Endothelial autophagy: an effective target for radiation-induced cerebral capillary damage
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Toxicity to central nervous system tissues is the common side effects for radiotherapy of brain tumor. The radiation toxicity has been thought to be related to the damage of
cerebral endothelium. However, because of lacking a suitable high-resolution _vivo_ model, cellular response of cerebral capillaries to radiation remained unclear. Here, we present the
_flk:eGFP_ transgenic zebrafish larvae as a feasible model to study the radiation toxicity to cerebral capillary. We showed that, in living zebrafish larvae, radiation could induce acute
cerebral capillary shrinkage and blood-flow obstruction, resulting brain hypoxia and glycolysis retardant. Although _in vivo_ neuron damage was also observed after the radiation exposure,
further investigation found that they didn’t response to the same dosage of radiation _in vitro_, indicating that radiation induced neuron damage was a secondary-effect of cerebral vascular
function damage. In addition, transgenic labeling and qPCR results showed that the radiation-induced acute cerebral endothelial damage was correlated with intensive endothelial autophagy.
Different autophagy inhibitors could significantly alleviate the radiation-induced cerebral capillary damage and prolong the survival of zebrafish larvae. Therefore, we showed that radiation
could directly damage cerebral capillary, resulting to blood flow deficiency and neuron death, which suggested endothelial autophagy as a potential target for radiation-induced brain
toxicity. SIMILAR CONTENT BEING VIEWED BY OTHERS INTRAVENOUS INJECTION OF CYCLOPHILIN A REALIZES THE TRANSIENT AND REVERSIBLE OPENING OF BARRIER OF NEURAL VASCULATURE THROUGH BASIGIN IN
ENDOTHELIAL CELLS Article Open access 29 September 2021 ACETYLATION OF Α-TUBULIN RESTORES ENDOTHELIAL CELL INJURY AND BLOOD–BRAIN BARRIER DISRUPTION AFTER INTRACEREBRAL HEMORRHAGE IN MICE
Article Open access 07 May 2025 BRAIN-DERIVED ENDOTHELIAL CELLS ARE NEUROPROTECTIVE IN A CHRONIC CEREBRAL HYPOPERFUSION MOUSE MODEL Article Open access 18 March 2024 INTRODUCTION Most of
patients with primary brain tumors or metastases tumors had to undergo radiotherapy, such as whole-brain radiotherapy (WBRT), stereotactic radio surgery (SRS) and intensity modulated
radiation therapy (IMRT)1,2. Although radiotherapy was an effective treatment for brain tumors3,4,5, experimental and clinical evidence suggested that more than 40% of people receiving
radiotherapy would suffer from brain damage and mainly manifested as cognitive dysfunction6. Radiation-induced cognitive dysfunction refers to the dysfunction of memory, learning, processing
speed, and attention, occurring in 50–90% of these patients and gradually becoming irreversible2,7,8. Although radiotherapy-induced brain damage has been discussed in many studies, the
cellular and molecular mechanisms of radiation-induced brain damage is still controversial. Radiation can damage neuronal, glial and vascular compartments of the brain and may lead to
molecular, cellular and functional changes9. The neurovascular unit (NVU), composed of neurons, interneurons, astrocytes, endothelial cells, basal lamina, and extracellular matrix, is the
basal structure of brain homeostasis10. these cells work together intimately and be closely coordinated, which ensure dynamic linkages in reciprocal way under physiological conditions11.
Although the radiotherapy would induce the damage of all components, the endothelial cells were more sensitive to radiation12,13 and showed significant increased caspase-3/7 activity in a
radiation dose-dependent manner, whereas microglia and neuronal cells exhibited lower ratio of dead cells at each dose studied14. As the brain is a constant metabolic demand organ15
(consuming 20% of the body), endothelium damage and decreased microcirculation might lead to poor perfusion of brain tissue, which could cause death of other cells (including neurons and
glial cells) within the neurovascular unit. Varies animal models have been applied to study the radiation induced vascular damage, they found that radiation therapy could induce
dose-dependent endothelial apoptosis16, disruption of the blood-brain barrier17, vascular rarefaction, and cognitive deficits18. However, few can reveal the _in vivo_ morphological and
functional changes of cerebral capillaries after exposing to ionizing radiation. The zebrafish has been served as an important animal model to study the geneses of the vascular and nervous
system disease19,20. The small size, optical translucency, and conservative gene of the zebrafish larvae, in combination with a variety of fluorescent labeling transgenic lines permit
real-time and high-resolution observation of morphological and gene-expression changes in endothelial cells _in vivo_. Therefore, we established a zebrafish model using the larvae of
transgenic zebrafish (_flk:eGFP_) to study radiation-induced cerebrovascular damage. Continuously confocal tracking astonishingly discovered the morphologically and functionally changes in
cerebral capillaries after radiation. 3D coculture of zebrafish neurons and endothelial cells suggested that endothelial cells were more sensitive to radiation compared with other cells in
brain. Whole-brain living images of _fli1a:mCherry-GFP-LC3_ transgenic zebrafish larvae and q-PCR data indicated the association of endothelial autophagy with the radiation induced damage to
cerebral capillary. Finally, autophagy inhibition experiments highlighted the use of autophagy inhibitors in relieving the damage of cerebral capillaries and the subsequent effect to the
neurons and glia after the radiotherapy. MATERIALS AND METHODS CLINICAL RESEARCH From the database of West China Hospital, a patient who was diagnosed with glioma and underwent Computed
Tomography angiography (CTA) before and after brain radiation was enrolled. The interval time of the patient from first to last radiation was within 40 days, the times of radiation was 10,
and total dosage of radiotherapy was 40 Gy. The clinical research has received approval from the institutional review board of the Medical Faculty at the West China Hospital, Sichuan
University and all methods were carried out in accordance with relevant guidelines and regulations. The written informed consent was obtained from this patient. TRANSGENIC ZEBRAFISH
MAINTENANCE AND ESTABLISHMENT Lines used in this study included _flk:eGFP_ (zfin: s843Tg), _HuC:mCherry_ (zfin: nia02Tg). _GFAP:mCherry_ and _fli1a:mCherry-GFP-LC3_ were established using
multisite gateway system (Life Technologies) and Tol2 kit21. Zebrafish were maintained at 28.5 °C with a 14-hour light/10- hour dark cycle as previously described22. Embryos were kept in
incubator at 28.5 °C, and treated with 0.1 Mm 1-phenyl-2-thiourea (PTU, Sigma P5272) to inhibit pigment formation beyond 24 hours post-fertilization. All zebrafish experiments were conducted
in accordance with the guidelines of the Animal Care and Use Committee of Sichuan University (Chengdu, Sichuan, China) and approved by the institutional review board of the Medical Faculty
at the West China Hospital, Sichuan University. 3-D CULTURE OF TRANSGENIC ZEBRAFISH NEURONS AND ENDOTHELIAL CELLS _flk:eGFP_ and _HuC:mCherry_ transgenic zebrafish were incrossed and the
embryos were collected. At 80% epiboly/tailbud stage, embryos were washed with PBS containing 5X antibiotics. All embryos were then dechorionated at six hours post fertilization using
pronase enzyme degradation. Twenty embryos in each group were then dissociated into single cells with continuous pipetting. Zebrafish embryos cells were then seeded into matrigel in 48-well
plates for 1 week. The cells were cultured by complete DMEM/F12 medium (Gibco) supplemented with epidermal growth factor (EGF, 20 ng/ml, Peprotech), basic fibroblast growth factor (bFGF, 10
ng/ml, Peprotech) and B27 (1 × , Invitrogen). Before or after the ionizing radiation, the cells were generally maintained at 28 °C with 5% CO2. X-RAY RADIATION Radiation experiments were
performed according to the Radiation Safety Manual of West China Hospital, Sichuan University. Briefly, zebrafish larvae (8dpf, days post fertilization) were stably mounted in the central of
2.5 cm thick 3% Sodium carboxymethlycellulose (SCMC). Larvae mounted in 6-well dish were then directly exposed to X-Ray Radiation (ELEKTA, Versa HD, Sweden) with 10 cm × 10 cm of filed size
at West China Hospital. Larvae in the radiated group received a single 5 Gy and 10 Gy dose radiation respectively. Control larvae were treated identically but received no radiation. The
larvae were put back into incubator within 30 min after radiation exposure. IMAGING STRATEGY AND QUANTIFICATION For imaging cerebral vasculature of zebrafish _in vivo_, selected embryos were
mounted in low melting point (LMP) agarose (1%, wt/vol) at the bottom of a 29 mm glass bottom dish and covered with fish water at 33 °C. The real-time tracking images were taken with Zeiss
LSM 880 Confocal Microscope with Airyscan (Carl Zeiss, Germany). The midbrain region of each brain were imaged with a confocal microscope under 40× lens with a total depth of 100 μm. We
counted LC3 puncta on all blood vessels, and calculated the number of puncta on each 100μm vessels. We calculated the number of all perfused vessel branches and vascular diameter basing on
fluorescent images by ZEN blue and analyzed by the Prism software. BRAIN ANGIOGRAPHY AND CEREBRAL BLOOD VOLUME MONITOR For intracranial angiography of zebrafish larva _in vivo_, fluorescein
sodium (Sigma F6337, 376 Da) and Dextran blue (MW, 10 kDa) were dissolved in PBS to final concentrations of 2 mg/ml and 12.5 mg/ml respectively. Zebrafish larvae were anesthetized with 0.2
mg/ml Tricaine (Sigma A5040) before the tracker injection. For brain angiography, about 3 nl of tracers were injected into each larva from pericardium and confocal live images were taken
within 1–2 hours post the tracer injection. The whole brain perfusion images were taken with Confocal Microscope. The brain of zebrafish was moved to the center of the field of vision, and
all images generally had a same depth (100 μm). We calculated number of all perfused vessel branches in fluorescent images and quantified by the Prism software. For the cerebral blood-volume
monitor assay, 1 hour after the tracer injection, the brains of the zebrafish larvae (n = 6 in each group) were dissected out using tweezers under a stereomicroscope. The brains were then
completely digested using 100 μl of mixture of 0.25% trypsin, 2.5 mg/ml collagenase IV and 1 mg/ml DNAses. After high-speed centrifuge (2000 rpm/min) for 10 min, the fluorescent intensity of
fluorescein sodium and Dextran blue in the supernatants were measured using a fluorescence microplate reader (FLx800™). HYPOXIA DETECTION ASSAY AND NAD(P)H AUTOFLUORESCENCE ASSESSMENT
Intracranial hypoxia was evaluated using a hypoxia-detecting probe (MAR, Goryo Chemical, Japan) as the manufacturer described23. Briefly, hypoxia-detecting probe was injected directed into
the zebrafish brain at 4-day post radiation (dpr) and the injected zebrafish were then maintained in incubator for 2 hours. Then the zebrafish larvae were fixed with 4% PFA and the brains
were isolated with tweezers under a stereomicroscope. After staining, the fluorescence intensity of the zebrafish brain was analyzed by Confocal (Leica SP5). For NAD(P)H assessment, the
brain samples were fixed with 4% PFA for 4 hours, and then washed with PBS for 1 hour. NAD(P)H had its own autofluorescence, and it absorbs light of wavelength 340 ± 30 nm and emits
fluorescence at 460 ± 50 nm24. The specific fluorescent intensity of NAD(P)H in each sample was imaged using a confocal (Leica SP5), and quantified by image J. QUANTITATIVE REAL-TIME PCR The
cerebral endothelial cells of flk:eGFP transgenic zebrafish larvae are showing green fluorescence and the cells were isolated by FACS. Total RNA was extracted using an RNeasy column
(Axygen), and cDNA was obtained using SuperScript III reverse transcriptase (Invitrogen). Quantitative PCR (qPCR) was then performed on an ABI7300 thermocycler (Applied Biosystems) using
SYBR green Master Mix (Applied Biosystems). The relative fold change of autophagy related genes, including _lc3, beclin1, atg5_ and _ambra_, were calculated in 0, 5 and 10 Gy group at 2-day
and 4-day post radiation. The following primers were used: _β-actin_ (CTTCTTGGGTATGGAATCTTGC and GTACCACCAGACAATACAGTG); _lc3_ (GAGAAGTTTTTGCCGCCTCT and ACCTGTGTCCGAACATCTCC); _atg5_
(AGGATACCCGCCTGTTTCAC and TCCCTCGTGTTCAAACCACA); _beclin1_ (CATCACTGAGAACGAGTGCCA and CTGTGGTTGCGTCCCTCATC); _ambra_ (CTGCTGCTCATTGCCACC and CTGCTCCTCATGCTGACC). FLOW CYTOMETRY To test
survival of neurons and glial cells after radiation, the zebrafish brains were dissected and digested on 4day post radiation as previous described. DAPI/AnnexinV-FITC staining was performed
according to the standard method25. Flow Cytometry data were acquired by FACSVerse (BD Biosciences) and analyzed by FlowJo software (Tree Star). The apoptosis test of neurons and endothelial
cells in 3-D culture system was performed using the same DAPI/AnnexinV-FITC staining in a similar way. DRUG ADMINISTRATION DETAILS A clinical anti-vasoconstriction drug nimodipine and three
autophagy inhibitors (Wortamanin, Ly294002 and Chloroquine) (Selleck). These small molecular inhibitors were added directly into fish water. Nimodipine (5 μM) was immediately added into
fish water after the radiation exposure, and the drug was changed daily for continuing 4 days. Living brain imaging by confocal was done at 2 and 4dpr. For the autophagy inhibitor treat
groups, zebrafish embryos were soaked in fish water containing autophagy inhibitors (Wortamanin (1 μM), Ly294002 (10 μM) and Chloroquine (100 μM)) for 6 hours each day, and then washed and
changed with fresh water until the death of these radiated larvae. STATISTICAL ANALYSIS Prism software was used for statistical analysis. All datasets were challenged by a normality test.
Datasets with a Normal distribution were analyzed by unpaired t test. For zebrafish survival studies, Kaplan–Meier survival curves were generated and analyzed for statistical significance
with GraphPad Prism. A level of *P < 0.05 or **P < 0.01 was regarded as statistically significant. RESULTS WHOLE-BRAIN-RADIATION THERAPY IMPAIRS THE INTRACRANIAL BLOOD-PERFUSION IN A
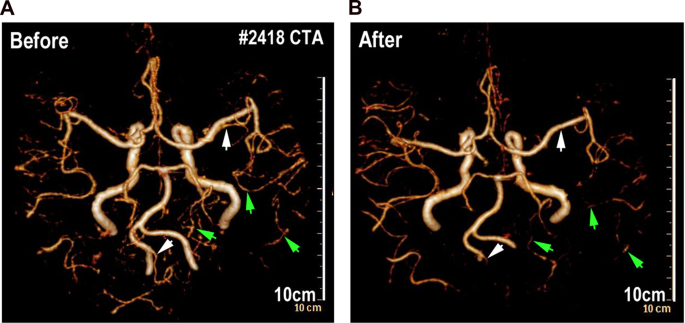
GLIOMA PATIENT A patient from West China Hospital was collected in the present study. This patient was diagnosed with glioma and underwent Computed Tomography angiography (CTA) before and
after brain radiation. The patient totally received 10 times of whole brain radiation (the total radiotherapy dosage is 40 Gy) within 40 days from June 2016 to July 2016. After comparing
3-dimensional computed tomography angiography (Fig. 1), we found that the intracranial vessels and blood-perfusion was significantly affected after the last time of radiation. Some big
intracranial vessels (such as left post cerebral artery) (Fig. 1) shrank after 10 times of brain radiation and partial small cerebral vessels (such as branches of right middle cerebral
artery and branches of post cerebral artery) (Fig. 1) could no longer be detected by CTA after the radiation. This piece of clinical data from this glioma patient implies that intensive
brain radiation might result damage of intracranial vessels and reduction of the cerebral blood-perfusion. X-RAY RADIATION RESULTS ACUTE SHRINKAGE OF CEREBRAL CAPILLARIES OF ZEBRAFISH LARVA
Zebrafish has been envisioned as a popular vertebrate model system for studying radioprotection26. Here, to establish a zebrafish X-ray Radiation damage model, 8dpf zebrafish embryos were
stably mounted in the central of 2.5 cm thick 3% Sodium carboxymethlycellulose (SCMC). Mounted Larvae were exposed to a single dose of X-Ray Radiation (5 Gy and 10 Gy), and then were
released to fresh water for viability and behavior assessment (Fig. 2A). At 2dpr, we found, although the zebrafish morphology looks normal, most of the radiated-larvae had obvious balance
and coordination problems (Fig. 2B). Larvae couldn’t keep themselves steady in the water and kept readjusting their bodies (Fig. 2B, upper panel). In addition, by track the swim
trajectories, we found that the radiated-larvae were reluctant to swim, comparing with the control larvae at the same age (Fig. 2B, lower panel). Then, to investigate the acute changes of
cerebral vasculature of the zebrafish larvae exposing to radiation, we employed the _flk:eGFP_ transgenic zebrafish. The endothelial cells of the _flk:eGFP_ zebrafish were GFP labeled and
the zebrafish larvae were transparent, which enable us to image and reconstruct the whole cerebral vasculature of zebrafish larva at single-cell resolution by confocal. Interestingly, living
images of the brain vasculature revealed that the general diameter of cerebral capillaries of the radiated-larvae were smaller than that in the untreated zebrafish (Fig. 2C). Quantitative
analysis indicated that the shrinkage of cerebral capillaries was radiation dosage dependent (Fig. 2C,D). In addition, time-series evaluation results indicated that the radiation-induced
capillary shrinkage was irreversible over time (Fig. 2D) and most of the radiated-larvae (5 Gy and 10 Gy) would die, within 6 days post the radiation (Fig. 2E). We also imaged and evaluated
the cardiovascular system and the cardiac function of the larvae after exposing to radiation. Fluorescent images indicated that the size and movement of Bulbus Arteriosus (BuA) during a
cardiac cycle was similar to the untreated larvae (Fig. 2F) and the heart beat rate was also not affected (p = 0.57, Fig. 2F). These results suggested the relatively normal cardiovascular
function of zebrafish larvae after exposing to radiation. Altogether, our results showed that single dose of X-ray radiation could specifically induce an irreversible cerebral capillary
damage in zebrafish larvae. RADIATION-INDUCED CEREBRAL CAPILLARY SHRINKAGE AFFECTS CEREBRAL BLOOD-PERFUSION IN ZEBRAFISH LARVAE To monitor the cerebral blood flow in zebrafish larva after
exposing to radiation, Dextran blue (10,000 MW) and fluorescein sodium (376 MW) were pericardially injected into _flk:eGFP_ zebrafish larva at different time points before the imaging and
evaluation. The whole brain confocal images revealed that the blue-dextran-labeled blood-flow in the cerebral capillaries continuing declining after exposing to the radiation (Fig. 3A,B). In
addition, to quantify the changes of blood volume in the zebrafish brain, at different time points (0, 2, 4 dpr), we dissected the larva brains (n = 10 in each group at every time point)
under a stereomicroscope. The brains in each group were completely digested and the fluorescence intensity (dextran blue and fluorescein sodium) of the supernatant was normalized and
measured (Fluorescence Microplate Reader, Thermo). Consistent with the angiography of cerebral vessels, we found that the fluorescent intensity of the radiated-brains supernatant was much
lower than that in the untreated group (Fig. 3C,D), suggested a reduction of the blood volume in the radiated brains. Brain blood-volume reduction could be resulted from the cerebral
capillary shrinkage that induced by radiation. To clarify this relationship, we tried to rescue the brain blood flow defect by using a clinical anti-vasoconstriction drug-Nimodipine.
Nimodipine is a calcium channel blocker and has some selectivity for cerebral vasculature. Nimodipine (5 μM) was directly added into fish water after the radiation exposure, and the drug was
changed daily for continuing 4days. Living brain imaging by confocal was done at 2 and 4dpr. As expected, with the Nimodipine treatment, obvious vasodilation of the cerebral capillaries of
the radiated-larvae was observed at 2dpr and the cerebral blood-perfusion showed a marked improvement (Fig. 3E,F). However, this improvement only lasts for a short time. At 4dpr, even with
the continuing Nimodipine treatment, the cerebral blood-perfusion in the radiated-larva was dramatically dropped and severely vascular shrinkage was detected (Fig. 3E,F). As a result, the
short-term effect of Nimodipine on the cerebral vasoconstriction only resulted in a limited over-all survival improvement after the radiation (Fig. 3G). RADIATION-INDUCED CEREBRAL
BLOOD-PERFUSION DEFICIENCY RESULTS SECONDARY NEURON AND GLIAL CELLS DAMAGE IN ZEBRAFISH BRAIN To investigate the influence of radiation-induced cerebral vascular damage, we carried out a
series of _in vivo_ and _in vitro_ experiments. Firstly, by measuring intensity of the NAD(P)H-related autofluorescence in the zebrafish brain27, we monitored the changes of cerebral energy
metabolism of zebrafish larvae after exposing to radiation. The NADPH auto fluorescent signaling is mainly located in the telencephalon, diencephalon and mesencephalon of zebrafish brain
(Fig. 4), which is generally overlapped with the regions with high neuron metabolism. In accordance with the shrinkage of the cerebral capillaries, Confocal images showed that the
NAD(P)H-related fluorescence in the radiated-brains was dramatically reduced at 4dpr (Figs. 4A, S1). Next, by using the hypoxia detection probe together with the neuron-labeled transgenic
zebrafish (_HuC:mCherry_), we revealed the severe hypoxia (green channel) in the zebrafish brains at 4dpr (Fig. 4B). At the same time, we found that rare healthy neurons (mCherry+) were
detected in the radiated-brains at 4dpr, compared with the untreated zebrafish brains (Fig. 4B). Vertebrate neurons are generally viewed as among the most anoxia-sensitive of all cells28,29.
The death of neurons from hypoxia and glucose deprivation in the radiated brains can follow quickly from necrosis or in other slower ways, such as apoptosis. To determine the possible role
of programmed cell death in the radiated zebrafish brains, we then applied annexin V apoptosis assay. To monitor the neuron and glial cells in the zebrafish brain, we used either
neurons-labeled _HuC:mCherry_ or the glia-labeled _GFAP:mCherry_ transgenic zebrafish in the radiation test. At 2dpr, we digested the brains of the transgenic zebrafish larvae (n = 10 in
each group) that with or without radiation exposure (10 Gy) and staining of DAPI/FITC-annexin V was then applied. Flow cytometry results indicated that higher apoptosis ratio of neurons and
glial cells in the brains with radiation exposure. To confirm that the neuron damage was mainly a secondary effect of radiation-induced cerebral blood flow deficiency, rather than a direct
toxicity of radiation. We co-cultured the zebrafish neurons and endothelial cells in 3D Matrigel and exposed these cells to X-ray radiation at the same dose as we tested in zebrafish (5 and
10 Gy). The co-cultured neurons and endothelial cells were maintained at 28 °C with 5% CO2, supplied with sufficient glucose and essential growth factors (methods and materials). At 4dpr, we
evaluated the status of endothelial cells (_flk:eGFP_) and neurons (_Huc:mCherry_). As showed in the fluorescent channel (Fig. 4D), control endothelial cells in the 3D Matrigel behaved as
normal, forming endothelial sprouts and connecting with each other. However, the radiated endothelial sprouts were much thinner and were more retracted than the control (Fig. 4D).
Endothelial sprouts in the Matrigel were then digested into single cells and counted. The result indicated that, except for the morphological changes, there were actually less endothelial
cells survived in the wells after the radiation and the cell number reduction was radiation-dose dependent (Fig. 4E). On the other hand, we also evaluated the cell status of the neurons in
the 3D Marigel after the radiation as we did with endothelial sprouts. Interestingly, contrasts to the endothelial cells, neurons were more resistant to radiation. No detectable
morphological or cell number changes were observed (Fig. 4F,G). In addition, annexin V apoptosis assay indicated that no significant apoptosis was initiated in the culturing neurons after
exposing to radiation (Fig. 4H). These results together indicated that endothelial cells, compared with neurons, are more sensitive to radiation. And it’s highly possible that the _in vivo_
neuron death after the radiation was attributed to the radiation-induced cerebral damage and intracranial ischemia. RADIATION INDUCES INTENSIVE AUTOPHAGY FLUX IN THE CEREBRAL ENDOTHELIAL
CELLS IN ZEBRAFISH LARVAE The radiation-induced endothelial damage results in severe cerebral capillary shrinkage, which leads to intracranial ischemia and the death of neurons and glial
cells. However, the mechanism of radiation induced endothelial damage remains unclear. Previous studies showed that radiation almost uniformly promotes autophagy in tumor cells30 and
extensive autophagy has been related to programmed cell death31. Thus, to track the possible endothelial autophagy flux after radiation exposure, we established a transgenic zebrafish line
(fli:mCherry-GFP-LC3), in which the endothelial cells specifically expressing chimeric LC3 fused to both GFP (acid sensitive) and mCherry (acid stable). This chimeric LC3 protein has been
used to monitor autophagosome transport32,33. The fli:mCherry-GFP-LC3 transgenic zebrafish larvae (8dpf) were exposed to X-ray radiation at different dosages as we did before. Confocal
images at 4dpr showed that, contrasts to the untreated larvae, abundant LC3 puncta were detected in the cerebral endothelium of radiated zebrafish larvae (Fig. 5A). Quantitative analysis
indicated that the number of the LC3 puncta were radiation dosage dependent (Fig. 5B). In addition, in the 5 Gy group, partial of the puncta were GFP and mCherry double positive, indicating
that these autophagosomes had not yet fused with the lysosomes. And in the 10 Gy group, most of the LC3 puncta were mCherry single positive, indicating the maturation of these autophagosomes
(Fig. 5A,B). To confirm the activation of endothelial autophagy after the X-ray radiation, we then isolated cerebral endothelial cells by FACS from zebrafish brains with or without
radiation exposure. The mRNA expression levels of autophagy related genes (_lc3, beclin1, atg5_ and _ambra1_) were then evaluated by qPCR. At both 2 and 4 dpr, the qPCR results showed that
the expression of evaluated autophagy-related genes were elevated in the cerebral endothelial cells of radiated brains, compared with that in the control larvae. Previous studies have
correlated the intensive autophagic flux with various forms of cell death34. Here, in our experiment, we found that the cerebral endothelial cells in the radiated zebrafish brains also
showed a higher expression of apoptotic genes (Fig. S2). Then, using AnnexinV/DAPI apoptosis assay, we indeed found a higher apoptotic ratio and lower percentage of living endothelial cells
in the radiated zebrafish brains were than that in the control brains (Fig. 5D,E). Hence, these results together suggested a role of radiation-induced endothelial autophagic damage or
autophagic death in the radiation-induced cerebral vascular damage and intracranial ischemia. INHIBITION OF AUTOPHAGY ALLEVIATES RADIATION-INDUCED CEREBRAL VASCULAR DAMAGE AND EXTENDS THE
SURVIVAL OF ZEBRAFISH LARVAE Autophagy is a double-edged sword, either keeping the cells survived through adverse microenvironment or directly incorporating in various forms of cell death.
Here, we revealed the general cerebral endothelium damage after exposing to radiation at different dosages (5 and 10 Gy), we rationalized that radiation-induced endothelial autophagy was
positively correlated to the cerebral capillary damage. To test this hypothesis, we tried several autophagy inhibitors: Wortamanin, Ly294002 and Cloroquine. PI3K is required for autophagy35
and inhibition of PI3K with LY294002 or Wortamanin can inhibit autophagic sequestration. Chloroquine inhibits autophagy as it raises the lysosomal pH, which leads to inhibition of both
fusion of autophagosome with lysosome and lysosomal protein degradation36. After exposing to radiation, zebrafish larvae were soaked in culturing water supplied with Wortamanin (1 μM),
Ly294002 (10 μM) or Cloroquine (100 μM) for 6 hours per day until the death of these radiated larvae. The dosages of anti-vasoconstriction drug Nimodipine and autophagy inhibitors
Wortamanin, Ly294002 and Cloroquine were determined by zebrafish maximum tolerated concentration and maximum patient’s plasma concentration of each compound. At 4dpr, Confocal images of
_fli:mCherry-GFP-LC3_ transgenic zebrafish larvae showed that that number of fluorescent LC3 puncta in the cerebral endothelium were significantly reduced with the treatment of autophagy
inhibitors (Fig. 6A,C) after radiation exposure. In addition, by using the _flk:eGFP_ transgenic larvae and the Dextran-blue angiography, we evaluated the changes of cerebral capillaries and
blood flow. As expected, with the treatment of autophagy inhibitors, the blood-perfusion in the brains of radiated larvae was significantly improved (Fig. 6B,D) and the survival of radiated
zebrafish larvae were also significantly extended, especially for the group that treated with ly294002 (Fig. 6E, p < 0.01). To study the change of zebrafish behavior caused by radiation
after using autophagy inhibitors, we evaluate their stability and moving tracks. We found the control radiated-larvae had obvious balance and coordination problems, and the radiated
zebrafish larvae using autophagy inhibitors showed more active and could keep balance (Fig. S3). These results indicate that radiation-induced intensive endothelial autophagy is positively
correlated to the cerebral vascular damage and could be served as a target for anti-radiation-induced brain toxicity. DISCUSSION Previous studies have suggested that endothelial cells were
more sensitive to radiation compared with neurons and glial cells; therefore, brain injury induced by radiotherapy may be related to vascular damage12. To test brain vascular pathogenicity
_in vivo_, we developed a transgenic zebrafish radiation model to study the radiation-induced cerebrovascular damage, which allowed real-time living tracking of endothelial damage and blood
perfusion in brain followed by ionizing radiation. In particular, the _(fli:mCherry-GFP-LC3)_ transgenic zebrafish and the qPCR analysis indicated that cerebral endothelial autophagy was
significantly associated with the radiation-induced cerebral capillary damage. Further autophagy inhibition experiments demonstrated a vital role of autophagy in radiation-induced
endothelial damage, highlighting the therapeutic potential of regulating autophagy for the treatment of radiation-induced brain injury. Using _flk:eGFP_ fish, we demonstrated the death of
cerebral vascular endothelial cells induced by radiation appeared in a dose-dependent pattern. Recent studies suggested radiation induced cerebral vascular damage begins with progressive
endothelial cells loss. In a rat radiation model, single dose of 5–20 Gy radiation in brain lead to a 15% decrease of endothelial cell number within 1 day, then followed by continuous
endothelial cells loss within a month37. In addition, we observed contraction of cerebral capillaries induced by radiation, and radiation could further result in cerebral capillaries network
simplification over time. Initial studies demonstrated that fractionated radiotherapy induced a rarefaction of vessels within the Cornu Ammonis 1(CA1), Cornu Ammonis 3(CA3) and dentate
gyrus region of the hippocampus (an area important for spatial memory), which is closely related to supply of oxygen, nutrients and trophic factors to brain, and thus result in deficits in
spatial learning and memory38. However, in some mouse radiation damage models, the blood perfusion of tissue cannot be accurately assessed, and the damage of vascular function cannot be
evaluated at the whole brain level. In addition, the tissue fixation and section staining would alter the vascular structure. Meanwhile, because of the relatively low resolution of CTA and
MRA for human patients, morphological and functional changes of micro vessels after radiation could not be detected either. In our study, the whole brain of transgenic zebrafish can be
directly imaged under a fluorescent microscope or confocal due to their small size and transparency. Therefore, using zebrafish radiation model, the 3-D _in vivo_ tracking of brain
vasculature provides chances for us to preciously evaluate the cerebral endothelium and blood perfusion after exposing to ionize radiation. The molecular mechanism of cerebral vascular
damage by ionizing radiation is still controversial. Radiation could induce the damage of DNA followed by increased expression of apoptosis signals in many vascular endothelial cells,
resulting in angiogenesis compromise14, which is partially consistent with our results. The DNA damage response coordinates many processes, including DNA repair, regulation of cell cycle
checkpoints, and ultimately induction of a programmed cell death, most often apoptosis, when DNA damage cannot be repaired. Many researchers suggest, that autophagy, a catabolic process
considered to be a cellular survival mechanism, is a central player in the regulation of DNA damage response. For mild stress, activation of DNA damage response may evoke autophagy as an
early adaptive response. For serious stress, such as radiotherapy, autophagy did not promote survival, but induced apoptosis. We found that the apoptosis of endothelial cells increased to 8%
after radiation compared with control group (2%) in zebrafish brain. Besides, the strongly upregulation of autophagy genes39, including atg5, beclin-1, LC3 and ambra1, demonstrate that the
endothelial autophagy is activated by ionizing radiation. To lively image the radiated-induced endothelial autophagy in zebrafish, we establish the fli:mCherry-GFP-LC3 transgenic zebrafish
to monitor the autophagy process specifically in endothelial cells after X-ray radiation. Interestingly, our results demonstrated that radiation could lead to acceleration of autophagic flux
and apoptosis in cerebral endothelial cells (Fig. 5). However, in an _in vitro_ study, Kalamida _et al_. found ionizing radiation could trigger perinuclear accumulation of the
autophagosomal proteins and repression of the autophagolysosomal flux in human umbilical vein endothelial cells, suggesting against the blockage of autophagic flux may contribute to
radio-resistance40,41. The difference might owe to the difference between _in vivo_ and _in vitro_ studies, and different radiation dosage and animal models. Autophagy is a highly conserved
cytoprotective and complex process, and several pharmacological and nutritional interventions are available to inhibit autophagy at the nucleation, elongation, fusion or degradation phase.
Although chloroquine, ly294002 and wortamannin are all considered as autophagy inhibitors, each functions differently in the autophagy process. Chloroquine42 is a inhibitor of lysosomal
function, ly29400243 is VPS34 inhibitor, and they can pass through blood-brain barrier. Compared with ly294002, wortamannin44 had no blood-brain barrier permeant and had relatively bad
selectivity. Although chloroquine and ly294002 are able to significantly repress the formation of autophagosomes in the cerebral endothelium, they had systemically toxic to zebrafish.
Commercial autophagy drug library contains hundreds of autophagy related compounds. Take advantage of the high throughput drug screening ability of zebrafish model, we may find an autophagy
inhibitor that could significantly alleviate the radiation-induced cerebral vascular damage but has relatively low toxicity in the future. Our study also had some limitations. For instance,
in our current study, the major molecular mechanism of radiation induced vascular damage was tested in zebrafish model, which should be further tested in mammals and human cerebral
endothelial cells. In addition, because of the tiny size of zebrafish, the same dose of radiation in zebrafish might be different in patients, which need to further addressed in the
following studies. Despite these limitations, it was the first time that revealing the radiation-induced morphological change of cerebral vessels at the whole-brain level. We also tested a
mechanism of radiation-induced endothelial damage in transgenic zebrafish model, suggesting a novel therapeutic strategy for radiation-induced cerebral vascular damage. REFERENCES *
Greene-Schloesser, D., Moore, E. & Robbins, M. E. Molecular pathways: radiation-induced cognitive impairment. _Clin. Cancer Res._ 19, 2294–2300,
https://doi.org/10.1158/1078-0432.CCR-11-2903 (2013). Article CAS PubMed PubMed Central Google Scholar * Greene-Schloesser, D. _et al_. Radiation-induced brain injury: A review. _Front.
Oncol._ 2, 73, https://doi.org/10.3389/fonc.2012.00073 (2012). Article CAS PubMed PubMed Central Google Scholar * McTyre, E., Scott, J. & Chinnaiyan, P. Whole brain radiotherapy
for brain metastasis. _Surgical Neurol. Int._ 4, S236–244, https://doi.org/10.4103/2152-7806.111301 (2013). Article Google Scholar * Lippitz, B. _et al_. Stereotactic radiosurgery in the
treatment of brain metastases: the current evidence. _Cancer Treat. Rev._ 40, 48–59, https://doi.org/10.1016/j.ctrv.2013.05.002 (2014). Article PubMed Google Scholar * Khuntia, D., Brown,
P., Li, J. & Mehta, M. P. Whole-brain radiotherapy in the management of brain metastasis. _J. Clin. Oncol._ 24, 1295–1304, https://doi.org/10.1200/JCO.2005.04.6185 (2006). Article CAS
PubMed Google Scholar * Pease, N. J., Edwards, A. & Moss, L. J. Effectiveness of whole brain radiotherapy in the treatment of brain metastases: a systematic review. _Palliat. Med._
19, 288–299, https://doi.org/10.1191/0269216305pm1017oa (2005). Article CAS PubMed Google Scholar * Davis, C. M. _et al_. Effects of X-ray radiation on complex visual discrimination
learning and social recognition memory in rats. _PLoS one_ 9, e104393, https://doi.org/10.1371/journal.pone.0104393 (2014). Article ADS CAS PubMed PubMed Central Google Scholar *
Schultheiss, T. E. & Stephens, L. C. Invited review: permanent radiation myelopathy. _Br. J. Radiol._ 65, 737–753, https://doi.org/10.1259/0007-1285-65-777-737 (1992). Article CAS
PubMed Google Scholar * Attia, A., Page, B. R., Lesser, G. J. & Chan, M. Treatment of radiation-induced cognitive decline. _Curr. Treat. Options Oncol._ 15, 539–550,
https://doi.org/10.1007/s11864-014-0307-3 (2014). Article PubMed Google Scholar * Kovacs, R., Heinemann, U. & Steinhauser, C. Mechanisms underlying blood-brain barrier dysfunction in
brain pathology and epileptogenesis: role of astroglia. _Epilepsia_ 53(Suppl 6), 53–59, https://doi.org/10.1111/j.1528-1167.2012.03703.x (2012). Article CAS PubMed Google Scholar *
Abbott, N. J. & Friedman, A. Overview and introduction: the blood-brain barrier in health and disease. _Epilepsia_ 53(Suppl 6), 1–6, https://doi.org/10.1111/j.1528-1167.2012.03696.x
(2012). Article PubMed PubMed Central Google Scholar * Dimitrievich, G. S., Fischer-Dzoga, K. & Griem, M. L. Radiosensitivity of vascular tissue. I. Differential radiosensitivity of
capillaries: a quantitative _in vivo_ study. _Radiat. Res._ 99, 511–535 (1984). Article ADS CAS PubMed Google Scholar * Belka, C., Budach, W., Kortmann, R. D. & Bamberg, M.
Radiation induced CNS toxicity–molecular and cellular mechanisms. _Br. J. Cancer_ 85, 1233–1239, https://doi.org/10.1054/bjoc.2001.2100 (2001). Article CAS PubMed PubMed Central Google
Scholar * Ungvari, Z. _et al_. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial
cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. _J. Gerontol. A Biol. Sci. Med. Sci_ 68, 1443–1457,
https://doi.org/10.1093/gerona/glt057 (2013). Article CAS PubMed PubMed Central Google Scholar * Ruben, J. D. _et al_. Cerebral radiation necrosis: incidence, outcomes, and risk factors
with emphasis on radiation parameters and chemotherapy. _Int. J. Radiat. oncology, biology, Phys._ 65, 499–508, https://doi.org/10.1016/j.ijrobp.2005.12.002 (2006). Article ADS Google
Scholar * Li, Y. Q., Chen, P., Haimovitz-Friedman, A., Reilly, R. M. & Wong, C. S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. _Cancer
Res._ 63, 5950–5956 (2003). CAS PubMed Google Scholar * Li, Y. Q., Chen, P., Jain, V., Reilly, R. M. & Wong, C. S. Early radiation-induced endothelial cell loss and blood-spinal cord
barrier breakdown in the rat spinal cord. _Radiat. Res._ 161, 143–152 (2004). Article ADS CAS PubMed Google Scholar * Shi, L. _et al_. Spatial learning and memory deficits after
whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. _Radiat. Res._ 166, 892–899, https://doi.org/10.1667/RR0588.1 (2006). Article ADS CAS
PubMed Google Scholar * Wilkinson, R. N. & van Eeden, F. J. The zebrafish as a model of vascular development and disease. _Prog. Mol. Biol. Transl. Sci._ 124, 93–122,
https://doi.org/10.1016/B978-0-12-386930-2.00005-7 (2014). Article CAS PubMed Google Scholar * Duboc, V., Dufourcq, P., Blader, P. & Roussigne, M. Asymmetry of the Brain: Development
and Implications. _Annu. Rev. Genet._ 49, 647–672, https://doi.org/10.1146/annurev-genet-112414-055322 (2015). Article CAS PubMed Google Scholar * Kwan, K. M. _et al_. The Tol2kit: a
multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. _Dev. Dyn._ 236, 3088–3099, https://doi.org/10.1002/dvdy.21343 (2007). Article CAS PubMed Google
Scholar * Westerfield, M. _The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio)_. (Inst Of Neuro Science, 2007). * Piao, W. _et al_. Development of azo-based
fluorescent probes to detect different levels of hypoxia. _Angew. Chem. Int. Ed. Engl._ 52, 13028–13032, https://doi.org/10.1002/anie.201305784 (2013). Article CAS PubMed Google Scholar
* Blacker, T. S. _et al_. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. _Nat. Commun._ 5, 3936, https://doi.org/10.1038/ncomms4936 (2014). Article ADS CAS
PubMed Google Scholar * Zembruski, N. C., Stache, V., Haefeli, W. E. & Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. _Anal. Biochem._ 429, 79–81,
https://doi.org/10.1016/j.ab.2012.07.005 (2012). Article CAS PubMed Google Scholar * Hwang, M., Yong, C., Moretti, L. & Lu, B. Zebrafish as a model system to screen radiation
modifiers. _Curr. Genomics_ 8, 360–369, https://doi.org/10.2174/138920207783406497 (2007). Article CAS PubMed PubMed Central Google Scholar * Evans, N. D., Gnudi, L., Rolinski, O. J.,
Birch, D. J. & Pickup, J. C. Non-invasive glucose monitoring by NAD(P)H autofluorescence spectroscopy in fibroblasts and adipocytes: a model for skin glucose sensing. _Diabetes Technol.
Ther._ 5, 807–816, https://doi.org/10.1089/152091503322527012 (2003). Article CAS PubMed Google Scholar * Bickler, P. E. & Donohoe, P. H. Adaptive responses of vertebrate neurons to
hypoxia. _J. Exp. Biol._ 205, 3579–3586 (2002). CAS PubMed Google Scholar * Lipton, P. Ischemic cell death in brain neurons. _Physiol. Rev._ 79, 1431–1568 (1999). Article CAS PubMed
Google Scholar * Paglin, S. _et al_. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. _Cancer Res._ 61, 439–444 (2001). CAS PubMed Google
Scholar * Liu, Y. & Levine, B. Autosis and autophagic cell death: the dark side of autophagy. _Cell Death Differ._ 22, 367–376, https://doi.org/10.1038/cdd.2014.143 (2015). Article
CAS PubMed Google Scholar * Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3.
_Autophagy_ 3, 452–460 (2007). Article CAS PubMed Google Scholar * Pankiv, S. _et al_. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates
by autophagy. _J. Biol. Chem._ 282, 24131–24145, https://doi.org/10.1074/jbc.M702824200 (2007). Article CAS PubMed Google Scholar * Kroemer, G. & Levine, B. Autophagic cell death:
the story of a misnomer. _Nat. Rev. Mol. Cell Biol._ 9, 1004–1010, https://doi.org/10.1038/nrm2529 (2008). Article CAS PubMed PubMed Central Google Scholar * Blommaart, E. F., Krause,
U., Schellens, J. P., Vreeling-Sindelarova, H. & Meijer, A. J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. _Eur.
J. Biochem._ 243, 240–246 (1997). Article CAS PubMed Google Scholar * Shintani, T. & Klionsky, D. J. Autophagy in health and disease: a double-edged sword. _Science_ 306, 990–995,
https://doi.org/10.1126/science.1099993 (2004). Article ADS CAS PubMed PubMed Central Google Scholar * Ljubimova, N. V., Levitman, M. K., Plotnikova, E. D. & Eidus, L. Endothelial
cell population dynamics in rat brain after local irradiation. _Br. J. Radiol._ 64, 934–940, https://doi.org/10.1259/0007-1285-64-766-934 (1991). Article CAS PubMed Google Scholar *
Warrington, J. P. _et al_. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. _Am. J. Physiol. Heart Circ. Physiol_ 300, H736–744,
https://doi.org/10.1152/ajpheart.01024.2010 (2011). Article CAS PubMed Google Scholar * Marino, G., Niso-Santano, M., Baehrecke, E. H. & Kroemer, G. Self-consumption: the interplay
of autophagy and apoptosis. _Nat. Rev. Mol. Cell Biol._ 15, 81–94, https://doi.org/10.1038/nrm3735 (2014). Article CAS PubMed PubMed Central Google Scholar * Kalamida, D., Karagounis,
I. V., Giatromanolaki, A. & Koukourakis, M. I. Important role of autophagy in endothelial cell response to ionizing radiation. _PLoS one_ 9, e102408,
https://doi.org/10.1371/journal.pone.0102408 (2014). Article ADS PubMed PubMed Central Google Scholar * McRobb, L. S. _et al_. Ionizing radiation reduces ADAM10 expression in brain
microvascular endothelial cells undergoing stress-induced senescence. _Aging (Albany NY.)_ 9, 1248–1268, https://doi.org/10.18632/aging.101225 (2017). Article CAS Google Scholar * Cui, C.
M. _et al_. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. _Mol. Med. Rep._ 12, 2323–2328,
https://doi.org/10.3892/mmr.2015.3611 (2015). Article CAS PubMed Google Scholar * Cui, D. R. _et al_. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in
the rat hippocampus through the NF-kappaB/p53 signaling pathway. _Neuroscience_ 246, 117–132, https://doi.org/10.1016/j.neuroscience.2013.04.054 (2013). Article CAS PubMed Google Scholar
* Zhao, H. _et al_. Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. _Mol. Biol. Rep._ 40, 819–827, https://doi.org/10.1007/s11033-012-2120-z (2013). Article
CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Wusheng Tang for the zebrafish husbandry. This work was supported by grants from the National Natural Science
Foundation of China (Grant no. 81703064; 81773097; 81872068; 81872391). This work was supported by grants from the National Natural Science Foundation of China (Grant no. 81703064; 81773097;
81872068; 81872391;81872466). AUTHOR INFORMATION Author notes * These authors contributed equally: Xiaolin Ai and Zengpanpan Ye. AUTHORS AND AFFILIATIONS * State Key Laboratory of
Biotherapy and Cancer Center, West China Hospital, Sichuan University, and Collaborative Innovation Center for Biotherapy, Chengdu, Sichuan, P. R. China Xiaolin Ai, Zengpanpan Ye, Xi Huang,
Jian Zhong & Chengjian Zhao * Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan, P. R. China Xiaolin Ai, Zengpanpan Ye, Chao You & Jianguo Xu *
West China School of Public Health, No.4 West China Teaching Hospital, Sichuan University, Chengdu, Sichuan, P. R. China Yuqin Yao, Xuejiao Song & Huashan Shi * State Key Laboratory of
Biotherapy and Department of Head and Neck Oncology, West China Hospital, West China Medical School, Sichuan University, Chengdu, Sichuan, P. R. China Jianghong Xiao * Department of Imaging,
West China Hospital of Sichuan University, Chengdu, Sichuan, P. R. China Min Fan * Department of Gynecology and Obstetrics, State Key Laboratory of Biotherapy, West China Second University
Hospital, Sichuan University and Collaborative Innovation Center for Biotherapy, Chengdu, China Dongmei Zhang Authors * Xiaolin Ai View author publications You can also search for this
author inPubMed Google Scholar * Zengpanpan Ye View author publications You can also search for this author inPubMed Google Scholar * Yuqin Yao View author publications You can also search
for this author inPubMed Google Scholar * Jianghong Xiao View author publications You can also search for this author inPubMed Google Scholar * Chao You View author publications You can also
search for this author inPubMed Google Scholar * Jianguo Xu View author publications You can also search for this author inPubMed Google Scholar * Xi Huang View author publications You can
also search for this author inPubMed Google Scholar * Jian Zhong View author publications You can also search for this author inPubMed Google Scholar * Min Fan View author publications You
can also search for this author inPubMed Google Scholar * Xuejiao Song View author publications You can also search for this author inPubMed Google Scholar * Huashan Shi View author
publications You can also search for this author inPubMed Google Scholar * Dongmei Zhang View author publications You can also search for this author inPubMed Google Scholar * Chengjian Zhao
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.J.Z., X.L.A., H.S.S. and C.Y. conceived the initial concept for the study. C.J.Z.,
X.L.A., H.S.S. and J.G.X. designed the study. X.L.A., Z.P.P.Y., C.J.Z., H.S.S., Y.Q.Y., J.H.X., J.Z., D.M.Z. and X.H. carried out the experiments. C.J.Z. and Z.P.P.Y. wrote the manuscript.
M.F., Z.P.P.Y. and X.J.S. revised the manuscript. CORRESPONDING AUTHORS Correspondence to Dongmei Zhang or Chengjian Zhao. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ai, X., Ye, Z., Yao, Y. _et al._ Endothelial Autophagy: an Effective Target for Radiation-induced Cerebral Capillary Damage. _Sci Rep_ 10,
614 (2020). https://doi.org/10.1038/s41598-019-57234-9 Download citation * Received: 26 March 2019 * Accepted: 11 December 2019 * Published: 17 January 2020 * DOI:
https://doi.org/10.1038/s41598-019-57234-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative