Characterization of cereblon-dependent targeted protein degrader by visualizing the spatiotemporal ternary complex formation in cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Targeted protein degradation (TPD) through a proteasome-dependent pathway induced by heterofunctional small molecules is initiated by the formation of a ternary complex with
recruited E3 ligases. This complex formation affects the degradation ability of TPD molecules, and thus we tested for visualization of the intracellular dynamics of ternary complex
formation. In this study, we applied the fluorescent-based technology detecting protein-protein interaction (Fluoppi) system, in which detectable fluorescent foci are formed when ternary
complex formation induced by TPD molecules occurs in cells. We show here that cells coexpressing BRD4 and cereblon (CRBN) tagged with the Fluoppi system formed detectable foci in both live
and fixed cells only when treated with BRD4-targeting degraders utilizing CRBN as an E3 ligase in dose- and time-dependent manners. Notably, the maintenance and efficacy of TPD
molecule-induced foci formation correlated with the ability to degrade target proteins. Furthermore, we demonstrated that BRD4-targeting and FKBP12F36V-targeting degraders formed ternary
complexes mainly in the nucleus and cytoplasm, respectively, suggesting that TPD molecules utilize the proteasome to degrade target proteins in their corresponding localized region. Our
results also suggest that the Fluoppi system is a powerful tool for characterizing TPD molecules by visualizing the spatiotemporal formation of ternary complex. SIMILAR CONTENT BEING VIEWED
BY OTHERS SNAPSHOTS AND ENSEMBLES OF BTK AND CIAP1 PROTEIN DEGRADER TERNARY COMPLEXES Article 16 November 2020 AFFINITY AND COOPERATIVITY MODULATE TERNARY COMPLEX FORMATION TO DRIVE TARGETED
PROTEIN DEGRADATION Article Open access 13 July 2023 MOLECULAR MECHANISMS OF CAND2 IN REGULATING SCF UBIQUITIN LIGASES Article Open access 26 February 2025 INTRODUCTION Removing a protein
of interest such as a disease-causative undruggable protein through targeted protein degradation (TPD) is anticipated to become a new therapeutic modality1,2,3,4. TPD molecules are
bifunctional molecules composed of target-binding and E3 ligase-binding components connected by a linker, and these three moieties have been refined in order to augment the abilities of TPD
molecules and thereby discover efficacious new drugs5,6,7. To confirm the potency of TPD molecules _in vitro_, the capacities not only to degrade the target proteins in cells, but also to
induce a ternary complex with a target protein, a TPD molecule and an E3 ligase, are often examined. Because the ternary complex formation initiates the ubiquitination of target proteins
followed by their degradation in cells, it is important to analyze the ternary complex formation in order to improve the design of TPD molecules, potentially leading to efficacious new
drugs8,9,10. For this purpose, binding assays such as amplified luminescent proximity homogeneous assay (AlphaLISA)11 and surface plasmon resonance (SPR)12 techniques have been performed in
cell-free systems. Furthermore, a recent report described the measurement of bromodomain and extra-terminal (BET) protein degraders’ capacity for ternary complex formation or ubiquitination
in cells using NanoBRET systems13 and that time-lapse protein-protein interaction imaging via the separation of phases-based protein interaction reporter (SPPIER) visualized early ternary
complex formation induced by BET protein degraders14. Even if these techniques can measure the ternary complex formation ability, it is difficult to clarify the maintenance and efficacy of
intracellular spatiotemporal dynamics of ternary complex formation induced by TPD molecules. Therefore, we utilize the fluorescent-based technology detecting protein-protein interaction
(Fluoppi) system to clarify such dynamics in cells. The Fluoppi system is a tool for detecting protein-protein interactions in live and fixed cells15,16,17. Briefly, it involves the
coexpression of an Azami-Green (AG) fused protein and another Assembly helper (Ash)-tagged protein in cells. AG-derived fluorescence is uniformly distributed in the absence of interaction.
However, once the interaction occurs, AG-derived fluorescence forms detectable foci because of the excess assembly of two proteins through Ash tags. In this paper, we present data showing
not only that TPD molecules form specific foci with the Fluoppi system, which enables determination of where and when the TPD molecules induce ternary complexes in cells, but also that the
duration of foci formation is correlated with the degradation abilities of TPD molecules. RESULTS CEREBLON (CRBN)-DEPENDENT BET PROTEIN DEGRADERS INDUCE SPECIFIC FOCI FORMATION WITH THE
FLUOPPI SYSTEM To assess the possibility that the Fluoppi system could be applied to evaluate TPD molecules, we first chose ARV-82518, which was composed of BRD4-binding OTX01519 and
CRBN-binding pomalidomide20 and degraded BRD4 by recruiting CRBN as an E3 ligase. We constructed Fluoppi expression vectors coding BRD4 fused to the Ash tag at the C-terminus (BRD4-Ash) and
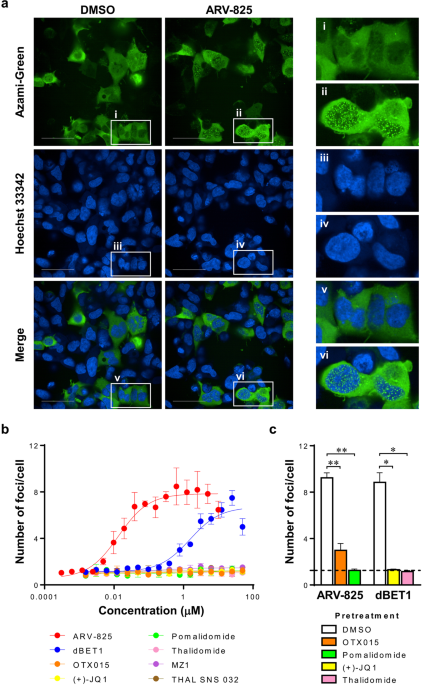
CRBN fused to AG at the C-terminus (CRBN-AG), and cotransfected these vectors into 293A cells. We then treated these cells with 0.5% DMSO or 0.1 µM ARV-825 for 30 min and fixed them,
followed by confocal microscopic analysis. As shown in Fig. 1a, ARV-825 induced specific foci mainly in the nucleus, but DMSO did not. Next, we treated these cells with various compounds and
determined the number of foci per cell. Figure 1b indicates that only TPD molecules, ARV-825 and dBET111, which bound both BRD4 and CRBN, induced foci formation in a dose-dependent manner.
This suggests that neither parts of TPD molecules such as BRD4-binding (+)-JQ121, OTX015, CRBN-binding thalidomide22 and pomalidomide nor TPD molecules such as MZ123 bound to BRD4 and
von-Hippel-Lindau (VHL) and THAL SNS 03224 bound to CDK9 and CRBN form specific foci. Furthermore, to confirm the specificity of TPD molecule-induced foci formation, cells were treated with
an excess amount of the moieties of ARV-825 or dBET1 prior to the treatment with these TPD molecules (Fig. 1c). As expected, pretreatment with the parts of TPD molecules remarkably reduced
the foci formation, suggesting that the foci induced by TPD molecules represent the ternary complex formation. We also checked the opposite combination of Fluoppi tags, with the results
showing that AG-fused BRD4 formed foci with its binders even in the absence of Ash-fused CRBN (Supplementary Fig. S1), suggesting that AG-fused BRD4 could nonspecifically form the foci when
treated with BRD4 binders (see Discussion). FOCI FORMED IN THE FLUOPPI SYSTEM ARE TIME-DEPENDENT AND REPRESENT TERNARY COMPLEXES TO BE DEGRADED BY PROTEASOME Next, we investigated the time
course of foci formation induced by ARV-825 or dBET1. As shown in Fig. 2a, both molecules induced foci as early as 5 min after stimulation and the levels of foci formation peaked within 1 h.
Intriguingly, foci induced by dBET1 were diminished in 6 h, whereas ARV-825 maintained foci formation 24 h later, suggesting that dBET1 could not keep the ternary complex in cells for
longer than 6 h. Previous reports suggest that the proteasome inhibitor MG132 blocks targeted protein degradation by TPD molecules13,18. Therefore, we pretreated cells cotransfected with
BDR4-Ash and CRBN-AG with MG132 before the stimulation with ARV-825 or dBET1 and investigated the formation of foci (Fig. 2b). Surprisingly, inhibition of protein degradation through the
proteasome sustained or upregulated foci formation, even after the treatment with dBET1 for 6 h. These results indicated that TPD molecules maintained the ternary complex in the absence of
targeted protein degradation. To confirm that the foci formation correlates with the degradation of target proteins, we next checked the time-dependent protein degradation levels with these
molecules. To this end, we utilized the HiBiT tag system, which easily enabled measurement of the endogenous protein expression levels in combination with the CRISPR/Cas9 knock-in system25.
Cells harboring the HiBiT sequence in the N-terminus of the BRD4 gene were treated with ARV-825 or dBET1 for 3, 8 and 24 h (Fig. 2c). The obtained results show that ARV-825 continuously
reduced the protein levels of BRD4 for 24 h, while dBET1 lost its degradation ability after 3 h, which was correlated with the rapid reduction of foci formation as shown in Fig. 2a and
consistent with the observation that dBET1 was unstable when the proteasome pathway was active (Fig. 2b). Taken together, these results imply that the Fluoppi system detects the TPD
molecule-induced ternary complex amenable to proteasomal degradation. FLUOPPI ANALYSIS REVEALS WHERE TERNARY COMPLEX FORMATION OCCURS IN CELLS BRD4 is a member of the BET family, which
localizes in the nucleus26. In this paper, we show that the foci formation induced by BRD4 targeting degraders mainly occurred in the nucleus (Fig. 1a). We also tested another TPD molecule,
dTAG-1327,28, which targets FKBP12 containing F36V mutation (FKBP12F36V) and located in the cytoplasm29 and binds CRBN as an E3 ligase. Cells coexpressing FKBP12F36V fused to Ash tag at the
N-terminus (Ash-FKBP12F36V) and CRBN-AG were treated with 1 µM dTAG-13 for 1 h and fixed, followed by confocal microscopic analysis (Fig. 3a,b). As expected, dTAG-13 induced specific foci
mainly in the cytoplasm. We also investigated the time course of foci formation by dTAG-13 (Fig. 3c). The obtained results indicated that dTAG-13 continuously increased the foci formation
until 24 h after treatment. In addition, ARV-825 continuously formed foci for 24 h, while dBET1 lost such formation after 6 h, as shown in Fig. 2a. Our results indicate that the occurrence
and duration of foci formation are dependent on the properties of TPD molecules. TERNARY COMPLEX FORMATION OCCURS IMMEDIATELY AFTER THE TREATMENT OF TPD MOLECULES To visualize the formation
of ternary complex at early time points after the addition of TPD molecules, we utilized real-time monitoring. Cells coexpressing BRD4-Ash and CRBN-AG were prepared for live-cell imaging,
and AG and Hoechst 33342 images were monitored after the addition of DMSO or ARV-825 for 1 h (Fig. 4a,b and Supplementary Movies S1–S3). The obtained results showed that treatment with
ARV-825 (0.1 µM) initiated the formation of foci in the nucleus within a few minutes and this peaked within 30 min, which is similar to the results depicted in Fig. 2a. However, treatment
with a lower concentration of ARV-825 (0.01 µM) gradually increased foci formation, indicating that the early formation of ternary complexes induced by TPD molecules occurred in time- and
dose-dependent manners. DISCUSSION TPD molecules link target proteins to E3 ligases to initiate ubiquitination of the target proteins, followed by degradation of the target proteins via a
ubiquitin-proteasome dependent pathway. To date, TPD molecules’ abilities have been mainly evaluated in terms of capacities such as binding to target proteins and E3 ligases in cell-free
systems7,11 or by cell-based BRET13 and SPPIER14 assays, or in terms of the potency for degrading target proteins in cells5. Because of the complexity of the cellular environment, it is also
highly beneficial for the design of TPD molecules to monitor the dynamics of the ternary complex formation in cells8,9. From the perspective of evaluating the intracellular ternary complex
formation, the NanoBRET system has advantages for enabling the real-time monitoring of ternary complex formation biochemically with a high bioluminescent signal and for acquiring robust data
because of the use of the acceptor/donor channel emission ratio as an evaluation criterion13. However, compared with fluorescent protein-based assays, such as the Fluoppi and SPPIER
systems, the NanoBRET system has lower signal-to-noise ratios due to it being based on Förster resonance energy transfer (FRET) and it remains difficult to monitor the intracellular dynamics
of the complex visually. One potential reason for this is the degradation of the target protein itself, resulting in loss of the energy donor or acceptor in the NanoBRET system, while
fluorescent protein-based assays form fluorescent spots with assembled target proteins and the disruption of a target protein appears to have little effect on the foci formation. In addition
to these advantages of robustness to the degradation of target proteins and high brightness with condensed fluorescent proteins, the SPPIER system can monitor two interacting proteins with
different fluorescent proteins, which facilitates the understanding of individual molecular dynamics14. However, this approach has the disadvantages of being applicable only to live-cell
imaging and that its spatial resolution still needs to be improved. In fact, SPPIER-tagged BRD4 appeared to be localized in the cytosol despite BRD4 being a nuclear protein26, which was
probably due to huge protein aggregation or the specific characteristics of the fluorescent protein tags used in the SPPIER system. The Fluoppi system also utilizes the assembly of
fluorescent proteins and, for this reason, has advantages similar to those of the SPPIER system in detecting the ternary complex. However, the Fluoppi system has better spatial resolution,
such as enabling the intracellular localization of target proteins (see below), and also enables spatiotemporal visualization of ternary complex formation with both live and fixed cells,
contributing to the further development of flexible assays; for example, fixed foci-formed cells can be subjected to staining with the antibodies against other proteins. However, the Fluoppi
system has the disadvantage that it requires preliminary examinations to eliminate the possibility of nonspecific foci formation (see below). Previous studies suggested that ARV-825 can
more efficiently decrease the expression of BRD4 and the proliferation of leukemia cells than dBET130,31. Our results also indicated that ARV-825 induced specific foci formation more
effectively than dBET1 (Fig. 1b). It is conceivable that the difference in EC50 values of foci formation among TPD molecules reflects the ability to downregulate the target protein. It is
supposed that the time course of foci formation by these two BET protein degraders was strongly correlated with maintenance of the levels of degradation of the target proteins (Fig. 2a,c).
These results suggest a strong correlation between the level of time-dependent formation of ternary complex and the level of targeted protein degradation, supporting the notion that a stable
ternary complex leads to efficient targeted protein degradation8. In addition, dBET1 has been reported to partially cancel the degradation of BRD4 upon the treatment of cells for 24 h due
to the instability of the phthalimide component11, which is consistent with our observations (Fig. 2c). Because the inhibition of proteasome-dependent targeted protein degradation by a
proteasome inhibitor sustained or increased the foci formation induced by dBET1 (Fig. 2b), the data obtained with Fluoppi analysis first revealed that the dissociation of dBET1 from the
ternary complex by the proteasome-dependent degradation of BRD4 caused the instability of dBET1 in cells, resulting in the rapid loss of its degradation ability (Fig. 2a,c). Moreover, the
Fluoppi system also allows us to monitor early foci formation in live cells (Fig. 4 and Supplementary Movies S1–S3), and we show that the peak time of foci formation after the addition of
ARV-825 depends on its concentration (Fig. 4b), which is similar to the results obtained with other systems13,14. Collectively, these findings show that the Fluoppi system is a useful tool
for evaluating the efficacy and longevity of TPD molecules in cells. It has remained unclear where the TPD molecules form ternary complexes and degrade the target proteins. We utilized BRD4
as an example of a nuclear protein26 and FKBP12 as a cytoplasmic protein29, and clearly showed that BRD4-degrader and FKBP12F36V-degrader formed ternary complexes mainly in the nucleus (Fig.
1a and Fig. 4a) and cytoplasm (Fig. 3a,b), respectively. As previously reported, the ubiquitin-proteasome systems function in the cytoplasm and nucleus32,33; our results therefore clearly
suggest that TPD molecules utilize the proteasome to degrade target proteins in their corresponding localized region. To summarize, our data presented in this report reveal that the Fluoppi
system can characterize TPD molecules by investigating when and where ternary complex formation takes place. The Fluoppi system enables the visualization of protein-protein interaction in
the form of foci created by the accumulation of AG with Ash tags. Specific foci were induced by TPD molecules when appropriate target proteins and E3 ligases fused with Fluoppi tags were
coexpressed (Fig. 1b); however, nonspecific foci formed when AG-fused protein accumulated alone in the presence of its binder (Fig. S1). We speculate that this formation of nonspecific foci
with AG-fused BRD4 occurred for the following reasons. BRD4 binds the acetylated histone H3 and H4 in steady-state conditions34; however, its binding is disrupted by BET inhibitors such as
(+)-JQ1 and OTX015, followed by the rapid accumulation of dissociated free BRD4 in the nucleus18,21,30. Moreover, BRD4 molecules interact with each other35, so it is conceivable that BET
inhibitor-induced free AG-fused BRD4 molecules accumulate in the nucleus and associate with each other to form fluorescent foci even in the absence of Ash-tagged CRBN. It is also suggested
by another report that tetrameric AG-fused oligomeric proteins could potentially form huge foci in cells36. Therefore, to avoid nonspecific foci formation in the study of TPD molecules with
the Fluoppi system, it is important to confirm in advance that AG-fused protein cannot form foci by self-oligomerization and that TPD molecules or binders of target proteins or E3 ligases
used as moieties of TPD molecules cannot induce foci in cells expressing AG-fused protein alone. In this study, we have confirmed that AG-fused CRBN could not form foci in the steady state
or upon stimulation with CRBN binders such as pomalidomide or thalidomide (Fig. 1); therefore, the combination of AG-fused CRBN and Ash-tagged target protein can be applied for assessing
various CRBN-dependent TPD molecules. We summarize the workflow for the evaluation of TPD molecules with the Fluoppi system in Supplementary Table S1. Lastly, this Fluoppi system can be
adapted for a readout method in high-throughput screening. Using a 384-well plate format, even though we performed both kinetic live-cell imaging (Fig. 4) and microscopic analysis of fixed
cells (Fig. 1) with the transiently transfected cells, it is preferable to use cell lines stably expressing the Fluoppi-tagged proteins for high-throughput screening because the transfection
efficiency in each experiment strongly affects the quantification of foci per cell. Given that the spatiotemporal visualization by the Fluoppi system enables detailed analysis of a TPD
molecule’s potency, our approach amenable to high-throughput screening permits large-scale _in vitro_ assessment, which would be highly valuable to the drug discovery process such as lead
optimization. We have now investigated the ternary complex formation induced by TPD molecules recruiting another E3 ligase with the Fluoppi system. TPD is an emerging technology with
therapeutic applications and is rapidly developing in terms of its use for drug production. Therefore, multiple methods for characterizing TPD molecules will soon be needed, and our results
provide a new option for evaluating TPD molecules’ properties by visualizing the spatiotemporal formation of ternary complex in cells. METHODS REAGENTS ARV-825, dBET1, (+)-JQ1, OTX015 and
pomalidomide were purchased from Cayman. MZ1, THAL SNS 032, dTAG-13 and MG132 were purchased from Tocris Bioscience. Thalidomide was purchased from Sigma. All compounds were dissolved with
DMSO (Merck Millipore). FLUOPPI VECTOR CONSTRUCTION Fluoppi vectors (phAG-MNL, pAsh-MNL or pAsh-MCL) were provided by Medical & Biological Laboratories. ORF cDNAs of human BRD4, FKBP12
or cereblon (CRBN) were obtained from Flexi ORF Clone (Promega). FKBP12F36V single point mutation was inserted using PrimeSTAR Mutagenesis Basal Kit (Takara Bio), in accordance with the
manufacturer’s instructions. ORFs amplified with PrimeSTAR Max DNA Polymerase (Takara Bio) were inserted into Fluoppi vectors using In-Fusion HD cloning kit (Takara Bio), in accordance with
the manufacturer’s instructions. CELL PREPARATION FOR FLUOPPI ANALYSIS 293A cells (Thermo Fisher Scientific) were cultured with Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific)
containing 10% FBS (HyClone), MEM Non-Essential Amino Acids Solution (Thermo Fisher Scientific) and GlutaMax (Thermo Fisher Scientific) within a humidified incubator with 5% CO2 at 37 °C.
Cells were harvested and 5 × 105 of them were plated on a six-well plate (Corning). The next day, phAG and pAsh vectors were cotransfected with Lipofectamine 3000 reagents (Thermo Fisher
Scientific), in accordance with the manufacturer’s instructions. The ratios of phAG and pAsh vectors were as follows: phAG-MNL-CRBN and pAsh-MNL-BRD4 were used at a ratio of 3:1 for the
BRD4/CRBN assay and phAG-MNL-CRBN and pAsh-MCL-FKBP12F36V were used at a ratio of 9:1 for the FKBP12F36V/CRBN assay. The following day, cells were harvested and counted. A total of 2 × 104
cells were plated on a 384-well plate (PerkinElmer) or frozen using Cell Banker (Wako) for live-cell imaging analysis. MICROSCOPIC ANALYSIS OF FIXED CELLS TREATED WITH COMPOUNDS Compounds
and DMSO were directly added to cultured cells with a D300e digital dispenser (Tecan). In some experiments, cells were pretreated with MG132 or moieties of TPD molecules for 1 h before the
treatment with TPD molecules. After incubation for the indicated times, cells were fixed with 20 µL of Mildform 20N (Wako) containing 1 µg/mL Hoechst 33342 (Sigma) for 15 min, followed by
washing with 50 µL of PBS (Thermo Fisher Scientific) twice. After washing, the cells were immersed in 50 µL of PBS and the plate was sealed. AG and Hoechst 33342 images were acquired with
Opera Phenix (PerkinElmer). Twenty-five fields of view per well were acquired using a ×63 water-immersed objective lens. The numbers of foci formed by AG and Hoechst 33342-positive cells
were quantified and the number of foci per cell was calculated with Harmony 4.9 software (PerkinElmer). EC50 values of foci formation of TPD molecules were calculated with Prism 6 software
(GraphPad Software). KINETIC LIVE-CELL IMAGING OF ARV-825 Frozen cells were thawed and 2.5 × 104 of them were plated on a 384-well plate. The next day, cells were washed with culture medium
and incubated with 20 µL of culture medium containing 2 µg/mL Hoechst 33342 for more than 30 min. The culture plate was set into Cell Voyager 7000 S (CV7000S, Yokogawa) with a humidified 5%
CO2 incubator at 37 °C. After the addition of 20 µL of culture medium containing 0.1% DMSO or 0.02 or 0.2 µM ARV-825 in 0.1% DMSO, three fields of view in a well were acquired with a ×60
water-immersed objective lens for 1 h at 45-s intervals. CellPathfinder software (Yokogawa) was used to create imaging movies and count the foci that formed. MEASUREMENT OF INTRACELLULAR
BRD4 PROTEIN LEVELS 293A cells harboring a HiBiT tag sequence25 in the N-terminus of the BRD4 gene (293A_HiBiT-BRD4) were established using the CRISPR-Cas9 system in our laboratory. Four
thousand cells per well were plated on a 384-well white plate (Greiner). The next day, cells were treated with DMSO or BET protein degraders at the indicated concentrations and incubated for
3, 8 and 24 h. After incubation, cells were treated with Nano Glo HiBiT Lytic Detection System (Promega) to detect the HiBiT-BRD4-derived luminescent signals with EnVision Xcite
(PerkinElmer). The number of cells in each well was estimated based on DNA content measured using CellTox Green Reagent (Promega) with EnVision Xcite and the luminescent intensity of
HiBiT-BRD4 was normalized with the fluorescent signals of CellTox Green Reagent. The BRD4 expression levels in BET protein degrader-treated cells were calculated as the percentage of those
in DMSO-treated cells. STATISTICAL ANALYSIS Statistical analyses using unpaired two-tailed Student’s t-test were performed with KaleidaGraph software (Synergy Software). REFERENCES * Lai, A.
C. & Crews, C. M. Induced protein degradation: an emerging drug discovery paradigm. _Nat. Rev. Drug Discov._ 16, 101–114 (2017). Article CAS Google Scholar * Huang, X. & Dixit,
V. M. Drugging the undruggables: exploring the ubiquitin system for drug development. _Cell Res._ 26, 484–498 (2016). Article CAS Google Scholar * An, S. & Fu, L. Small-molecule
PROTACs: An emerging and promising approach for the development of targeted therapy drugs. _EBioMedicine._ 36, 553–562 (2018). Article Google Scholar * Ohoka, N., Shibata, N., Hattori, T.
& Naito, M. Protein knockdown technology: Application of ubiquitin ligase to cancer therapy. _Curr. Cancer Drug Targets._ 16, 136–146 (2016). Article CAS Google Scholar * Sakamoto, K.
M. _et al_. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. _Proc. Natl. Acad. Sci. USA_ 98, 8554–8559 (2001). Article
ADS CAS Google Scholar * Collins, I., Wang, H., Caldwell, J. J. & Chopra, R. Chemical approaches to targeted protein degradation through modulation of the ubiquitin-proteasome
pathway. _Biochem. J._ 474, 1127–1147 (2017). Article CAS Google Scholar * Gadd, M. S. _et al_. Structural basis of PROTAC cooperative recognition for selective protein degradation. _Nat.
Chem. Biol._ 13, 514–521 (2017). Article CAS Google Scholar * Bondeson, D. P. _et al_. Lessons in PROTAC design from selective degradation with a promiscuous warhead. _Cell Chem. Biol_.
18, 78–87.e5 (2018). * Paiva, S. L. & Crews, C. M. Targeted protein degradation: elements of PROTAC design. _Curr. Opin. Chem. Biol._ 50, 111–119 (2019). Article CAS Google Scholar *
Hines, J., Lartigue, S., Dong, H., Qian, Y. & Crews, C. M. MDM2-recruiting PROTAC offers superior, synergistic antiproliferative activity via simultaneous degradation of BRD4 and
stabilization of p53. _Cancer Res._ 79, 251–262 (2019). Article CAS Google Scholar * Winter, G. E. _et al_. Drug development. Phthalimide conjugation as a strategy for _in vivo_ target
protein degradation. _Science._ 348, 1376–1381 (2015). Article ADS CAS Google Scholar * Roy, M. J. _et al_. SPR-measured dissociation kinetics of PROTAC ternary complexes influence
target degradation rate. _ACS Chem. Biol._ 14, 361–368 (2019). Article CAS Google Scholar * Riching, K. M. _et al_. Quantitative live-cell kinetic degradation and mechanistic profiling of
PROTAC mode of action. _ACS Chem. Biol._ 13, 2758–2770 (2018). Article CAS Google Scholar * Chung, C. I., Zhang, Q. & Shu, X. Dynamic imaging of small molecule induced
protein-protein interactions in living cells with a fluorophore phase transition based approach. _Anal. Chem._ 90, 14287–14293 (2018). Article CAS Google Scholar * Koyano, F. _et al_.
Ubiquitin is phosphorylated by PINK1 to activate parkin. _Nature._ 510, 162–166 (2014). Article ADS CAS Google Scholar * Yamano, K. _et al_. Site-specific interaction mapping of
phosphorylated ubiquitin to uncover parkin activation. _J. Biol. Chem._ 290, 25199–25211 (2015). Article CAS Google Scholar * Watanabe, T. _et al_. Genetic visualization of protein
interactions harnessing liquid phase transitions. _Sci. Rep._ 7, 46380, https://doi.org/10.1038/srep46380 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Lu, J. _et al_.
Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. _Chem. Biol._ 22, 755–763 (2015). Article CAS Google Scholar * Noel, J. K. _et al_. Development of the BET
bromodomain inhibitor OTX015. _Mol. Cancer Ther._ 12, C244, https://doi.org/10.1158/1535-7163.TARG-13-C244 (2014). Article Google Scholar * Lopez-Girona, A. _et al_. Cereblon is a direct
protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. _Leukemia._ 26, 2326–2335 (2012). Article CAS Google Scholar * Filippakopoulos, P.
_et al_. Selective inhibition of BET bromodomains. _Nature._ 468, 1067–1073 (2010). Article ADS CAS Google Scholar * Ito, T. _et al_. Identification of a primary target of thalidomide
teratogenicity. _Science._ 327, 1345–1350 (2010). Article ADS CAS Google Scholar * Zengerle, M., Chan, K. H. & Ciulli, A. Selective small molecule induced degradation of the BET
bromodomain protein BRD4. _ACS Chem. Biol._ 10, 1770–1777 (2015). Article CAS Google Scholar * Olson, C. M. _et al_. Pharmacological perturbation of CDK9 using selective CDK9 inhibition
or degradation. _Nat. Chem. Biol._ 14, 163–170 (2018). Article CAS Google Scholar * Schwinn, M. K. _et al_. CRISPR-mediated tagging of endogenous proteins with a luminescent peptide. _ACS
Chem. Biol._ 13, 467–474 (2018). Article CAS Google Scholar * Dey, A. _et al_. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. _Mol.
Cell Biol._ 20, 6537–6549 (2000). Article CAS Google Scholar * Erb, M. A. _et al_. Transcription control by the ENL YEATS domain in acute leukaemia. _Nature._ 543, 270–274 (2017). Article
ADS CAS Google Scholar * Nabet, B. _et al_. The dTAG system for immediate and target-specific protein degradation. _Nat. Chem. Biol._ 14, 431–441 (2018). Article CAS Google Scholar *
Harding, M. W., Galat, A., Uehling, D. E. & Schreiber, S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. _Nature._ 341, 758–760 (1989). Article
ADS CAS Google Scholar * Xu, L. _et al_. Targetable BET proteins- and E2F1-dependent transcriptional program maintains the malignancy of glioblastoma. _Proc. Natl. Acad. Sci. USA_ 115,
E5086–5095 (2018). Article CAS Google Scholar * Zhang, X. _et al_. Protein targeting chimeric molecules specific for bromodomain and extra-terminal motif family proteins are active
against pre-clinical models of multiple myeloma. _Leukemia._ 32, 2224–2239 (2018). Article CAS Google Scholar * Reits, E. A., Benham, A. M., Plougastel, B., Neefjes, J. & Trowsdale,
J. Dynamics of proteasome distribution in living cells. _EMBO J._ 16, 6087–6094 (1997). Article CAS Google Scholar * Pack, C. G. _et al_. Quantitative live-cell imaging reveals
spatio-temporal dynamics and cytoplasmic assembly of the 26S proteasome. _Nat. Commun._ 5, 3396, https://doi.org/10.1038/ncomms4396 (2014). Article ADS CAS PubMed Google Scholar * Dey,
A., Chitsaz, F., Abbasi, A., Misteli, T. & Ozato, K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. _Proc. Natl. Acad. Sci. USA_ 100,
8758–8763 (2003). Article ADS CAS Google Scholar * Wang, R., Li, Q., Helfer, C. M., Jiao, J. & You, J. Bromodomain protein Brd4 associated with acetylated chromatin is important for
maintenance of higher-order chromatin structure. _J. Biol. Chem._ 287, 10738–10752 (2012). Article CAS Google Scholar * Karasawa, S., Araki, T., Yamamoto-Hino, M. & Miyawaki, A. A
green-emitting fluorescent protein from Galaxeidae coral and its monomeric version for use in fluorescent labeling. _J. Biol. Chem._ 278, 34167–34171 (2003). Article CAS Google Scholar
Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Biochemistry Research Group, Biological Research Department, Daiichi Sankyo RD Novare Co., Ltd., 1-16-13 Kitakasai,
Edogawa-ku, Tokyo, 134-8630, Japan Tomohiro Kaji, Hiroshi Koga, Mutsumi Kuroha, Toshihiko Akimoto & Kenji Hayata Authors * Tomohiro Kaji View author publications You can also search for
this author inPubMed Google Scholar * Hiroshi Koga View author publications You can also search for this author inPubMed Google Scholar * Mutsumi Kuroha View author publications You can also
search for this author inPubMed Google Scholar * Toshihiko Akimoto View author publications You can also search for this author inPubMed Google Scholar * Kenji Hayata View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.K. designed and conducted most of the experiments, and wrote most of the paper. H.K. conducted
live-cell imaging and edited the manuscript. M.K. established genome-edited cells and measured protein expression levels. T.A. and K.H. edited the manuscript and supervised the study. All
authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Tomohiro Kaji. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION. SUPPLEMENTARY MOVIE S1. SUPPLEMENTARY MOVIE S2. SUPPLEMENTARY MOVIE S3. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kaji, T., Koga, H., Kuroha, M. _et al._ Characterization of cereblon-dependent
targeted protein degrader by visualizing the spatiotemporal ternary complex formation in cells. _Sci Rep_ 10, 3088 (2020). https://doi.org/10.1038/s41598-020-59966-5 Download citation *
Received: 10 October 2019 * Accepted: 05 February 2020 * Published: 20 February 2020 * DOI: https://doi.org/10.1038/s41598-020-59966-5 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative