Adsorptive removal of tetracycline by sustainable ceramsite substrate from bentonite/red mud/pine sawdust
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In this study, a novel, sustainable and efficient ceramsite substrate of constructed wetlands (CWs) were prepared for tetracycline (TC) removal by employing bentonite (Ben) and red
mud (Rm) as the main materials and pine sawdust (Ps) as the additive. The optimal parameters for Ben/Rm/Ps ceramsite preparation were obtained via orthogonal and one-factor experimental
designs, and the optimal parameters were presented as follows: mass ratio of Ben: Rm: Ps = 4:1:0.9, preheating temperature = 240 °C, preheating time = 20 min, calcining temperature = 1150
°C, and calcining time = 14 min. The properties of Ben/Rm/Ps-op ceramsite (obtained at the optimal condition) were first analyzed, including XRD and SEM, and demonstrated a microporous
structure with some crystal strength components. Neutral condition and higher temperature were indicated conducive to improve the TC removal efficiency, while coexisting ions (Na+ or Ca2+)
showed adverse effect for TC adsorption by Ben/Rm/Ps-op. In addition, adsorption kinetics and isotherm could be well described by the second-order kinetics and linear isothermal model,
respectively, which suggested chemisorption and multilayer adsorption thickness increased infinitely. The theoretical maximum TC adsorption capacity of Ben/Rm/Ps-op at 20 °C reached up to
2.5602 mg/g. In addition, Ben/Rm/Ps-op could effectively remove TC as the CWs substrate under a dynamic flow condition. Further, Ben/Rm/Ps-op exhibited high reusability capability and
stability for TC removal, and the adsorption amount still remained for 2.13 mg/g (_C_0 = 80 mg/L) after three consecutive cycles. SIMILAR CONTENT BEING VIEWED BY OTHERS BIOCHAR MMT ZNAL LDH
COMPOSITE MATERIALS DERIVED FROM SOLID WASTE FOR HEAVY METAL REMOVAL IN ARTIFICIAL ACID MINE DRAINAGE Article Open access 28 April 2025 ADSORPTION PROPERTIES OF PB(II) AND CD(II) IN ACID
MINE DRAINAGE BY OYSTER SHELL LOADED LIGNITE COMPOSITE IN DIFFERENT MORPHOLOGIES Article Open access 21 May 2024 STUDY ON THE PREPARATION MECHANISM AND DEPHOSPHORIZATION EFFECT OF
SW-CERAMSITE Article Open access 22 March 2025 INTRODUCTION As the most widely used spectrum antibiotics, tetracycline (TC) can effectively inhibit the activities of chlamydia, mycoplasma,
protozoan parasites, rickettsiae, gram-negative bacteria and gram-positive bacteria1. Therefore, it is widely and frequently applied in medical and health, poultry breeding and other
industries for disease treatment2. Nevertheless, animals usually fail to fully metabolize TC, and most of ingested TC are discharged into the environment, which would pose potent pollution
danger to environment consequently3,4,5,6. Long-term exposure to TC in the environment has caused serious concern about toxicity and bacterial resistance, which leads to ecological damage
and threatens human health through bioaccumulation in the food chain7. It has been proved that TC can interact with phospholipids and be adsorbed by biological cell membranes, which can also
lead to complications of human allergic reactions. Despite of short half-life of TC, the current sewage treatment system can not completely remove TC4, which has caused reports of TC
residues in surface water, groundwater and soil around the world, and even in many foods7. Therefore, it is of great significance to develop effective methods to remove TC in water
environment. To date, there are a lot of techniques such as filtration, ion exchange, adsorption, electrochemical treatment, membrane separation, catalytic degradation, etc. that have been
developed for the removal of antibiotics, toxic heavy metals and dye pollutants from wastewater8,9,10. Among them, adsorption is one of the most reliable methods due to its ease of
operation, low process cost, high removal efficiency and regeneration ability11. Many prepared adsorbents have been reported for the removal of pollutants in water, for example, the removal
of As (V)12 and Cr (VI)13,14,15 by prepared adsorbent with high removal rates. Therefore, this study intends to employ adsorption mechanism to remove TC. With the emergence of constructed
wetlands (CWs) called “earth artificial kidney”, people are more inclined to use this ecological method to further treat tail water of municipal wastewater treatment plant. In addition, CWs
were designed and built to take advantage of natural processes involving wetland vegetation, soil and associated microbial combinations16, which was recognized as a relatively natural,
low-cost, energy-efficient, and ecological technology for treating municipal or domestic wastewaters17. CWs are mainly purified by the synergy of microorganisms, plants and substrates. They
often showed better removal efficiency for nitrogen, phosphorus and organic compounds, etc. Wetland substrate is the skeleton of wetland ecosystem, which is generally composed of gravel,
sand, soil and artificial matrix, accounting for almost 80% of the total volume of wetland. The efficiency and effect of constructed wetland water treatment will be greatly improved by using
the filler matrix with good selectivity. Preparing ceramic products via high temperature calcination of raw materials is a promising method to improve the performance and safety of
materials and popularize applications. Ceramic production can bring a lot of benefits to the product, such as improving mechanical strength by curing/stabilization; stabilizing harmful
metals by adding embedded crystals; removing organic pollutants/pathogenic agents by pyrolysis and gasification; and improving adsorption capacity by generating microporous
structures18,19,20. Bentonite has a good adsorption capacity for pollutants, and can be used to remove petroleum toxins, purify gasoline and kerosene, and treat wastewater. The main active
mineral ingredient of bentonite is montmorillonite with high grade content of 85–90%. High content of SiO2 and Al2O3 makes bentonite meet the requirement for sintering ceramsite as the main
material21. However, low fusing composition (8.33%) can not guarantee the good performance of ceramics with bentonite as the only material, while more energy will be consumed and costs
increase. Therefore, it is necessary to mix other materials to improve the performance of sintered ceramics, reduce energy consumption and reduce production costs. Red mud, a polluting waste
residue discharged from alumina extraction in aluminium industry, contains a large amount of iron oxide. In addition, its main minerals are aragonite and calcite, with the content of
60–65%. Mixing red mud into bentonite for ceramsite preparing could bring two benefits: (1) high content of Al2O3 contributes to forming more mullite with high strength and further enhancing
the mechanical strength of ceramsite; (2) more melting aids can improve the firing properties of ceramsite. Further, in order to remove TC efficiently, ceramsite requires strong adsorption
capacity, which is closely related to its specific surface area and internal porosity. Pine sawdust (Ps), as we all know, can produce CO, CO2 and other gases by pyrolysis at high
temperature, which would improve the expansion and porosity of ceramsite. Therefore, Ps may act as a functional additive to improve the sintering process and properties of Ben/Rm ceramsite.
At present, some studies have reported the use of red mud with other raw materials such as fly ash, clay and other fired ceramics or ceramic bricks22,23. Especially, Zhang _et al_.
demonstrated ceramsite prepared from red mud, bagasse, powdered glass and molasses alcohol wastewater an effective and regenerable material used for TC adsorption treatment24. However,
bentonite/red mud/pine sawdust (Ben/Rm/Ps) ceramsite has not been reported yet. To address this knowledge gap, this study prepared Ben/Rm ceramsite and investigated the manufacture
parameters through orthogonal experimental designs. Ps, as a functional additive, was then added to the recipe and the optimal parameters were ascertained via one-factor experimental design.
In addition, the TC adsorption capacity of Ben/Rm/Ps ceramsite was explored, as well as adsorption mechanism. As such, the overall goal of this study was to develop a stable ceramsite with
a good adsorption capacity of TC, which can improve TC immobilization of CWs substrate and extend its lifetime. MATERIALS AND METHODS MATERIALS All chemicals applied in this study were of
analytical grade or higher. Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were obtained from Shanghai Bio-Chem Technology Co., Ltd (Shanghai, China). TC solution was prepared from TC
hydrochloride power, and TC hydrochloride with a molecular formula of C22H25ClN2O8, and molecular weight of 480.90 g/mol was purchased from Aladdin Bio-Chem Reagent Company (Shanghai,
China). Bentonite (Ben) was purchased from Jushi mining Company (Baoding, Hebei, China). Red mud (Rm) was collected from waste of Weiqiao Pioneer Group Co., Ltd. (Binzhou, Shandong, China).
Pine sawdust (Ps) was obtained from pine branches (Shanghai, China). Deionized (DI) water (18.25 MΩ∙cm) was come into being from a water purification system (EMD Millipore Corp., Merck KGaA,
Darmstadt, Germany). PREPARATION OF CERAMSITE Three raw materials (Ben, Rm and Ps) were dried at 110 °C for 3 h and passed through an 80 mesh sieve. Raw materials mixture with different
mass ratio was mixed with running water (30–40 wt%), stirred evenly and twisted into 6–8 mm pellets, then dried at 105 °C for 2 h. The ceramic blank pellets subsequently undergone preheating
(120–240 °C) for 15–30 min and roasting (1000–1150 °C) for 5–30 min successively with a KSL-1200 × -S box type high temperature sintering furnace (Hefei Kejing Materials Technology Co.,
Ltd., Anhui, China). After cooling, the finished ceramsite was obtained. MATERIALS CHARACTERIZATION Elemental analysis of Ben and Rm was determined by X-ray fluorescence spectrometry (XRF)
using a Spectro Midex system (Spectro Analytical Instrument Company, Germany). Elemental analysis of Ps was detected through a Vario EL III system (Elementar, Germany). The crystal phases of
the materials were analyzed using an X’Pert PRO MRD/XL system (XRD) (Panalytical, Almelo, the Netherlands). The surface morphologies of materials were imaged using a scanning electron
microscope (SEM) (JSM-840A electron microscope, JEOL, Tokyo, Japan). Thermogravimetric analysis (TG) was conducted on a TA instrument (Netzsch, Selb, Germany). The bulk density and apparent
density of ceramsite were determined by GB/T 17431.1-2010 standard method25. The compressive strength of ceramsite was measured according to the reported method26. The toxicity analysis of
heavy metal leaching was carried out using HJ/T 299–2007 method27, and GB 3838-2002 standard28 and GB 5085.3-2007 standard29 were employed as the evaluation criteria. STATIC ADSORPTION
EXPERIMENTS All the adsorption experiments were carried out in dark using brown glass vials (total volume = 150 mL) with 50 mL TC solution and ceramsite on an HZQ-120H heating oscillator
(Yiheng Scientific Instrument Co., Ltd., Shanghai, China) with a speed of 160 rpm. pH was adjusted using dilute HCl and NaOH aqueous solution (aq.). TC adsorption kinetics studies were
conducted at pH = 7 and temperature = 20 °C with an initial concentration of TC of 80 mg/L and ceramsite dosage of 20 g/L. At predetermined times (5–600 min), the vials were sacrificially
sampled. Besides, to investigate adsorption thermodynamics, the adsorption kinetics assays were carried out at 30 °C and 40 °C as well. For TC adsorption isotherm experiment, the initial
concentration of TC was varied from 2 to 80 mg/L with a fixed ceramsite dosage of 20 g/L, and the mixture (pH = 7) was shaken for 24 h at 20 °C to reach the adsorption equilibrium. To
explore effect of ceramsite dosage on adsorption, different doses of ceramsite (5–50 mg/L) were added into TC solution (80 mg/L), and the mixture (pH = 7) was shaken for 24 h at 20 °C. To
probe effect of pH on adsorption, the equilibrium tests were conducted with an initial TC concentration of 80 mg/L, a ceramsite dosage of 20 g/L, and finial solution pH 2–10 at 20 °C for 24
h. To examine effect of ionic strength, 0–0.25 mol/L NaCl or CaCl2 were added into TC solution (80 mg/L) with ceramsite dosage of 20 g/L at pH = 7, temperature of 20 °C and shaking for 24 h.
After adsorption is completed, the solution was filtered through a 0.22 μm microfiltration membrane. The concentrations of TC were detected via SP-756P Ultraviolet-Visible Spectrophotometer
at 355 nm. The adsorption amount at predetermined time _t_ (_q__t_, mg/g) and equilibrium adsorption amount (_q__e_, mg/g) of TC on materials and removal efficiency (R, %) were calculated
via: $${q}_{t}=\frac{({C}_{0}-{C}_{t})V}{m}$$ (1) $${q}_{e}=\frac{({C}_{0}-{C}_{e})V}{m}$$ (2) $$R=\frac{({C}_{0}-{C}_{{\rm{e}}})}{{C}_{0}}\times 100 \% $$ (3) where _C__t_ (mg/L) is the
residual concentration in the liquid phase at sampling time _t_ (min); _C_0 and _C__e_ (mg/L) are the initial and equilibrium concentrations of TC, respectively; _V_ (L) is the total volume
of the solution; and _m_ (g) is the mass of ceramsite. TC concentration in the solution phase (_C__d_, mg/L) was determined upon centrifugation and filtration, and the percent of TC desorbed
as calculated via: $$D=\frac{{C}_{d}}{({C}_{0}-{C}_{{\rm{e}}})}\times 100 \% $$ (4) DYNAMIC COLUMN EXPERIMENTS To better appraise the adsorption efficiency of Ben/Rm/Ps-op for TC, dynamic
column experiments were carried out. A glass column wrapped in aluminium foil was employed in the tests, with a height of 50 cm and an internal diameter of 5 cm. Ceramsites were loaded into
the column with a height of 20 cm, and the corresponding filter volume was 393 mL. The initial concentration of TC was set as 4 mg/L, and the solution was bumped into the column in an
up-flow mode. For the effect of HRT, column tests were carried out at different HRTs (5, 10 and 15 h), and the solution samples were collected daily for detecting TC concentration. For the
effect of packing height, the column experiments were conducted with different packing heights (10, 15 and 20 cm) at HRT of 0.5 h, and the solution samples were collected daily for detecting
TC concentration. CERAMSITE REGENERATION STUDIES Regeneration and recyclability are crucial parameters for industrial application of adsorbents. In this study, regeneration of ceramsite was
investigated in a batch test. The used adsorbent Ben/Rm/Ps-op were regenerated by washing with N, N-dimethylformamide (DMF) several times and freeze-drying for 24 h. The
adsorption-regeneration experiment was repeated 3 times in 80 mg/L TC solution at 20 °C and pH = 7. RESULTS AND DISCUSSION PREPARATION AND OPTIMIZATION OF BEN/RM CERAMSITE Elemental
compositions of Ben and Rm were determined by XRF (Table 1). High contents of SiO2 and Al2O3 were found in Ben (SiO2 = 60.8% and Al2O3 = 11.9%) and Rm (SiO2 = 15.2% and Al2O3 = 22.2%), which
are sufficient to meet the requirements of sintering ceramsite21. In addition to SiO2 and Al2O3, some fluxing components (e.g., MgO, CaO, Na2O, and K2O) and volatiles also present in Ben
and Rm. About 10.48% and 50.68% of fluxing components are respectively contained in Ben and Rm. Therefore, Ben and Rm can be used as the main materials for firing ceramsite. Moreover, the
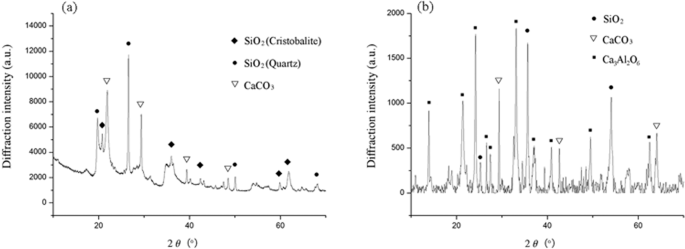
mineral constituent of Ben and Rm were appraised by XRD. For Ben, the peak strength of SiO2 crystal phase is most intense, and crystal peaks of CaO3 can also be observed (Fig. 1a). Since
other major components of Ben such as Al2O3 and MgO did not form crystal morphology, corresponding peaks were not detected. For Rm, large amount of crystal SiO2 and a certain amount of CaCO3
and Ca3Al2O6 were detected (Fig. 1b). According to elemental compositions in Table 1, we calculated the content of SiO2, Al2O3, fluxing components and SiO2 + Al2O3 in the mixture of Ben and
Rm with different ratios (1:1, 2:1, 3:1, 4:1, 5:1, 6:1 and 7:1) (Table 2). With the increase of proportion (Ben: Rm), the content of SiO2 and SiO2 + Al2O3 would increase, while the content
of Al2O3 and fluxing components would decrease. Taking into account Riley three-phase diagram30 and the actual situation of adding as much Rm as possible to the raw materials, four ratios
(3:1, 4:1, 5:1 and 6:1) were adopted to prepare Ben/Rm ceramsite. To optimize the preparation of CFA/WS ceramsite and identify the critical factors of determining the ceramsite properties,
an orthogonal experimental design of five factors (mass ratio of Ben: Rm, preheating temperature and time, calcining temperature and time) and four levels (L16(4)5) were conducted and bulk
density of ceramsite was employed as the evaluation index. Relatively lower bulk density is preferable, which demonstrates higher porous ceramsite bodies31. The significance levels of
different influencing factors on the ceramsite bulk density were clarified through the range analysis32. Table 3 summed up the results of L16(4)5 orthogonal design. The _K_ value for each
level of a parameter was the average of four bulk density values, and the range value (_R_) for each factor was the difference between the maximal and minimal _K_ values of the four levels.
The range analysis suggested Ben: Rm ratio was the most important factor and followed by preheating time and calcining temperature, while others factors were not of significance. As depicted
in Table 3, with the increase of Ben: Rm ratio, the bulk density of ceramic displayed a decline trend, which may be related to the change of sintering and volatile compositions. In
addition, when the preheating time was within 15–25 min, ceramsite bulk density increased obviously with the increase of time. However, increasing the preheating time from 25 to 30 min, the
bulk density showed a slightly decrease. Under certain conditions, increasing preheating time facilitated softening ceramsite, which ensured ceramsite produced enough gas in the roasting
stage, thus reduced the bulk density. However, when preheating time is too long, organic matter and carbonate will decompose and volatilize to produce gas in the preheating stage, which will
reduce the gas amount produced in roasting stage, then the bulk density increased. PREPARATION AND OPTIMIZATION OF BEN/RM/PS CERAMSITE Ps was characterized by ultimate analysis (Table 4)
and TG (Fig. 2). The TG curve shows that the Ps mass nearly did not change below 260 °C, while a drastic decline occurred within 260–468 °C, and above 468 °C little Ps was residual (Fig. 2).
Therefore, decomposition of Ps is expected to occur in the initial stage of ceramic calcination stage, which can improve the sintering performance and promote the formation of ceramic
porous structure. The aforementioned L16(4)5 orthogonal experimental design indicated the mass ratio of raw materials and preheating time were the most significant influence factors on
ceramic sintering. Therefore, for the preparation of Ben/Rm/Ps ceramsite, the mass ratio of raw materials and preheating time were optimized by using one-factor experimental design, while
the other fixed parameters were adopted the optimal ones based on L16(4)5 results, i.e., preheating temperature = 240 °C, calcining temperature = 1150 °C, calcining time = 14 min. For the
optimization of mass ratio of raw materials, the preheating time was fixed at 15 min, and four levels of Ben: Rm were employed, i.e., 3 g:1 g, 4 g:1 g, 5 g:1 g and 6 g:1 g. Different amounts
of Ps (0.1–1 g) were added to the raw materials to sinter ceramsite. Apparent density, bulk density and compressive strength of ceramsite were determined to appraise the influence of raw
materials ratio (Fig. 3a–c). Besides, a three-phase diagram based on the three parameters was presented in Fig. 3d, during which 19, 36 and 8 represent mass ratios of Ben: Rm: Ps = 4:1:0.9,
6:1:0.6 and 3:1:0.8, respectively. Combining the three-phase diagram (Fig. 3d) with apparent density, bulk density and compressive strength curves (Fig. 3a–c), 8, 19 and 36 displayed
outstanding performance among all ratios. Besides, 19 and 36 have little difference in compressive strength and apparent density, while showed much more excellent than 8. In addition,
comparing with 36, 19 exhibited smaller bulk density and possessed more red mud, which is conducive to waste utilization. Therefore, the Ben: Rm: Ps ratio of 4:1:0.9 was chosen for the
follow-up experiment. For the optimization of preheating time, the optimal ratio of Ben: Rm: Ps (4:1:0.9) was employed and the preheating time was varied from 10 min to 30 min. TC adsorption
capacity of obtained ceramsite was assessed by static adsorption experiments. The TC adsorption capacity of ceramsite showed a trend of firstly increasing and then decreasing with the
increase of preheating time (Fig. 4). From 10 min to 20 min, the TC adsorption capacity of ceramsite showed a steady increase, and reached the maximum value at 20 min. With the increase of
preheating time, softening degree of ceramsite raises, which is conducive to ensuring enough gas producing in the roasting stage, thus reducing bulk density. Continuously increase the
preheating time from 20 min to 30 min, the TC adsorption capacity of ceramsite displayed a quick decline. This can be explained that the organic matter and carbonate will decompose and
volatilize to produce gas in the preheating stage for too long preheating time, which will reduce the amount of gas produced in the roasting stage and increase the stacking density. To sum
up, the optimal parameters for preparation of Ben/Rm/Ps ceramsite were determined as: Ben: Rm: Ps = 4:1:0.9, preheating temperature = 240 °C, preheating time = 20 min, calcining temperature
= 1150 °C, and calcining time = 14 min. The Ben/Rm/Ps ceramsite prepared at this optimal condition (hereafter referred to as Ben/Rm/Ps-op) had an apparent density of 1.41 g/cm3, bulk density
of 0.54 g/cm3 and compressive strength of 19.45 MPa. SEM analysis (Fig. 5) showed that both the surface and cross-section of Ben/Rm/Ps-op was mainly distributed by macroporous and
microporous structures with different sizes, indicating a large specific surface area and widely distributed adsorption sites. The SEM results demonstrated that ceramsite was a porous
ceramsite and might be a good adsorbent for wastewater treatment. XRD detected the formation of several crystal phases in Ben/Rm/Ps-op including SiO2, Ca3Si2O7, Ca2Si2O5(OH)2 and Ca2SiO4
(Fig. 6). The crystal compositions are helpful to improve the strength of ceramsite; the active components such as SiO2, Si2O76−, Si2O5(OH)24− and SiO44− can be adsorption sites of TC via
ion exchange. Since TC contain a positively charged group in the structure, regardless of the zero net charge or negative net charge, it is likely that the molecule arranges at the surface
in such a way that the positively charged group is located very close to the surface. SiO2 is negatively charged at the solution of pH > 2.5, which could easily combine with TC. In
addition, negatively charged groups Si2O76−, Si2O5(OH)24− and SiO44− also showed excellent binding ability with TC. Moreover, heavy metal leaching toxicity analysis (Table 5) revealed the
concentrations of leached heavy metals from Ben/Rm/Ps-op were far below hazardous wastes standard (GB 5085.3-2007)29, and basically met the surface water quality of Class III (GB
3838-2002)28, recommending Ben/Rm/Ps-op will not cause secondary pollution to the aquatic environment. Therefore, Ben/Rm/Ps-op is a safe ceramsite with great mechanical strength and
adsorption capacity. ADSORPTION OF TC ADSORPTION KINETICS It is observed in Fig. 7 that Ben/Rm/Ps-op could catch most of TC from aqueous solution in the initial 180 min and reach equilibrium
after 360 min. After calculation, 64% TC was removed at equilibrium and the equilibrium adsorption capacity was as high as 2.5602 mg/g. Pseudo-first-order and pseudo-second-order models
were tested to analyze the kinetics results, which are expressed as33,34: Pseudo-first-order model: $$\mathrm{ln}\,{q}_{e}-{q}_{t}=ln{q}_{e}-{k}_{1}t$$ (5) Pseudo-second-order model:
$$\frac{t}{{q}_{t}}=\frac{1}{{k}_{2}{{q}_{e}}^{2}}+\frac{t}{{q}_{e}}$$ (6) where _q__t_ and _q__e_ (mg/g) are the adsorption capacities of TC at time _t_ (min) and equilibrium, respectively;
_k_1(min−1) is the rate constant for pseudo-first-order model and _k_2(g/(mg∙min)) is for pseudo-second-order model, respectively. As a result, the pseudo-second-order model showed higher
R2 = 0.9996, compared with R2 = 0.9789 for the pseudo-first-order model (Table 6, Fig. 8). Besides, _q__e,cal_ (2.6889 mg/g) of pseudo-second-order model was closer to _q__e,exp_ value
(2.5602 mg/g) than _q__e,cal_ (1.7591 mg/g) of pseudo-first-order model did. Consequently, the adsorption of TC by Ben/Rm/Ps-op conforms to pseudo-second-order model, indicating that the
rate controlling step for adsorption was a chemical interaction35. ADSORPTION MECHANISM The pseudo-second-order model, including all processes of adsorption (external liquid film diffusion,
surface adsorption, intraparticle diffusion and so on), could not accurately reflect the mechanism of this adsorption process36. For further exploring adsorption mechanism of TC on
Ben/Rm/Ps-op, intraparticle diffusion model was employed to determine the type of rate-controlling step. This model can be delivered as follows: $${q}_{t}={k}_{int}{t}^{0.5}+Const$$ (7)
where _k__int_ (mg/(g∙min0.5)) is the constants for the intraparticle diffusion model, and _Const_ (mg/g) is a constant proportional to the extent of boundary layer thickness. Figure 9
expressed the linear plots involving two adsorption stages with different slopes. _k_int_1_ refers to the external adsorption rate constant in first step, and _k_int_2_ indicates the
internal adsorption rate constant of the second stage by diffusion between particles into the adsorbent. The value of _k_int_1_ was higher than that of _k_int_2_ owing to a rapid increase in
adsorption during the initial phase, with increased active sites available. This result is related to changes in mass transfer rate during adsorption process. The linear portion did not
pass through the origin, suggesting that the adsorption mechanism of the TC onto Ben/Rm/Ps-op is not only restrained by the intraparticle diffusion step37. ADSORPTION ISOTHERM Adsorption
isotherm result at 20 °C was presented in Fig. 10. With the increase of the initial concentration of TC, adsorption capacity of Ben/Rm/Ps-op for TC increased overtly, while TC removal rate
significantly descended. The isotherm results were further analyzed using linear, Langmuir, Freundlich, Tempkin and D-R (Dubinin-Radushkevich) isotherm models, as expressed below38,39: The
linear isotherm model indicates that amount of adsorption is linearly proportional to the equilibrium solution concentration, which can be depicted as: $${q}_{e}={K}_{d}{C}_{e}$$ (8) The
Langmuir isotherm model assumes that the adsorption sites on the surface of the monolayer are uniform and equivalent, with no interaction between adsorbate molecules at adjacent locations,
which is expressed as40: $$\frac{{C}_{e}}{{q}_{e}}=\frac{{C}_{{\rm{e}}}}{{q}_{m}}+\frac{1}{{q}_{m}{K}_{L}}$$ (9) where _q__m_ (mg/g) is the maximum adsorption capacity, and _K__L_ (L/mg) is
the Langmuir constant associatd with the affinity of binding site and is also a measure of the free energy of adsorption. The Freundlich isotherm model is used to depict the adsorption on an
energy heterogeneous surface, and could be expressed as41: $$ln{q}_{e}=ln{K}_{F}+\frac{1}{n}ln{C}_{e}$$ (10) where _n_ is the heterogeneity factor indicating the adsorption strength of the
adsorbent, and _K__F_ (mg/g∙(L/mg)1/n) is the constant in connection with the adsorption capacity. Tempkin and Pyzhev assumed that some indirect adsorbate/adsorbate interactions had effect
on adsorption isotherms and suggested that the adsorption heat of all the molecules in the layer would decrease linearly with coverage due to these interactions. The Tempkin isotherm has
been used as below: $${q}_{e}=\frac{RT}{b}lnA{C}_{e}$$ $${q}_{e}=\frac{RT}{b}lnA+\frac{RT}{b}ln{C}_{e}$$ $$B=\frac{RT}{b}$$ A plot of _q__e_ versus ln_C__e_ could determine the constants _A_
and _B_. The constant B is related to the adsorption heat42. The D-R empirical equation put forward by Dubinin and Radushkevich, has been widely employed to depict the gases and vapours
adsorption on microporous solids. In the case of liquid phase adsorption, several researches have indicated that the adsorption energy can be estimated via D-R equation. Assuming only
monolayer adsorption occurs in micropores adsorption and the D-R equation is applicable, the adsorption capacity per unit surface area of the adsorbent at equilibrium, _q__e_, can be
described as43: $${q}_{e}={q}_{0}\exp (\,-\,B{\varepsilon }^{2})$$ $$\varepsilon =RT\,\mathrm{ln}(1+\frac{1}{{C}_{e}})$$ where _B_ is the constant related to the adsorption energy, _q_0 is
the ultimate capacity per unit area of adsorbent micropores, and _ε_ is the Polenyi potential. The most probable adsorption energy, _E_, has been shown as: $$E={(2B)}^{-\frac{1}{2}}$$ As
Fig. 11 depicted, the linear, Langmuir, Freundlich, Tempkin and D-R isotherm models were used to fit TC adsorption data onto Ben/Rm/Ps-op. The parameter values were based on the regression
of the isotherm equations and were summarized in Table 7. The results showed that Langmuir isotherm model was not suitable for the adsorption, demonstrating that the adsorption of TC by
Ben/Rm/Ps-op was not monolayer adsorption, neither Tempkin nor D-R isotherm models. Whereas, linear isotherm model was suitable for the determination of data due to the higher correlation
coefficient _R_2 (0.98689) than _R_2 (0.95035) of Freundlich. For linear model, the coverage of monolayer and the initial amount of multilayer adsorption appear to be superimposed. Since
there is no platform in linear model, it indicates that the adsorption does not reach saturation, and the multilayer adsorption thickness seems to increase indefinitely. ADSORPTION
THERMODYNAMICS Thermodynamic properties of TC onto Ben/Rm/Ps-op were further investigated, which could be described via Gibb’s free energy (_ΔG_°), enthalpy (_ΔH_°) and entropy (_ΔS_°). The
thermodynamic were estimated using the following relations38,44: $${K}_{C}=\frac{{C}_{Ae}}{{C}_{e}}$$ (11) $$\varDelta {G}^{^\circ }=-\,{\rm{RT}}ln{K}_{c}$$ (12)
$$ln{K}_{{\rm{c}}}=-\,\frac{\varDelta {H}^{^\circ }}{RT}+\frac{\varDelta {S}^{^\circ }}{R}$$ (13) where _R_ (8.314 J/K mol) is the gas constant; _T_ (K) is temperature; _K__c_ is the
equilibrium constant; _C__e_ is the equilibrium concentration of TC in the solution (mg/L); and _C__Ae_ is the amount of adsorbed TC on the adsorbent at equilibrium (mg/L). _C__Ae_ and
_C__e_ are obtained from _q__e_ values of the pseudo-second-order model (Fig. 12). Δ_S_° and Δ_H_° were acquired from the slope and intercept of linear plot of ln_K_c versus 1/T according to
Eq. (13) (Fig. 13). Figure 14 described adsorption capacity of ceramiste at different temperatures, and demonstrated that increasing temperature would promote adsorption capacity of TC by
ceramsite. Table 8 presented the thermodynamic parameters at different temperatures. The negative values of Δ_G_° (−1.751, −2.322 and −2.806 kJ/mol for 293.15, 303.15 and 313.15 K
respectively) suggested that the adsorption of TC onto Ben/Rm/Ps-op was spontaneous and thermodynamically favorable. Besides, Δ_G_° value decreased with an increase in temperature,
indicating that the spontaneous nature of the adsorption was inversely proportional to the temperature and higher temperature enhanced the adsorption45. Moreover, the positive value of Δ_H_°
suggested that the adsorption of TC onto Ben/Rm/Ps-op was an endothermic process. The positive value of Δ_S_0 revealed increased randomness at the solid/solution interface during the
adsorption of TC onto Ben/Rm/Ps-op46. EFFECTS OF PH, BEN/RM/PS-OP DOSAGE AND IONIC STRENGTH For the effect of pH on adsorption, the adsorption capacity and removal rate of TC increase within
pH 2–7, and decrease during pH 7–10 (Fig. 15a). Under strong acid condition (pH 2–4), TCH3+ is the main form of TC, which could be combined with silicon dioxide with negative charge in
ceramsite. While hydrogen ions in the solution are more competitive than TC in binding to the ceramisite voids at the same time. So the amount of TC adsorbed by ceramisite (from 1.86 to 1.94
mg/g) and removal rate (from 46.5% to 48.5%) did not increase significantly in the range of pH 2–4. With increasing pH from 4 to 7, the adsorption capacity of TC sharply increased by 0.62
mg/g and the removal rate climbed to 64% at pH 7. This because H+ decreased in an order of magnitude, and the adsorption of TC by ceramsite increased obviously. However, from pH 7 to pH 10,
the removal rate of TC by a large margin reduced to 53% with an adsorption capacity of 2.12 mg/g. During this period, the morphology of TC gradually changed from TCH2° to TCH−, and the
negative charge ratio on the surface of ceramsite increased, which were unfavorable to the adsorption of TC by ceramsite. This result indicates the adsorption capacity of Ben/Rm/Ps-op will
be largely impacted by pH, and the adsorption performance was excellent at the neutral condition. Besides, the adsorption of TC was conducted in the presence of different dosage of
Ben/Rm/Ps-op. As Fig. 15b described, the removal rate of TC significantly increased from approximately 21.38% to 78.75% with increasing Ben/Rm/Ps-op dosage from 5 to 50 g/L. However, the TC
adsorption capacity gradually decreased from approximately 3.42 to 1.26 mg/g. Given the efficiency and economy of such operation, the optimum Ben/Rm/Ps-op dosage is 20 g/L, under which both
the adsorption efficiency and capacity were kept high. In addition, adsorption experiments on the effect of ionic strength were conducted using 80 mg/L TC solution containing 0–0.25 mol/L
NaCl or CaCl2 at pH = 7 and the temperature of 20 °C. Figure 15c describes the adsorption behavior of TC versus ironic strength. The existence of NaCl (or CaCl2) decreases the adsorption
capacity of TC onto _Ben/Rm/Ps-op_, which may be due to the competitive effect between Na+ (or Ca2+) and TC on the adsorption sites. Parolo _et al_. observed that it can be explained that
metal cations in solution could easily chelate with TC47, and electrolyte can produce electrostatic shielding effect, thus affect adsorption48,49. In addition, increasing Na+ (or Ca2+)
concentration can bring in the contraction of adsorbent pores, leading to that some adsorbate could not enter into pores, and the reduction of surface adsorption sites of
_Ben/Rm/Ps-op_50,51,52. Further, it is clear that NaCl, a univalent electrolyte, had less negative impact on TC adsorption than a divalent CaCl2 under identical conditions. Thus, it can be
concluded that coexisting ions had adverse effect for TC adsorption onto _Ben/Rm/Ps-op_. DYNAMIC ADSORPTION OF TC The effect of hydraulic retention time (HRT) and packing height on TC
removal were investigated, and the result was shown in Fig. 16. It can be seen from the figure that HRT had a great influence on the removal of TC by Ben/Rm/Ps-op. When HRT = 5, 10 and 15 h,
the average removal rates of TC by ceramsite got to 69.0%, 77.7% and 81.1% respectively. With the increase of HRT, the removal rate of TC by ceramsite increases. The reason is that the
increase of retention time of solution through the packed column will lead to more sufficient contact reaction between ceramsite and TC, which makes the total amount of TC adsorbed by
ceramsite increase. In addition, with the increase of HRT, the amount of TC adsorbed by ceramsite increases, but the degree of increase decreases (77.7–69.0% > 81.1–77.7%). This could be
interpreted as that with the prolongation of adsorption time, the adsorption sites decrease and the adsorption difficulty increase. Since the HRT of a CWs system is usually longer than 3
days53 and the removal efficiency have already reached 81.1% at HRT = 15 h, the Ben/Rm/Ps-op has the high potential to effectively remove TC as the CWs substrate under a dynamic flow
condition. Figure 16b presents TC concentration of Ben/Rm/Ps-op packed column at different packing heights. The TC concentration at different packing heights showed a similar change trend
versus the operation time, i.e., rapidly increasing in the initial days, then reaching a relatively stable level, and gradually increasing in the late stage. However, the initial rapidly
increasing takes different time. TC concentration quickly increase in the initial 5, 7 and 8 days, respectively for height = 10, 15, 20 cm. This may be attributed to that with the increase
of the quality of adsorbent, the adsorption sites on the surface of adsorbent increase, which lead to prolonging the adsorption time of TC. We could also see in Fig. 16b, the lower section
played the key role in the adsorption of TC, and it also arrive at saturation first during the continuously operation. REGENERATION OF BEN/RM/PS-OP Seen in Fig. 17, good adsorption
capability of the regenerated adsorbent was still retained after three rounds of sorption-desorption cycles. In addition, TC removal rates for repeated three times were 61.7%, 58.4% and
53.2% respectively. The decrease (2.3–5.2% in every cycle) of removal rate might have been owing to the loss of irreversible occupation of partial-adsorption sites54. Nevertheless, the
adsorption amount of ceramsites still remained at a high value (2.13 mg/g, _C_0 = 80 mg/L) after three consecutive cycles, suggesting the high reusability capability and stability of
Ben/Rm/Ps-op for TC removal. CONCLUSIONS In this study, a kind of CWs Ben-Rm-Ps ceramsite was prepared to remove TC in effluent. Ben/Rm/Ps-op ceramsite was prepared with the condition Ben:
Rm: Ps = 4:1:0.9, preheating temperature = 240 °C, preheating time = 20 min, calcining temperature = 1150 °C, and calcining time = 14 min, which possessed microporous structure and low heavy
metal leaching toxicity. The second-order kinetics and linear isothermal model can well simulate the adsorption of TC by Ben/Rm/Ps-op, and the maximum adsorption capacity can reach for
2.5602 mg/g. In addition, TC adsorption onto Ben/Rm/Ps-op was demonstrated a spontaneous endothermic process and higher temperature enhanced the adsorption. Further, Ben/Rm/Ps-op has been
also proved the high potential to effectively remove TC as the CWs substrate under a dynamic flow condition and high reusability capability and stability for TC removal under batch tests.
This study combines basic theory and engineering application research, and has important value for the research and development of CWs matrix filler. The research results of this subject
will provide an important reference for the research and application of artificial calcined ceramsite as a light aggregate in water pollution control. In addition, the preparation and
application of new ceramsite matrix can not only enhance the pollutant removal function of CWs, but also utilize solid wastes such as red mud and biomass. Therefore, the research and
development of ceramsite products also has comprehensive economic, social and environmental benefits. REFERENCES * Chopra, I. & Roberts, M. Tetracycline Antibiotics: Mode of Action,
Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. _Microbiology and Molecular Biology Reviews_ 65, 232–260, https://doi.org/10.1128/mmbr.65.2.232-260.2001 (2001).
Article CAS PubMed PubMed Central Google Scholar * Ahmed, M. B. _et al_. Competitive sorption affinity of sulfonamides and chloramphenicol antibiotics toward functionalized biochar for
water and wastewater treatment. _Bioresource Technology_ 238, 306–312 (2017). Article CAS Google Scholar * Zhu, Y. G. _et al_. Diverse and abundant antibiotic resistance genes in Chinese
swine farms. _Pnas_ 110, 3435–3440 (2013). Article ADS CAS Google Scholar * Chen, Y., Zhang, H., Luo, Y. & Song, J. Occurrence and dissipation of veterinary antibiotics in two
typical swine wastewater treatment systems in east China. _Environmental Monitoring & Assessment_ 184, 2205–2217 (2012). Article CAS Google Scholar * Zhou, L. J. _et al_. Excretion
masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. _Science of the Total Environment_ 444, 183–195 (2013). Article ADS CAS Google Scholar
* Hamscher, G. _et al_. Different behavior of tetracyclines and sulfonamides in sandy soils after repeated fertilization with liquid manure. _Environmental Toxicology & Chemistry_ 24,
861–868 (2010). Article Google Scholar * Hu, X., Zhou, Q. & Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from
organic vegetable bases, northern China. _Environmental Pollution_ 158, 2992–2998 (2010). Article CAS Google Scholar * Li, W. _et al_. Superb fluoride and arsenic removal performance of
highly ordered mesoporous aluminas. 198, 143–150 (2011). * Shi, Y. J. _et al_. The fabrication of TiO2 supported on slag-made calcium silicate as low-cost photocatalyst with high adsorption
ability for the degradation of dye pollutants in water. 281, 21–28 (2017). * Li, N. _et al_. Electro-adsorption of tetracycline from aqueous solution by carbonized pomelo peel and composite
with aniline. _Applied Surface Science_ 386, 460–466 (2016). Article ADS CAS Google Scholar * Zheng, J. Y. _et al_. Cubic nickel frames: one-pot synthesis, magnetic properties and
application in water treatment. _Crystengcomm_ 14, 7616–7620 (2012). Article CAS Google Scholar * Sahu, U. K. _et al_. Synthesis and characterization of magnetic bio-adsorbent developed
from Aegle marmelos leaves for removal of As(V) from aqueous solutions. _Environmental Science and Pollution Research_ 26, 946–958 (2019). Article CAS Google Scholar * Sahu, S., Sahu, U.
K. & Patel, R. K. Synthesis of thorium–ethanolamine nanocomposite by the co-precipitation method and its application for Cr(VI) removal. _New Journal of Chemistry_ 42, 5556–5569 (2018).
Article CAS Google Scholar * Sahu, S., Sahu, U. K. & Patel, R. K. Modified Thorium Oxide Polyaniline Core–Shell Nanocomposite and Its Application for the Efficient Removal of Cr(VI).
_Journal of Chemical & Engineering Data_ 64, 1294–1304 (2019). Article CAS Google Scholar * Sahu, S. _et al_. Synthesis of Polypyrrole-Modified Layered Double Hydroxides for Efficient
Removal of Cr(VI). _Journal of Chemical & Engineering Data_ 64, 4357–4368, https://doi.org/10.1021/acs.jced.9b00444 (2019). Article CAS Google Scholar * Gao, Y. _et al_. Intensified
nitrate and phosphorus removal in an electrolysis -integrated horizontal subsurface-flow constructed wetland. _Water Research_ 108, 39–45, https://doi.org/10.1016/j.watres.2016.10.033
(2017). Article CAS PubMed Google Scholar * Luo, P. _et al_. Phosphorus removal from lagoon-pretreated swine wastewater by pilot-scale surface flow constructed wetlands planted with
Myriophyllum aquaticum. _Science of the Total Environment_ 576, 490–497, https://doi.org/10.1016/j.scitotenv.2016.10.094 (2017). Article ADS CAS PubMed Google Scholar * Xu, G., Zou, J.
& Li, G. Ceramsite made with water and wastewater sludge and its characteristics affected by SiO2 and Al2O3. _Environmental Science & Technology_ 42, 7417–7423 (2008). Article ADS
CAS Google Scholar * Tang, Y., Chan, S.-W. & Shih, K. Copper stabilization in beneficial use of waterworks sludge and copper-laden electroplating sludge for ceramic materials. _Waste
Management_ 34, 1085–1091, https://doi.org/10.1016/j.wasman.2013.07.001 (2014). Article CAS PubMed Google Scholar * Yu, Y., Wu, R. & Clark, M. Phosphate removal by hydrothermally
modified fumed silica and pulverized oyster shell. _Journal of Colloid and Interface Science_ 350, 538–543, https://doi.org/10.1016/j.jcis.2010.06.033 (2010). Article ADS CAS PubMed
Google Scholar * Reed & JamesStalford. _Introduction to the Principles of Ceramic Processing_. (John Wiley & Sons, 1988). * Chen, X., Lu, A. & Qu, G. Preparation and
characterization of foam ceramics from red mud and fly ash using sodium silicate as foaming agent. _Ceramics International_ 39, 1923–1929 (2013). Article CAS Google Scholar * He, H. _et
al_. The effect of incorporation of red mud on the properties of clay ceramic bodies. _Applied Clay Science_ 70, 67–73 (2012). Article CAS Google Scholar * Zhang, L. Y. _et al_. Study on
adsorption of tetracycline by red mud-based ceramsite. _Journal of Water Supply Research Technology-Aqua_ 68, 39–50, https://doi.org/10.2166/aqua.2018.100 (2019). Article Google Scholar *
General Administration of Quality Supervision. (China Standard Press, Beijing, China (GB/T 17431.1–2010), 2010). * Li, Y. _et al_. Measurement and statistics of single pellet mechanical
strength of differently shaped catalysts. _Powder Technology_ 113, 176–184, https://doi.org/10.1016/S0032-5910(00)00231-X (2000). Article CAS Google Scholar * Environmental Protection
Agency of China. (China Standard Press, Beijing, China (HJ/T299-2007), 2007). * Environmental Protection Agency of China. (China Standard Press, Beijing, China (GB 3838-2002), 2002). *
Environmental Protection Agency of China. (China Standard Press, Beijing, China (GB 5085.3-2007), 2007). * Riley, C. M. Relation of Chemical Properties to the Bloating of Clays. _Journal of
the American Ceramic Society_ 34, 121–128 (2006). Article Google Scholar * Xu, G. R., Zou, J. L. & Li, G. B. Ceramsite obtained from water and wastewater sludge and its characteristics
affected by (Fe2O3 + CaO + MgO)/(SiO2 + Al2O3). _Water Research_ 43, 2885–2893, https://doi.org/10.1016/j.watres.2009.03.046 (2009). Article CAS PubMed Google Scholar * Cui, W., Li, X.,
Zhou, S. & Weng, J. Investigation on process parameters of electrospinning system through orthogonal experimental design. _Journal of Applied Polymer Science_ 103, 3105–3112,
https://doi.org/10.1002/app.25464 (2007). Article CAS Google Scholar * Ho, Y. S. & McKay, G. Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. _Journal
of Environmental Science and Health part A_ 34, 1179–1204, https://doi.org/10.1080/10934529909376889 (1999). Article Google Scholar * Fu, J. _et al_. Treating the dyeing wastewater of
Reactive Brilliant Red K-2BP by cetyltrimethylammonium chloride-modified bentonite with polyacrylamide flocculant. _Polish Journal of Environmental Studies_ 20, 61–66 (2011). CAS Google
Scholar * Liu, W. _et al_. Selective and irreversible adsorption of mercury(ii) from aqueous solution by a flower-like titanate nanomaterial. _Journal of Materials Chemistry A_ 3,
17676–17684, https://doi.org/10.1039/c5ta04521e (2015). Article CAS Google Scholar * Khaled, A. _et al_. Removal of Direct N Blue-106 from artificial textile dye effluent using activated
carbon from orange peel: adsorption isotherm and kinetic studies. _Journal of Hazardous Materials_ 165, 100–110 (2009). Article CAS Google Scholar * Sun, Y. _et al_. Characterization and
ciprofloxacin adsorption properties of activated carbons prepared from biomass wastes by H3PO4 activation. _Bioresource Technology_ 217, 239–244 (2016). Article CAS Google Scholar * Fu,
J. _et al_. Adsorption of disperse blue 2BLN by microwave activated red mud. _Environmental Progress & Sustainable energy_ 30, 558–566, https://doi.org/10.1002/ep.10506 (2011). Article
CAS Google Scholar * Langmuir, I. The constitution and fundamental properties of solids and liquids. Part II.—Liquids. _Journal of the American Chemical Society_ 38, 102–105 (1916). Google
Scholar * Trellu, C. _et al_. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: A critical review. _Journal of Hazardous Materials_ 306, 149–174 (2016).
Article CAS Google Scholar * Yang, K. _et al_. Adsorption of Polycyclic Aromatic Hydrocarbons by Carbon Nanomaterials. _Environmental Science & Technology_ 40, 1855–1861 (2006).
Article ADS CAS Google Scholar * Allen, S. J., Mckay, G. & Porter, J. F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. _Journal
of Colloid and Interface Science_ 280, 322–333 (2004). Article ADS CAS Google Scholar * Hsieh, C. T. & Teng, H. Liquid-Phase Adsorption of Phenol onto Activated Carbons Prepared with
Different Activation Levels. _Journal of Colloid and Interface Science_ 230, 171–175 (2000). Article ADS CAS Google Scholar * Akçay, M. Characterization and adsorption properties of
tetrabutylammonium montmorillonite (TBAM) clay: Thermodynamic and kinetic calculations. _Journal of Colloid and Interface Science_ 296, 16–21, https://doi.org/10.1016/j.jcis.2005.08.031
(2006). Article ADS CAS PubMed Google Scholar * Huang, W. _et al_. Phosphate removal from wastewater using red mud. _Journal of Hazardous Materials_ 158, 35–42,
https://doi.org/10.1016/j.jhazmat.2008.01.061 (2008). Article CAS PubMed Google Scholar * Han, R. _et al_. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue
adsorption onto natural zeolite. _Chemical Engineering Journal_ 145, 496–504, https://doi.org/10.1016/j.cej.2008.05.003 (2009). Article CAS Google Scholar * Parolo, M. E. _et al_.
Adsorption and circular dichroism of tetracycline on sodium and calcium-montmorillonites. _Colloids and Surfaces A: Physicochemical and Engineering Aspects_ 417, 57–64,
https://doi.org/10.1016/j.colsurfa.2012.10.060 (2013). Article CAS Google Scholar * Gao, Y. _et al_. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene
oxide. _Journal of Colloid & Interface Science_ 368, 540–546 (2012). Article ADS CAS Google Scholar * Vilar, V. J. P., Botelho, C. M. S. & Boaventura, R. A. R. Influence of pH,
ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. _Process Biochemistry_ 40, 3267–3275 (2005). Article CAS Google Scholar * Di, X., Zhou, X.
& Wang, X. Adsorption and desorption of Ni2+ on Na-montmorillonite: Effect of pH, ionic strength, fulvic acid, humic acid and addition sequences. _Applied Clay Science_ 39, 133–141
(2008). Article Google Scholar * Cheng, W. _et al_. Removal of malachite green (MG) from aqueous solutions by native and heat-treated anaerobic granular sludge. _Biochemical Engineering
Journal_ 39, 538–546 (2008). Article CAS Google Scholar * Feng, Y. _et al_. Adsorption behavior of EE2 (17α-ethinylestradiol) onto the inactivated sewage sludge: Kinetics, thermodynamics
and influence factors. _Journal of Hazardous Materials_ 175, 970–976 (2010). Article CAS Google Scholar * Ghosh, D. & Gopal, B. Effect of hydraulic retention time on the treatment of
secondary effluent in a subsurface flow constructed wetland. _Ecological Engineering_ 36, 1044–1051 (2010). Article Google Scholar * Guo, Y. _et al_. Removal of tetracycline from aqueous
solution by MCM-41-zeolite A loaded nano zero valent iron: Synthesis, characteristic, adsorption performance and mechanism. _Journal of Hazardous Materials_ 339, 22–32 (2017). Article CAS
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by China Postdoctoral Science Foundation (2018M641927), and National Natural Science Foundation of China
(41701541). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Environmental Science & Engineering, Fudan University, Shanghai, 200433, China Yanting Wang, Shengying Gong,
Yazhuo Li & Jie Fu * School of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China Zhang Li Authors * Yanting Wang View author
publications You can also search for this author inPubMed Google Scholar * Shengying Gong View author publications You can also search for this author inPubMed Google Scholar * Yazhuo Li
View author publications You can also search for this author inPubMed Google Scholar * Zhang Li View author publications You can also search for this author inPubMed Google Scholar * Jie Fu
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.F. designed the research; Y.W. planned the experiment methods; Y.W. and S.G. carried out
the experiments and sampling; Y.W., Y.L., Z.L. and S.G. analyzed data; Y.W. and J.F. wrote the paper. CORRESPONDING AUTHOR Correspondence to Jie Fu. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Wang, Y., Gong, S., Li, Y. _et al._ Adsorptive removal of tetracycline by sustainable ceramsite substrate from bentonite/red mud/pine sawdust. _Sci Rep_ 10, 2960 (2020).
https://doi.org/10.1038/s41598-020-59850-2 Download citation * Received: 08 November 2019 * Accepted: 04 February 2020 * Published: 19 February 2020 * DOI:
https://doi.org/10.1038/s41598-020-59850-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative