–2093 (2017). Article PubMed Google Scholar * Tu, C. _et al_. Rhizoremediation of a
dioxin-like PCB polluted soil by alfalfa: Dynamic characterization at temporal and spatial scale. _Chemosphere_ 189, 517–524 (2017). Article CAS ADS PubMed Google Scholar * Hou, J. _et
al_. PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. _Chemosphere_ 138, 592–598 (2015). Article CAS ADS PubMed Google Scholar
* Bergmann, G. T. _et al_. The under-recognized dominance of Verrucomicrobia in soil bacterial communities. _Soil Biol. Biochem._ 43, 1450–1455 (2012). Article CAS Google Scholar *
Debruyn, J. M., Nixon, L. T., Fawaz, M. N., Johnson, A. M. & Radosevich, M. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. _Appl. Environ.
Microbiol._ 77, 6295–6300 (2011). Article CAS PubMed PubMed Central Google Scholar * Halla, J., Soole, K. & Bentham, R. Hydrocarbon phytoremediation in the family Fabacea — A
Review. _Int. J. Phytoremediation_ 13, 317–332 (2011). Article CAS Google Scholar * Ji, Z. J. _et al_. Competition between rhizobia under different environmental conditions affects the
nodulation of a legume. _Syst. Appl. Microbiol._ 40, 114–119 (2017). Article PubMed Google Scholar * Sugiyama, A. & Yazaki, K. Root exudates of legume plants and their involvement in
interactions with soil microbes. _Signal. Commun. Plants_ 12, 27–49 (2012). Article Google Scholar * Reichman, S. M. The potential use of the legume-rhizobium symbiosis for the remediation
of arsenic contaminated sites. _Soil Biol. Biochem._ 39, 2587–2593 (2007). Article CAS Google Scholar * Hao, X. _et al_. Phytoremediation of heavy and transition metals aided by
legume-rhizobia symbiosis. _Int. J. Phytoremediation_ 16, 179–202 (2014). Article CAS PubMed Google Scholar * Sánchez-Pardo, B. & Zornoza, P. Mitigation of Cu stress by
legume-Rhizobium symbiosis in white lupin and soybean plants. _Ecotoxicol. Environ. Saf._ 102, 1–5 (2014). Article CAS PubMed Google Scholar * Piechalak, A., Tomaszewska, B.,
Baralkiewicz, D. & Malecka, A. Accumulation and detoxification of lead ions in legumes. _Phytochemistry_ 60, 153–162 (2002). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS This project was supported by the Putra-IPS grants GP-IPS/2017/9517500 (Rhizodegradation of petroleum oily sludge contaminated soil by hetero-rhizospheric bacteria from
_Cajanus cajan_ (Pigeon pea)). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400
UPM Serdang, Selangor, Malaysia Ibrahim Alkali Allamin, Nur Adeela Yasid, Siti Aqlima Ahmad & Yunus Shukor * Department of Microbiology, Faculty of Sciences, University of Maiduguri,
P.M.B. 1069, Maiduguri, Borno State, Nigeria Ibrahim Alkali Allamin * Department of Land Management, Faculty of Agriculture, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia Mohd
Izuan Effendi Halmi * Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, UKM Bangi 43600, Selangor, Malaysia Siti
Rozaimah Sheikh Abdullah Authors * Ibrahim Alkali Allamin View author publications You can also search for this author inPubMed Google Scholar * Mohd Izuan Effendi Halmi View author
publications You can also search for this author inPubMed Google Scholar * Nur Adeela Yasid View author publications You can also search for this author inPubMed Google Scholar * Siti Aqlima
Ahmad View author publications You can also search for this author inPubMed Google Scholar * Siti Rozaimah Sheikh Abdullah View author publications You can also search for this author
inPubMed Google Scholar * Yunus Shukor View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I.A.A., S.A.A, Y.S. and S.R.S.A. designed research.
I.A.A. conducted the experiments. N.A.Y., M.I.E.H. and Y.S analyzed the data. I.A.A. wrote the main manuscript and prepared figures and tables. All authors have reviewed the manuscript.
CORRESPONDING AUTHOR Correspondence to Yunus Shukor. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION DATASETS 1 TO 3. RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Allamin, I.A., Halmi, M.I.E., Yasid,
N.A. _et al._ Rhizodegradation of Petroleum Oily Sludge-contaminated Soil Using _Cajanus cajan_ Increases the Diversity of Soil Microbial Community. _Sci Rep_ 10, 4094 (2020).
https://doi.org/10.1038/s41598-020-60668-1 Download citation * Received: 28 April 2019 * Accepted: 12 February 2020 * Published: 05 March 2020 * DOI:
https://doi.org/10.1038/s41598-020-60668-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative