Early life cognitive development trajectories and intelligence quotient in middle childhood and early adolescence in rural western china
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The relationship of cognitive developmental trajectories during the dynamic first years with later life development outcomes remains unclear in low- and middle-income countries.
1388 Children born to women who participated in a randomized trial of antenatal micronutrient supplementation in rural China were prospectively followed. Cognitive development was assessed
six times between 3 and 30 months of age using Bayley Scales of Infant Development, and then in mid-childhood (7–9 years) and early adolescence (10–12 years) using Wechsler Intelligence
Scale for Children. We identified four distinct infant cognitive development trajectory subgroups using group-based trajectory modeling: (i) consistently above average, (ii) consistently
average, (iii) started below average and then improved, and (iv) started below average and then declined. LBW infants (<2500 g) were 10.60 times (95% CI 3.57, 31.49) more likely to be in
the trajectory group that started below average and then declined, while each grade increase in maternal education decreased the risk of being in this group by 73% (95% CI 54%, 84%). Infants
who performed consistently above average had 8.02 (95% CI 1.46, 14.59) points higher IQ in adolescence versus the declining trajectory group. These findings suggest that interventions to
improve early child development trajectories may produce long-term human capital benefits. SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURED EARLY CHILDHOOD EDUCATION EXPOSURE AND CHILDHOOD
COGNITION – EVIDENCE FROM AN INDIAN BIRTH COHORT Article Open access 05 June 2024 EARLY DIET IN PRETERM INFANTS AND LATER COGNITION: 10-YEAR FOLLOW-UP OF A RANDOMIZED CONTROLLED TRIAL
Article Open access 09 February 2021 DO THE PATHWAYS OF CHILD DEVELOPMENT BEFORE AGE THREE MATTER FOR DEVELOPMENT AT PRIMARY SCHOOL? EVIDENCE FROM RURAL CHINA Article Open access 20 November
2024 INTRODUCTION An estimated 250 million children under five years living in low- and middle- income countries (LMICs) failed to reach their full developmental potential1. Adversities and
risk factors during the first 1000 days of life lay the foundation for development and have long-term consequences across the lifecourse1,2,3,4. Studies have shown that suboptimal childhood
cognitive development is associated with higher risk of coronary heart disease, reduced human capital, and increased risk of mortality and poor health outcomes later in life5,6,7,8. A
number of modifiable risk factors for suboptimal development have been identified including poverty-related factors, inadequate stimulation, environmental and nutritional factors9,10.
Nevertheless, a limitation of studies that examined early life determinants of cognitive development typically only assess children at a single time-point, which does not capture the dynamic
process of child development. In fact, only a few studies from high-income countries focused on preterm infants have assessed cognitive development trajectories in early childhood. One
cohort study from UK and Ireland among 315 extremely preterm births found that impaired cognitive trajectory in infancy persisted into early adulthood and there was no evidence of
catch-up11. In contrast, a study in the US reported catch-up language trajectory from 3 to 12 years among very preterm infants12. To the best of our knowledge, no studies have examined the
relationship of early child development trajectories with later life development outcomes among the general population in LMICs. In this study, we used data from a rural Chinese birth
cohort, in which the cognitive development was assessed at 3, 6, 12, 18, and 24 months during the first two years of life and then at 30 months, middle childhood (7–9 years) and in
adolescence (10–12 years). The main aims of our analysis were to (1) identify distinct trajectories of cognitive development during the first two years of life, (2) examine predictors
associated with these trajectories, and (3) assess whether these trajectories were associated with long-term cognitive outcomes in middle childhood and early adolescence. RESULTS A total of
1388 children were included in group-based trajectory modelling (GBTM) analyses. Baseline characteristics of these participants are presented in Table 1. A total of 669 and 735 of these
participants were followed at middle childhood (7–9 years) and early adolescence (10–12 years), respectively (Supplementary Fig. S1). The mean age at middle childhood and adolescence were
7.8 years (SD ± 0.6) and 11.3 (SD ± 0.6) years, respectively. Most background characteristics were similar between individuals who completed the middle childhood and early adolescence
assessments and those who were lost to follow-up (Supplementary Table S1). IDENTIFICATION OF CHILD DEVELOPMENT TRAJECTORIES DURING THE FIRST TWO YEARS OF LIFE GBTM identified four trajectory
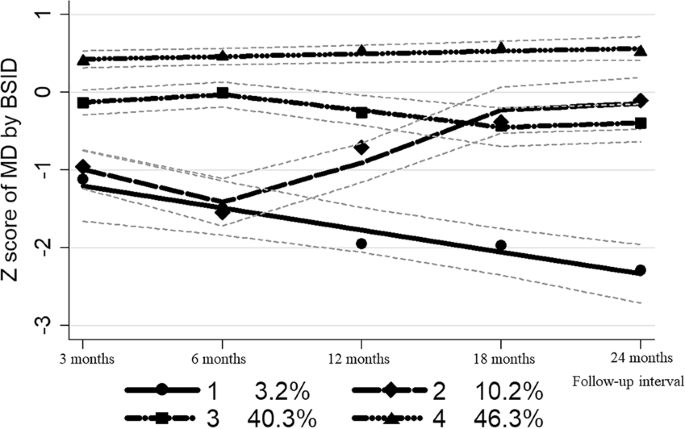
subgroups: (1) “Subgroup 1: Start below average-then decrease” (3.2% of all participants), (2) “Subgroup 2: Start below average-then increase” (10.2%), (3) “Subgroup 3: Consistently
average” (40.3%), (4) “Subgroup 4: Consistently above average” (46.3%). The fit indexes are presented in Supplementary Table S2 and Fig. 1 graphically presents the final trajectories of
cognitive test z scores during the first two years of life. PREDICTORS OF CHILD DEVELOPMENT TRAJECTORY GROUP We then compared the distribution of socioeconomic, pregnancy and birth outcome
characteristics between the trajectory groups (Supplementary Table S3 and Table 2).We determined that children born to mothers with increasing educational level were less likely to be in the
groups that started below average and then declined (RR 0.27, 95% CI 0.16, 0.46), started below average and then improved (RR 0.59, 95% CI 0.41, 0.85) and that performed consistently
average (RR 0.76, 95% CI 0.61, 0.94) than being in the group that performed consistently above average. Greater than 180 days of multiple micronutrients supplementation relative to folic
acid or folic acid plus iron supplementation <180 days during pregnancy was associated with decreased risk of being in group of children who started below average and then improved (RR
0.37, 95% CI 0.16, 0.90) and group of children who performed consistently average (RR 0.63, 95% CI 0.44, 0.89) compared to the group of children performing consistently above average.
Infants who were born low birth weight were 10.60 (95% CI 3.57, 31.49) times more likely to be in group of children who started below and then declined as compared to those who performed
consistently above average. We also examined these associations between Subgroup 1 and 2, and between Subgroup 3 and 4, respectively, and similar predictors were observed (Supplementary
Table S4). RELATIONSHIPS OF DEVELOPMENT TRAJECTORY GROUP WITH MIDDLE CHILDHOOD AND ADOLESCENT DEVELOPMENT OUTCOMES As shown in Table 3, the children who performed consistently above average
during the first 2 years of life had persistently higher test scores in middle childhood and early adolescence. Infants that were consistently above average during the first two years had
8.02 (95% CI 1.46, 14.59) and 2.52 (95% CI 0.62, 4.41) points higher cognitive test scores in adolescence as compared to those who started below average and then declined or those who were
consistently average, respectively. In the adjusted analyses (Table 3), infants in the trajectory group that started below average and then improved and the group that were consistently
average did not differ in cognitive outcomes in middle childhood or adolescence. However, the cognitive deficits of trajectory group that started below average and then declined relative to
group that started below average and then improved persisted into adolescence with an adjusted mean FISQ differences of −6.98 (95% CI −13.47, −0.49). We conducted a sensitivity analysis
using IPW to account for potential bias due to outcome censoring (loss to follow-up) and found there were no qualitative differences in our findings (Supplemental Table 5). We also examined
components of the FSIQ score and found similar associations within the VCI, WMI, PRI and PSI scores (Supplementary Tables S6 and Table S7). RELATIONSHIPS OF SINGLE-TIME POINT BSID-II SCORES
AT 12 AND 24 MONTHS WITH MIDDLE CHILDHOOD AND ADOLESCENT DEVELOPMENT OUTCOMES We observed statistically significant, but weaker correlations magnitude of association, between BSID-II
tertiles and development outcomes in middle childhood and early adolescence (Supplementary Table S8). Young children in the highest tertile of development scores at 12 and 24 months had 2.57
(95% CI: 0.31, 4.82) and 4.67 (95% CI: 2.01, 7.33) points higher scores in early adolescence as compared to those in the lowest tertile, respectively. DISCUSSION We identified four distinct
trajectories of infant cognitive development during the first two years of life in rural China: (1) children who started below average and then declined, (2) children who started below
average and then improved, (3) children who were consistently average and (4) children who performed consistently above average. Higher maternal education and supplementing antenatal
multiple micronutrients beyond 180 days were associated with reduced risk of being in suboptimal development trajectories; while, SGA and low birth weight birth increased the risk of being
in the suboptimal groups. The developmental advantages of Subgroup 4 (consistently above average) over the other three trajectory groups persisted through middle childhood into early
adolescence. In addition, the infants from subgroup 1 (started below average-then decreased) had the lowest test scores in middle childhood and early adolescence. These findings suggest that
infant cognitive development trajectories are robust predictors of children long-term development outcomes. We used a data-driven approach with five repeated measures in generally healthy
children to identify development trajectories. One longitudinal study from Australia reported similar trajectories in aspect of language development between 8 and 48 months of age, which
used the latent class analysis to address the categorized measures13. However, to the best of our knowledge, our study is the first to assess the relationship of early life development with
later life outcomes with these methods. This method advancement shows that trajectory modeling can identify subgroups of children who share similar important longitudinal changes in
development during the first two years of life. We also found the trajectory approach provided greater contrast between infants as compared to single time-point assessment of BSID-II scores.
These subgroups are also not distinct in studies that report development trajectory as age-specific averages11,12. As a result, trajectory modeling may better capture the dynamic process of
child development and thus provide stronger associations with long-term outcomes. We identified a pronounced catch-up pattern in Subgroup 2 (started below average-then increased) that would
have been missed with a single time point assessment. In this study, the catch-up development appeared to start early in infancy and provides additional evidence that the first two years of
life are critical for development. Of note, the catch-up pattern of cognitive development was similar to that of physical growth which also generally starts early in the first months of
life14,15. In addition, our risk factor analyses suggest that a combination of prenatal nutrition, socioeconomic, and environmental factors may affect catch-up development. Although this
finding requires replication in other studies, some studies reported that improvements in exclusive breastfeeding and longer partial breastfeeding were associated with better cognitive
development outcomes later in life16,17. In addition, the identification of Subgroup 1 (started below and then declined) indicates that the trajectory modeling can be used to identify
children with high risk of occurring delayed development. As a result, assessment of cognitive development trends may provide valuable information on children’s development and long-term
outcomes. Our study was also able to explore the early determinants that underlie the heterogeneity in these trajectories, which may help develop intervention strategies to reduce the risk
of high-risk children being in suboptimal trajectories. We found that infants from high-income household wealth were more likely to be in the above average group, which is similar to a study
conducted in the UK among subjects aged 2–1618. In addition, our finding that maternal education could independently act as a beneficial factor for child optimal development was in
agreement with previous studies19,20,21. It has been proposed that higher maternal education level was associated with less maternal depression, better child nutrition status, child-rearing
environment and ability to access and benefit from interventions. As described by the early nutrition programming22, antenatal micronutrient supplementation may improve child development,
but the evidence has been inconsistent in the studies that assessed outcome at a single point23,24,25. In the present study, infants born by women who consumed multiple micronutrients for
180 days or beyond during pregnancy were less likely to be in the suboptimal trajectories. This finding extends the current knowledge on mechanisms underlying the antenatal micronutrient
supplementation and long-term outcomes, which may have been linked during early life but would not be captured using the single-time assessment. We also identified SGA or LBW as another
predictor of development trajectories. These findings are consistent with prior studies26,27. Although some studies with short periods of follow-up reported that the influence of
intrauterine growth restriction on cognitive development appeared to diminish overt time28, our finding and another study from UK and Ireland supported that impaired cognitive trajectories
set in early life might persist into adolescence and early adulthood11. One recent systematic review from South Asia also reported that LBW children (<10 ys) relative to normal children
had 5 points lower cognitive scores with a dose-response relationship29. Further, the differences among them became bigger along with the increasing age29, which was similar to the tendency
observed in Subgroup 1 trajectory, i.e., linearly declining after birth. In the present study, this Subgroup 1 trajectory (started below average and then declined) was characterized by high
risk of occurring preterm, LBW, and/or SGA (Supplementary Table S3). Hence, these findings highlight the programs that aim to reduce the risk of adverse birth outcomes before and during
pregnancy. There remains debate as to the long-term functional outcomes of cognitive tests in infancy in LMIC settings30. After using structural equation model to account for measurement
error with 3 repeated measures among 130 infants, one longitudinal investigation from US reported that infant cognitive function moderately correlated with adolescent development outcomes
with a correlation coefficient of 0.5731, which was higher than that observed in our study (0.18 for 12 months and 0.30 for 24 months, respectively; Supplementary Table S8). Taken together,
these findings suggest that using single-time assessment of infant cognitive development has limitations to predict long-term outcomes and identify high-risk children for delayed
development. In the present study, adolescents from Subgroup 4 (consistently above average) had the highest cognitive test scores, suggesting that the developmental advantages established in
early life could persist through middle childhood into early adolescence. In addition, the infants from subgroup 1 (started below average-then decreased) had the lowest test scores in
middle childhood and early adolescence. These findings suggest that infant cognitive development trajectories are strong predictors of children long-term development outcomes, and highlight
the importance of providing appropriate interventions as early as possible, which could ameliorate restricted development and have important implications for human capital and well-being
across the life course. The results in the present study should be interpreted with a few limitations. First, the follow-up rate for children in middle childhood and adolescence in the
present study was approximately 50% of the original cohort and therefore bias due to dependent censoring is possible. Nevertheless, we found minimal to no differences in background
characteristics for children who had development assessed as compared to those who did not. Besides, a sensitivity analysis using IPW to account for outcome censoring also suggested the main
study findings were robust and the risk of bias due to censoring was likely minimal. Second, the use of data-driven approach allowed us to identify distinct cognitive trajectories over age
and was appropriate for several repeated measurements of the same individuals. However, the trajectory modelling approach shares inherent limitations including that extracting the optimal
number of subgroups, which is a process guided by statistical fit indices and some degree of investigators’ decision, and that the size of each trajectory was produced by the model that may
result in small sizes and consequently limited power to further analysis. Third, the cognitive development trajectories that we identified in our study population in rural China may not be
directly applicable to other settings. Finally, the underlying biological mechanisms between these predictors and cognitive trajectories cannot be examined in the present study. In summary,
we identified groups of distinct trajectories of cognitive development during the first two years of life in rural China. Prospectively, we found that these trajectory groups robustly
predicted development scores through middle childhood into adolescence. In addition, our risk factor analyses indicated that integrated of nutritional, environmental, and educational
interventions during the first 1,000 days of life may affect early life cognitive development trajectories and produce long-term effects on development and human capital across the life
course. METHODS PARTICIPANTS We used data from a prospective birth cohort of children born to women who participated in a randomized, double-blind trial of antenatal micronutrient
supplementation in rural western China. Children were followed in early childhood (age 3 to 30 months), middle childhood (age 7–9 years) and early adolescence (age 10–12 years). Details and
procedures of the trial and follow-up studies have been described elsewhere23,24,25,32. Briefly, all pregnant women across villages from two counties were randomized to take a daily capsule
of either folic acid, folic acid plus iron, or multiple micronutrients between August 2002 and February 2006. In the trial 4604 singleton births occurred, and 1400 births born in 2004–2006
were enrolled in long-term follow-up cohort. A total of 1388 was enrolled after excluding deaths (n = 3), birth defects (n = 7) and disabled parents (n = 2). Among them, 660 were followed at
7–9 years of age between October 2012 and September 2013, and 735 at 10–12 years of age between June 2016 and December 2016 for cognitive assessment. ASSESSMENTS OF COGNITIVE DEVELOPMENT At
the 3, 6, 12, 18, 24- and 30-month visit, mental development (MD) was assessed using a culturally appropriate, and locally validated Chinese version of Bayley Scales of Infant Development
(BSID-II)33. MD raw scores were transformed into age-standardized scores based on the data for infants in US34. In middle childhood and adolescence, we used the Wechsler Intelligence Scale
for Children, Fourth Edition (WISC-IV) to assess cognitive development35. According to Chinese norms of WISC-IV with satisfied reliability and validity, age-standardized full-scale
intelligence quotient (FSIQ), representing the general cognitive development, and aspects of verbal comprehension (VCI), perceptual reasoning (PRI), working memory (WMI), and processing
speed index (PSI) were derived36. Cognitive tests were standardly administered by public health graduates at subjects’ own home, local school or hospital meeting room that were free of
distractions. Field staff administering these cognitive tests were unaware of the socioeconomic background, randomized treatment allocation, birth outcomes or other health status of
participants. COVARIATES Information on socioeconomic status (parental age, occupation, education and household wealth), maternal nutrition status before pregnancy (mid-upper arm
circumference), randomized regimen (folic acid, folic acid plus iron, and multiple micronutrients), maternal parity and birth outcomes (preterm birth, low birth weight [LBW], small for
gestational age [SGA] birth and infant sex) was collected as part of the original trial using standard questionnaire, methods and/or procedures. These details are documented elsewhere23. A
wealth index was established from an inventory of 16 household assets or facilities by principal component analysis, which was then classified into thirds as an indicator of low-, middle-
and high-income households37. Preterm birth was defined as babies born alive before 37 weeks’ gestational age, and low birth weight was defined as a birth weight of less than 2500 g, as per
the World Health Organization (WHO) guidelines38,39. According to Intergrowth standards, SGA birth was defined as birth weight below the 10th percentile of weight-for-age and sex40. Given
our prior findings that multiple micronutrient supplementation could significantly improve cognitive development with the largest benefits observed with supplementation of at least 180
days25, we combined the randomized treatment regimens and duration into a categorical variable, i.e., folic acid or iron/folic acid lasting for <180 days (as reference), iron/folic acid
lasting for ≥180 days, multiple micronutrients lasting for <180 days, and multiple micronutrients lasting for ≥180 days. STATISTICAL ANALYSIS To increase comparability across ages, we
transformed the age-standardized cognitive test scores into z-scores based on age-specific medians and SD within the sample. We then used a group‐based trajectory modelling, specifically the
“traj” macro in Stata, to identify infant cognitive z-score developmental trajectories across 3, 6, 12, 18, and 24 months of age41,42,43. GBTM can identify subgroups of individuals who
share similar patterns of development42,43, and has been used to identify distinct trajectories of body composition and body mass index (BMI) that are associated with risks for obesity,
asthma, morbidity and mortality later in life44,45,46,47,48,49,50,51. Models with two or more subgroups were compared to identify the optimal number of subgroups and shapes that best
characterized the data, with maximum likelihood estimation accounting for missing z scores at any time point. The final model was selected based on general recommendations including: (i)
tests for parameter estimates for linear, quadratic and cubic terms, (ii) Bayesian and Akaike information criterion value, (ii) average of the posterior probabilities of group membership for
individuals assigned to each group, (iv) odds of correct classification based on the posterior probabilities of group membership, and (v) minimizing overlap in confidence intervals (CIs)
while summarizing the distinctive features of the data as parsimonious as possible43. Once the most appropriate trajectories were derived, the subgroup categories variable was used in all
subsequent analyses. Baseline characteristics by the trajectory groups were compared using Chi-squared tests or analysis of variance, and multivariate multinomial logistic regression models.
We then used generalized estimating equations with an independent correlation structure to assess the relationship of the trajectory groups with cognitive development outcomes at 30 months,
middle childhood and early adolescence. To hand the missing data of cognitive outcome in middle childhood and early adolescence and examine its potential to influence the results, we
applied the inverse probability weighting (IPW)52. We also conducted analyses using single-time point Bayley scores at 12 and 24 months to compare the magnitude of association for the GBTM
approach. Age-standardized FSIQ and VCI, WMI, PRI, and PSI scores were taken as the primary and secondary outcomes, respectively. All statistical analyses were performed using Stata 12.0
(Stata Corp, College Station, Texas, USA). ETHICAL APPROVAL The protocols of the original trial and all follow-up studies conformed to the ethical principles of the 1964 Declaration of
Helsinki, and were approved by UNICEF and the ethics committee of Xi’an Jiaotong University Health Science Center. Informed written content from pregnant women, and parents/caregivers, and
oral consent from children were obtained. DATA AVAILABILITY All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
REFERENCES * Black, M. M. _et al_. Early childhood development coming of age: Science through the life course. _Lancet._ 389, 77–90 (2017). Article PubMed Google Scholar * Wadhwa, P. D.,
Buss, C., Entringer, S. & Swanson, J. M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. _Semin Reprod Med._ 27,
358–368 (2009). Article CAS PubMed PubMed Central Google Scholar * Peter, D. G., Tatjana, B. & Mark, A. H. The Developmental Origins of Health and Disease (DOHaD) Concept: Past,
Present, and Future. _The Epigenome and Developmental Origins of Health and Disease_, https://doi.org/10.1016/B978-0-12-801383-0.00001-3 (2016). Google Scholar * Moody, L., Chen, H. &
Pan, Y. Early-life nutritional programming of cognition—the fundamental role of epigenetic mechanisms in mediating the relation between early-life environment and learning and memory
process. _Adv Nutr._ 8, 337–350 (2017). Article CAS PubMed PubMed Central Google Scholar * Deary, I. J., Whiteman, M. C., Starr, J. M., Whalley, L. J. & Fox, H. C. The Impact of
childhood intelligence on later life: Following up the Scottish mental surveys of 1932 and 1947. _J Pers Soc Psychol._ 86, 130–147 (2004). Article PubMed Google Scholar * McGurn, B.,
Deary, I. J. & Starr, J. M. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. _Neurology._ 71, 1051–1056 (2008). Article PubMed Google Scholar *
Gale, C. R., Deary, I. J., Schoon, I. & Batty, G. D. IQ in childhood and vegetarianism in adulthood: 1970 British cohort study. _BMJ._ 334, 245,
https://doi.org/10.1136/bmj.39030.675069.55 (2007). Article PubMed Google Scholar * Calvin, C. M. _et al_. Intelligence in youth and all-cause-mortality: Systematic review with
meta-analysis. _Int J Epidemiol._ 40, 626–644 (2011). Article PubMed Google Scholar * Walker, S. P. _et al_. Child development: Risk factors for adverse outcomes in developing countries.
_Lancet._ 369, 145–157 (2007). Article PubMed Google Scholar * Walker, S. P. _et al_. Inequality in early childhood: Risk and protective factors for early child development. _Lancet._
378, 1325–1338 (2011). Article PubMed Google Scholar * Linsell, L. _et al_. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: A
prospective, population-based cohort study. _Arch Dis Child._ 103, 363–370 (2018). Article PubMed Google Scholar * Luu, T. M. _et al_. Trajectories of receptive language development from
3 to 12 years of age for very preterm children. _Pediatrics._ 124, 333–341 (2009). Article PubMed Google Scholar * Ukoumunne, O. C. _et al_. Profiles of language development in pre-school
children: A longitudinal latent class analysis of data from the Early Language in Victoria Study. _Child: Care, Health Dev._ 38, 341–349 (2012). CAS Google Scholar * de Wit, C. C., Sas,
T. C., Wit, J. M. & Cutfield, W. S. Patterns of catch-up growth. _J Pediatr._ 162, 415–420 (2013). Article PubMed Google Scholar * Fitzhardinge, P. M. & Steven, E. M. The
small-for-date infant I. Later growth patterns. _Pediatrics._ 49, 671–681 (1972). CAS PubMed Google Scholar * Victora, C. G. _et al_. Association between breastfeeding and intelligence,
educational attainment, and income at 30 years of age: A prospective birth cohort study from Brazil. _Lancet Glob Health._ 3, e199–e205 (2015). Article PubMed PubMed Central Google
Scholar * Rochat, T. J. _et al_. Exclusive breastfeeding and cognition, executive function, and behavioural disorders in primary school-aged children in rural South Africa: A cohort
analysis. _PloS Med._ 13, e1002044, https://doi.org/10.1371/journal.pmed (2016). Article PubMed PubMed Central Google Scholar * von Stumm, S. & Plomin, R. Socioeconomic status and
the growth of intelligence from infancy through adolescence. _Intelligence._ 48, 30–36 (2015). Article Google Scholar * Wong, H. S. & Edwards, P. Nature or nurture: A systematic review
of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. _Matern Child Hlth J._ 17, 1689–1700 (2013). Article Google Scholar * Patra,
K., Greene, M. M., Patel, A. L. & Meier, P. Maternal education level predicts cognitive, language, and motor outcome in preterm infants in the second year of life. _Am J Perinatol._ 33,
738–744 (2016). Article PubMed PubMed Central Google Scholar * Harding, J. F. Increases in maternal education and low-income children’s cognitive and behavioral outcomes. _Dev Psychol._
51, 583–599 (2015). Article PubMed Google Scholar * Koletzko, B. _et al_. Long-term health impact of early nutrition: The power of programming. _Ann Nutr Metab._ 70, 161–169 (2017).
Article CAS PubMed Google Scholar * Zeng, L. _et al_. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural
western China: Double blind cluster randomised controlled trial. _BMJ._ 337, a2001, https://doi.org/10.1136/bmj.a2001 (2008). Article PubMed PubMed Central Google Scholar * Li, C. _et
al_. Prenatal micronutrient supplementation is not associated with intellectual development of young school-aged children. _J Nutr._ 145, 1844–1849 (2015). Article CAS PubMed Google
Scholar * Zhu, Z. _et al_. Association of antenatal micronutrient supplementation with adolescent intellectual development in rural western China: 14-Year follow-up from a randomized
clinical trial. _JAMA Pediatr._ 172, 832–841 (2018). Article PubMed PubMed Central Google Scholar * Farajdokht, F. _et al_. Very low birth weight is associated with brain structure
abnormalities and cognitive function impairments: A systematic review. _Brain Cogn._ 118, 80–89 (2017). Article PubMed Google Scholar * McKean, C. _et al_. Subgroups in language
trajectories from 4 to 11 years: The nature and predictors of stable, improving and decreasing language trajectory groups. _J Child Psychol Psyc._ 58, 1081–1091 (2017). Article Google
Scholar * Linsell, L., Malouf, R., Morris, J., Kurinczuk, J. J. & Marlow, N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth
weight: A systematic review. _JAMA Pediatr._ 169, 1162–1172 (2015). Article PubMed PubMed Central Google Scholar * Upadhyay, R. P. _et al_. Cognitive and motor outcomes in children born
low birth weight: A systematic review and meta-analysis of studies from South Asia. _BMC Pediatr._ 19, 35, https://doi.org/10.1186/s12887-019-1408-8 (2019). Article PubMed PubMed Central
Google Scholar * Mrozek-Budzyn, D., Kieltyka, M. A. & Majewska, R. Validity and clinical utility of children development assessment using milestones reported by mothers. _Przegl
Epidemiol._ 68(71–75), 153–155 (2014). Google Scholar * Yu, H., McCoach, D. B., Gottfried, A. W. & Gottfried, A. E. Stability of intelligence from infancy through adolescence: An
autoregressive latent variable model. _Intelligence._ 69, 8–15 (2018). Article Google Scholar * Li, Q. _et al_. Effects of maternal multimicronutrient supplementation on the mental
development of infants in rural western China: Follow-up evaluation of a double-blind, randomized, controlled trial. _Pediatrics._ 123, e685–e692 (2009). Article PubMed Google Scholar *
Huang, H. T. & Zhang, S. D. Standardization of bayley scales of infant development in Shanghai. _Chin J Child Health._ 1, 158–160 (1993). Google Scholar * Bayley, N. Bayley Scales of
Infant Development (Second ed.) (San Antonio,TX: Psychological Corp, 1993). * Wechsler D. The Wechsler Intelligence Scale for Children (Fourth ed.) (London: Pearson, 2004). * Chen, H.,
Keith, T. Z., Weiss, L., Zhu, J. & Li, Y. Testing for multigroup invariance of second-order WISC-IV structure across China, Hong Kong, Macau, and Taiwan. _Pers Indiv Differ._ 49, 677–682
(2010). Article Google Scholar * Filmer, D. & Pritchett, L. H. Estimating wealth effects without expenditure data or tears: An application to educational enrollments in states of
India. _Demography._ 38, 115–132 (2001). CAS PubMed Google Scholar * WHO. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of
a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. _Acta Obstet Gynecol Scand._ 56, 247–253 (1977). Google Scholar * WHO.
International statistical classification of diseases and related health problems, eleventh revision (ICD-11), https://icd.who.int/browse11/l-m/en (2018). * Villar, J. _et al_. International
standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. _Lancet._ 384, 857–868 (2014).
Article PubMed Google Scholar * Jones, B. L. & Nagin, D. S. A note on a stata plugin for estimating group-based trajectory models. _Sociol Method Res._ 42, 608–613 (2013). Article
MathSciNet Google Scholar * Nagin, D. S., Jones, B. L., Passos, V. L. & Tremblay, R. E. Group-based multi-trajectory modeling. _Stat Methods Med Res._ 27, 2015–2023 (2016). Article
MathSciNet PubMed Google Scholar * Nagin, D. S. & Odgers, C. L. Group-based trajectory modeling in clinical research. _Annu Rev Clin Psychol._ 6, 109–138 (2010). Article PubMed
Google Scholar * Ziyab, A. H., Karmaus, W., Kurukulaaratchy, R. J., Zhang, H. & Arshad, S. H. Developmental trajectories of Body Mass Index from infancy to 18 years of age: Prenatal
determinants and health consequences. _J Epidemiol Community Health._ 68, 934–941 (2014). Article PubMed Google Scholar * Pryor, L. E. _et al_. Developmental trajectories of body mass
index in early childhood and their risk factors: An 8-year longitudinal study. _Arch Pediatr Adolesc Med._ 165, 906–912 (2011). Article PubMed Google Scholar * Garden, F. L., Marks, G.
B., Simpson, J. M. & Webb, K. L. Body Mass Index (BMI) trajectories from birth to 11.5 years: Relation to Early Life Food Intake. _Nutrients._ 4, 1382–1398 (2012). Article PubMed
PubMed Central Google Scholar * Andersen, G. S. _et al_. Body composition growth patterns in early infancy: A latent class trajectory analysis of the ethiopian iABC birth cohort.
_Obesity._ 26, 1225–1233 (2018). Article PubMed Google Scholar * Liu, J. _et al_. Body mass index trajectories during the first year of life and their determining factors. _Am J Hum
Biol._ 31, e23188, https://doi.org/10.1002/ajhb.23188 (2019). Article PubMed Google Scholar * Liu, J. X. _et al_. Body mass index trajectories during infancy and pediatric obesity at 6
years. _Ann Epidemiol._ 27, 708–715 (2017). Article PubMed Google Scholar * Eny, K. M. _et al_. Breastfeeding duration, maternal body mass index, and birth weight are associated with
differences in body mass index growth trajectories in early childhood. _Am J Clin Nutr._ 107, 584–592 (2018). Article PubMed Google Scholar * Aris, I. M. _et al_. Pre-, perinatal, and
parental predictors of body mass index trajectory milestones. _J Pediatr._ 201, 69–77 (2018). Article PubMed PubMed Central Google Scholar * Seaman, S. R. & White, I. R. Review of
inverse probability weighting for dealing with missing data. _Stat Methods Med Res._ 22, 278–95 (2013). Article MathSciNet PubMed Google Scholar Download references ACKNOWLEDGEMENTS The
field work was supported by the National Natural Science Foundation of China (Grant Number 81872633, Lingxia Zeng) and National Key Research and Development Program of China (Grant Number
2017YFC0907200 and 2017YFC0907201, Hong Yan). This study was also supported by the China Scholarship Council (Grant Number 201806280188, Zhonghai Zhu). We also thank Nandita Perumal, PhD
MPH, from the Department of Global Health and Population, Harvard TH Chan School of Public Health for helpful comments and suggestions on an earlier version of the manuscript. AUTHOR
INFORMATION Author notes * These authors contributed equally: Zhonghai Zhu and Suying Chang. AUTHORS AND AFFILIATIONS * Department of Epidemiology and Biostatistics, School of Public Health,
Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, 710061, P.R. China Zhonghai Zhu, Qi Qi, Shaoru Li, Mohamed Elhoumed, Hong Yan & Lingxia Zeng * Department of Global
Health and Population, Harvard T.H. Chan School of Public Health, Boston, MA, USA Zhonghai Zhu, Wafaie W. Fawzi & Christopher R. Sudfeld * United Nations Children’s Fund, China Office,
Beijing, 100600, P.R. China Suying Chang * Department of Nutrition and Food Safety Research, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, Shaanxi, 710061,
P.R. China Yue Cheng * Nutrition and Food Safety Engineering Research Center of Shaanxi Province, Xi’an, 710061, Shaanxi, China Hong Yan * Key Laboratory of Environment and Genes Related to
Diseases, Xi’an Jiaotong University, Ministry of Education, Xi’an, 710061, Shaanxi, China Hong Yan & Lingxia Zeng * School of Public Health, University of Sydney, Sydney, New South
Wales, Australia Michael J. Dibley Authors * Zhonghai Zhu View author publications You can also search for this author inPubMed Google Scholar * Suying Chang View author publications You can
also search for this author inPubMed Google Scholar * Yue Cheng View author publications You can also search for this author inPubMed Google Scholar * Qi Qi View author publications You can
also search for this author inPubMed Google Scholar * Shaoru Li View author publications You can also search for this author inPubMed Google Scholar * Mohamed Elhoumed View author
publications You can also search for this author inPubMed Google Scholar * Hong Yan View author publications You can also search for this author inPubMed Google Scholar * Michael J. Dibley
View author publications You can also search for this author inPubMed Google Scholar * Wafaie W. Fawzi View author publications You can also search for this author inPubMed Google Scholar *
Lingxia Zeng View author publications You can also search for this author inPubMed Google Scholar * Christopher R. Sudfeld View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS Z.Z., S.C., Y.C., H.Y., M.J.D. and L.Z.: planned and designed the study; Z.Z., Q.Q., S.L. and M.E.: conducted the study; Z.Z., S.C., C.R.S. and W.W.F.:
analyzed data and interpreted results; Z.Z.: wrote the paper; L.Z.: had primary responsibility for final content; and all authors: reviewed, revised and approved the final paper.
CORRESPONDING AUTHOR Correspondence to Lingxia Zeng. ETHICS DECLARATIONS COMPETING INTERESTS Dr. Suying Chang is a nutrition specialist of UNICEF China Office. The other authors have no
conflicts of interest relevant to this article to disclose. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY ONLINE CONTENT RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhu, Z., Chang, S., Cheng, Y. _et al._ Early life cognitive development
trajectories and intelligence quotient in middle childhood and early adolescence in rural western China. _Sci Rep_ 9, 18315 (2019). https://doi.org/10.1038/s41598-019-54755-1 Download
citation * Received: 19 July 2019 * Accepted: 19 November 2019 * Published: 04 December 2019 * DOI: https://doi.org/10.1038/s41598-019-54755-1 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative