Enhancing the corrosion resistance of reinforcing steel under aggressive operational conditions using behentrimonium chloride
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Aggressive operational conditions e.g. saline media and acidic gases, e.g., CO2 can increase the corrosion rate of reinforcing steel. Accordingly, the necessity to protect the steel
under the above conditions without affecting the mechanical properties of the concrete is growing. Herein, the inhibition efficiency of a new corrosion inhibitor, behentrimonium chloride
(BTC, C25H54ClN), is explored in a simulated-concrete pore solution (SCP) with 3.5 wt.% NaCl at different pH using electrochemical impedance spectroscopy (EIS) and polarization methods.
Using only a 50 μmol L−1 of BTC, we are able to measure an inhibition efficiency of 91, 79, and 71% in SCP solution with 3.5% NaCl at pH of 12.5, 10 and 7, respectively without showing any
effect on the mechanical properties on the cured mortars. Temkin isotherm is used to describe the physisorption of BTC inhibitor on the steel surface. Also, the adsorption and influence of
the inhibitor on the metal surface are characterized using the scanning electron microscopy, atomic force microscopy, and X-ray photoelectron spectroscopy. In conclusion, this new inhibitor
shows high corrosion inhibition efficiencies under different aggressive conditions and can be used in concrete to reduce the corrosion rate of reinforcing steel without decreasing the
mechanical properties of the concrete. SIMILAR CONTENT BEING VIEWED BY OTHERS SYNTHESIS AND APPLICATIONS OF NOVEL SCHIFF BASE DERIVATIVES AS CORROSION INHIBITORS AND ADDITIVES FOR
IMPROVEMENT OF REINFORCED CONCRETE Article Open access 12 September 2023 AN EFFICIENT GREEN IONIC LIQUID FOR THE CORROSION INHIBITION OF REINFORCEMENT STEEL IN NEUTRAL AND ALKALINE HIGHLY
SALINE SIMULATED CONCRETE PORE SOLUTIONS Article Open access 03 September 2020 SYNERGISTIC INHIBITION EFFECT OF _CHLORELLA_ SP. AND BENZOTRIAZOLE ON THE CORROSION OF Q235 CARBON STEEL IN
ALKALINE ARTIFICIAL SEAWATER Article Open access 19 October 2024 INTRODUCTION Corrosion mitigation has attained a vast interest due to the high economic impact of replacing the damaged parts
with new ones especially in reinforced concrete structures1. It is well-known that once corrosion starts in reinforcing steel, the rust (corrosion product) occupies two to three times more
volume than the un-corroded steel. This higher volume induces pressures around the reinforcing bar and causes cracking of the surrounding concrete. In general, a concrete solution has an
alkaline nature (pH ~ 13) owing to the existence of sodium oxide (Na2O), and potassium oxide (K2O) in addition to calcium hydroxide as a result of the hydration reaction of calcium silicate
hydrate in cement (CSH) with water from the surrounding environment2,3,4,5,6,7. Accordingly, an oxide layer is existing on the reinforcing steel surface within concrete8,9,10,11. However,
penetrations of aggressive anions like chloride (Cl−) and sulfate (SO42−) ions can lead to localized damage of the passive film which increases the corrosion rate of steel1,8,10. A corrosion
current density (_i_corr), of around 0.2 μA cm−2, indicates active corrosion12, 0.1 μA cm−2, is safe for typical design life requirements of reinforced concrete structures13, while _i_corr,
less than 0.01 μA cm−2, is low enough to avoid corrosion-induced cracking indefinitely14. Consequently, the inhibitors to be used in simulated concrete pore solution should satisfy two
conditions; (i) a high inhibition efficiency in the existence of destructive ions, e.g. Cl− ions, at different pH values (from 7 to 12.5) and (ii) no influence on the mechanical attributes
of the concrete8,15,16. Abd El Haleem _et al_.9, used different inorganic inhibitors in saturated calcium hydroxide. The outcomes pointed out that the inhibition efficiency (_IE_%), of the
inhibitors, improved in the following order MoO4−2 > WO4−2 > HPO4−2 > CrO4−2. However, the disadvantages of using inorganic inhibitors in concrete environments are their toxicity to
living beings, cost, and inefficiency for localized corrosion8,17. Ormellese _et al_.1, has studied the long-term inhibition effectiveness of over 80 organic compounds from three main
categories: amines and alkanoamines, amino alcohols, and carboxylates in SCP solution containing 0.01 M NaOH at pHΣ12.6 in the absence of chloride ions. The results showed an increase in the
effectiveness of the inhibitors in the following order: carboxylates > amino acids > amines and alkanolamines. Abd El Haleem _et al_.10,15, highlighted the influence of benzotriazole
(C6H5N3) and its derivatives, 5-nitrobenzotriazole (C6H4N4O2) and 5-chlorobenzotriazole (C6H4ClN3), on reinforcing steel inhibition in an SCP solution containing 1 M NaCl. It was found that
the _IE_% of the explored inhibitors dwindled accordingly: 5-chlorobenzotriazole ˃ benzotriazole ˃ 5-nitrobenzotriazole. The maximum attained _IE_% was 69% in the presence of 5×10−4 M
5-chlorobenzotriazole. Interestingly, 0.0025% of deoxyribonucleic acid (DNA), showed _IE_% of 94% in SCP solution containing 3.5 wt. % NaCl with an increase of 3.61% in the compressive
strength (Fc), after 28 days18. Zhang _et al_.19, achieved an inhibition efficiency of 83.15% using maize gluten meal extract as an ecologically friendly inhibitor for reinforcing steel in
SPC containing 3.5 wt.%NaCl. The synthesized inhibitor of 4-(1-(4-methoxyphenyl) cyclohexyl)phenyl 9-oxodecanoate (MPOD) by Unnisa _et al_.20, exhibited and _IE_% of 71.81 in SCP solution
including 0.5 M NaCl. On the other hand, the chemi-physisorped polymethacrylic acid co-acrylamide corrosion inhibitor displayed an _IE%_ of 92.35 in SCP containing 1.8 wt.% chlorides21.
Shanmugapriya _et al_.22, achieved an _IE_% of 98 in SCP using an aqueous extract of turmeric. Anitha _et al_.23, used the extract of rosa damascene leaves as nature-friendly inhibitor in
SCP which achieved an _IE_% of 82. Wang _et al_.24, found that using 0.0008 mol L−1 of calcium lignosulfonate (CLS) showed a high _IE_% of 93.7 after immersion of carbon steel in SCP for
7200 h in comparsion to sodium oleate (SO), that exhibited an _IE_% of 40–60. Cao _et al_.25 explored the inhibition behaiour of phytic acid in carbonated concrete pore solution containing
0.6 mol L−1 NaCl on 20SiMn steel, which displayed an _IE%_ of 84.0 after immersion for 72 h. Asaad _et al_.26, prepared non- poisonous corrosion inhibitor of silver nano-particles doped palm
oil leaf extracts for renforcing steel in salin water. It was found that the addition of silver nanoparticles in the green inhibitor lead to increase the _IE%_ to 94.7 after immersion for
365 d, owing to the presence of excess calcium silicate hydrate and the enhancement of the pore construction and therefore decrease the conductivity of the pore solution. In this work, the
effectiveness of a new inhibitor (behentrimonium chloride, C25H54ClN) for the corrosion of reinforcing steel in highly saline SPCs at ambient temperature and different pH values is explored.
Behentrimonium chloride (BTC) is commonly used in hundreds of personal care products as conditioning and anti-static agents. Interestingly, Cameron _et al_.27, found that BTC is
biologically safe for humans when used in a concentration range up to 5%. However, the European Union recently restricted its use for more than ≥ 1%. Consequently, we, for the first time,
report the use of BTC as a corrosion inhibitor for reinforcing steel in saline SPC solutions of different pH values at significantly low concentrations of 2.5, 5, 10, and 20 ppm using
electrochemical and surface analysis techniques. EXPERIMENTAL The reinforcing steel samples were abraded by silicon carbide grit papers using a grinding machine (Jean Wirts TG 200, Germany),
sonicated with acetone, rinsed by deionized water and after that desiccate in air. The mild steel rebar contains (wt.%) C = 0.128, Si = 0.25, Mn = 0.7, Cu = 0.15, P = 0.04, S = 0.03, and
rest was Fe. Saturated calcium hydroxide (Ca(OH)2), were used as an electrolyte to mimic SCP in 3.5 wt.% NaCl. NaCl was purchased from Sigma Aldrich and Ca(OH)2 from Riedel-de Haën. The pH
of the SCP solution under investigation was 12.5 for the saturated Ca(OH)2, 10 or 7. The pH was reduced by addition of NaHCO3 powder to the SCP28. The electrochemical measurements were done
at ambient temperatures using a GAMRY 3000 potentiostat/galvanostat/ZRA (Warminster, PA, USA). EIS measurements were investigated in a frequency range of 100 kHz to 0.01 Hz with an AC
amplitude of 5 mV. In all electrochemical measurements, a saturated calomel electrode (SCE) and a graphite rod were employed as a reference and counter electrodes, respectively. The mild
steel samples with surface areas of 0.765 cm2 were subjected to the SCP. All the mild steel coupons were sited under open circuit (OCP), conditions for 30 min before initiating any
electrochemical test to attain the steady-state conditions. Polarization curves were attained from −0.25 to + 0.25 V against the open circuit potential (OCP), with a scan rate of 0.167 mV
s−1. Various concentrations (0, 2.5, 5, 10, and 20 ppm) of BTC (molecular weight = 404.164 g mol−1), which are equivalent to 12, 24, 37 and 50 μmol L−1, respectively, were synthesized in the
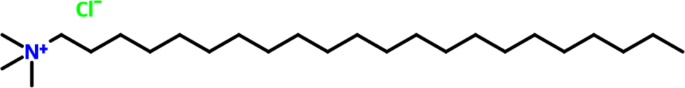
simulated saline SCP solutions. The BTC inhibitor was attained from Shanghai Dejun Chemical Technology Co., Ltd, Shanghai China, and its chemical formula is displayed in Fig. 1. Each
electrochemical measurement was repeated three times to confirm the reproducibility, and the average values were reported. MECHANICAL CHARACTERIZATION The effect of BTC inhibitor on the
compressive and flexural strength of the mortars prepared according to the ASTM C109/C109M and ASTM C348, respectively, was evaluated after different exposure times in the existence and
absence of the BTC corrosion inhibitor29,30. The compressive experiments were performed using a 300 KN Tecnotest 3 compression testing machine (Tecnotest, Modena, Italy). The flexural
strength experiments were utilized by a Lloyd LR 50 K universal testing machine (Ametec Inc. USA). The results are obtained by averaging three repeated tests. The mortar with 50 µmol L−1 of
BTC was prepared by the mechanical mixing, in a stainless steel mixer, one part mass of cement and one and a half part mass of standard sand, with a water/cement ratio of 0.48531. Then, the
mold was filled with the mixture under vibration to release air bubbles, and thereafter stored in a moist atmosphere for 2 days. After that, the demolding of the prepared specimens was
conducted, and the samples were kept under tap water over the test period32. The cured mortar samples were removed from the water and located in a drying oven at 60 °C for 24 h before the
strength test in order to shun the impact of the hydration of the concrete and to increase the strength of the measured samples33. RESULTS AND DISCUSSION EIS Figures 2 and 3 display the Bode
and Nyquist plots, respectively, for the reinforcing steel in SCP solutions containing 3.5 wt.%NaCl and BTC inhibitor concentrations of 12, 24, 37, 50 μmol L−1 at pH values of 12.5, 10 and
7 within a frequency range from 0.01 Hz to 100 k Hz at an Ac amplitude of 5 mV. Figure 4 exhibits the equivalent circuit (EC) utilized to fit the measured EIS data to obtain the different
parameters that explain the metal/solution interface. The parameters are listed in Table 1 in which _R_s and _R_ct, are credited to the electrolyte resistance and the charge transfer
resistance, respectively. However, the constant phase element is expressed by (_CPE_), which is used for a non-ideal double layer. The imperfectness behavior of the double layer is
accredited to the following parameters (i) a non-uniform surface coverage, (ii) surface roughness, and (iii) nonuniform current distribution or corrosion rate. The admittance and impedance
of the CPE is given by34,35: $$1/{Z}_{{\rm{CPE}}}={Y}_{{\rm{p}}}{({\rm{j}}\omega )}^{{\rm{n}}}$$ (1) where _Z_CPE is the CPE impedance (Ω cm−2); _Y_p is the numerical value of the admittance
1/│_Z_│, at _ω_ = 1 (rad s−1) and _j_2 = −1. _ω_ is the angular frequency and _n_ is the deviation element which varies from 0 and 1. When _n_ = 1 or 0, _Z_CPE is corresponding to an ideal
capacitor or resistor, respectively. The influence of the thickness and dielectric constant of the double layer is defined by the Helmholtz regime, given by the following formula:
$${C}_{{\rm{dl}}}=\frac{\varepsilon {\varepsilon }_{{\rm{o}}}\,A}{\delta }$$ (2) where _Ɛ_o and _Ɛ_ are the dielectric constant of air and electrolyte (mainly water), respectively and _A_ is
the surface area of the working electrode. The inhibition efficiency (_IE_%), is calculated using Eq. 3, $$IE \% =(\frac{{R}_{{\rm{ct}}1}-{R}_{{\rm{ct}}2}}{{R}_{{\rm{ct}}1}})\times 100$$
(3) Table 1 exhibits that the higher the corrosion inhibitor concertation is, the higher the _R_ct and lower Cdl values are which indicates that the ability of Cl− ions to attack the
reinforcing steel surface declines due to the presence of a protective adsorbed layer of BTC inhibitor. It is worth to mention that lowering the pH lead to alleviating the _IE_% from 91% at
pH=12.5 to 79% and 72% at pH 10 and 7, respectively. Three reasons can justify the chloride-induced loss of passivity of the reinforcing steel. First, an induced de-passivation owing to
adsorption of Cl− ion on the passive film at potential values higher than a critical value. Second, the penetration of Cl− ions into the oxide layer leading to the formation of
chloride-contaminated oxides. Finally, a mechanical film breakdown due to Cl− ions adsorption which can attenuate the surface tension, thus leading to a localized disturbance in the
mechanical stability of the passive layer36. The polarization curves for the reinforced steel in SCP solutions containing 3.5 wt.%NaCl and BTC inhibitor concentrations of 12, 24, 37, 50 μmol
L−1 at pH values of 12.5, 10 and 7 are shown in Fig. 5. The electrochemical corrosion factors such as as the corrosion free potential (_E_corr), pitting potential (_E_pit), corrosion
current density (_i_corr), the polarization resistance, (_R_p), cathodic and anodic Tafel slopes (_b_c and _b_a, respectively), the corrosion inhibition efficiency (_IE_%) and the surface
coverage area (_θ_), are calculated from Fig. 5, and recorded in Table 2. Additionally, the passive potential window is calculated using the following formula: $${E}_{pit}-{E}_{corr}$$ (4)
The corrosion inhibition efficiency (_IE_%), is calculated using the following formula37, $$IE \% =(\frac{{i}_{1}-{i}_{2}}{{i}_{1}})\times 100$$ (5) where _i_1 and _i_2 are the corrosion
current densities of reinforcing steel in the absence and existence of the BTC corrosion inhibitor, respectively. The surface coverage area (_θ_) is calculated utilizing Eq. 611, $$\theta
=\frac{IE \% }{100\,}$$ (6) and the polarization resistance (_R_p), was detected using the Stern–Geary equation37.
$${R}_{{\rm{p}}}=\frac{{b}_{{\rm{c}}}\,{b}_{{\rm{a}}}}{2.303\,{i}_{{\rm{corr}}}({b}_{{\rm{c}}}+{b}_{{\rm{a}}})}$$ (7) The polarization curves show the breakdown of the passive film before
and after the addition of BTC inhibitor at pH 12.5, see Table 2. However, increasing the inhibitor concentration shifts the pitting potentials (_E_pit), towards the more noble values
indicating that the passive layer formed more stabilized by the presence of BTC inhibitor. Moreover, the passive potential window at pH 12.5 is 0.18, which increased to 0.25, 0.27, 0.32 and
0.38 by the addition of 12, 24, 37, 50 μmol L−1 of the BTC inhibitor, respectively. The attack of chloride species to the reinforcing steel surface in SCP can lead to loss of its passive
layer if the concentration of the chloride species is adequately high. For reinforcing steel in concrete, the degree to which Cl¯ ions can damage the passive layer is related to the
alkalinity of the environment. In chloride-free alkaline conditions, the passive layer on the mild steel breaks down at a potential of +560 mV SCE38. The highest attained _IE_% was 88% at 50
μmol L−1 of the BTC inhibitor at pH = 12.5. It can be seen that the values of _b_a and _b_c diminished upon the addition of the inhibitor indicating that BTC is a mixed type inhibitor. it
is noteworthy to mention that the tabulated values of the corrosion density (_i_corr), shifts towards decreases with increasing the concertation of the BTC inhibitor. However, these values
are not in accordance with reported articles in references12,39. In fact, the diffusion of chloride species (Cl−), in cementitious materials immersed in saline water is a difficult process,
which includes numerous chemical and physical interactions. Cl− ions can bound chemically or physically through the cement paste, thus reducing the segment of free Cl− species that can
diffuse easily in the concrete pore solutions. Furthermore, the internal electric field generated from the anions and cations will accelerate the ions that possess low diffusion coefficients
and decelerate the ions that have high diffusion coefficients in order to keep the electro-neutrality status40. ADSORPTION ISOTHERM In order to understand and estimate the adsorption route
of BTC on steel surface, different adsorption isotherms are checked using the measured data from the poanlarization plots e.g. Langmuir, Frumkin and Temkin isotherms using the following
equations: Langmuir $$\frac{{C}_{{\rm{inh}}}}{\theta }=\frac{1}{{K}_{{\rm{ads}}}}+{C}_{{\rm{inh}}}$$ (8) Frumkin $$log\{C\times (\frac{{\rm{\theta }}}{1-{\rm{\theta
}}})\}=2.303\,logK+2\alpha {\rm{\theta }}\,$$ (9) Temkin $$\exp (-2\alpha {\rm{\theta }})={K}_{{\rm{ads}}}C$$ (10) where θ is the surface coverage of the reinforcement steel, _C_ is the
concentration of the BTC inhibitor species, _α_ is the adsorbate interaction factor and _K_ads is the adsorption– desorption equilibrium constant. The fitting outcomes showed that BTC
inhibitor obeys Temkin isotherm, see Fig. 6. After reorganizing Eq. (10), the following expression is attained: $$\theta =\frac{1}{-2\alpha }lnc+\frac{1}{-2\alpha }ln{K}_{ads}$$ (11) It can
be deduced from Eq. (11) that both of the slope and intercept are calculated from 1/−2_α_ and (1/−2_α)lnK__ads_, respectively. Knowing the _K_ads values at various pH values, the standard
Gibbs free energy change of adsorption (_∆G_°ads) are calculated using Eq. 12. $${K}_{{\rm{ads}}}=\frac{1}{55.5}{e}^{-\frac{\Delta {G}_{{\rm{ads}}}^{{\rm{o}}}}{RT}}$$ (12) Table 3 summarizes
the values of the _α_, _K_ads and _∆G_°ads. Values of _∆G_°ads ≥ −20 kJ mol−1, showing a physisorption adsorption, while _∆G_°ads ≤ −40 kJ mol−1 depicts chemisorption adsorption reactions.
Consequently, the intermediate values of _∆G_°ads shown in Table 3 (−32, −33 and −34 kJ mol−1), usually elucidate that chemi-physisorption of BTC inhibitor occurs on the reinforcing steel at
different pH values of 12.5, 10 and 7, respectively. However, since there is no free electron pair existing in the molecular structure of the BTC inhibitor that can form coordinated
covalent bond with the vacant d-orbitals in Fe (chemisorption), therefore it is more favorable that the adsorption mechanism is a strong physisorption ratherthan a chemi-physisorption one.
Physical adsorption takes place rapidly because of weak bondings such as Van der Waal’s or electrostatic attractive forces between inhibitor species and metal surface, and is directly
influenced by the electronegativity of the inhibitor compounds. The residence time for a physically adsorbed inhibitor is short, and its interaction with the steel surface is directly
associated with the corrosion free potential of the metal corrosion with respect to the potential of zero charge. Figure 7 exhibits the SEM of the reinforcing steel coupons after immersion
in SCP including 3.5 wt.% NaCl at different pH values of 12.5, 10, 7 in the existence and absence of 50 μmol L−1 of the BTC inhibitor for 24 h. It is clear that in case of the absence of the
corrosion inhibitor, deep pits were formed and their number is suppressed as the pH of the medium increases. Nonetheless, in the existence of the corrosion inhibitor, the number and pits
size are considerably reduced at the same pH values. Moreover, the pH values before and after immersion are measured. It is found that the pH values in the absence of the corrosion
inhibitors are lowered from 12.5, 10 and 7 to 10, 8.7 and 5.7, respectively. However, in the existence of the BTC inhibitor the pH values slightly dropped from 12.5, 10 and 7 to 11.5, 9.3
and 6.3, respectively. Surface topography and surface roughness of the reinforcing steel are explored after immersion in 3.5 wt.%NaCl of variable pH values for 24 h in the existence and
absence of 50 μmol L−1 of the BTC inhibitor using AFM, as depicted in Fig. 8. It is noted that the surface roughness (_R_a), escalates as the pH alleviates in the absence of the corrosion
inhibitor, see Table 4. However, _R_a is decreased significnatly in the existence of the BTC inhibitor in the deleterious medium signifying the construction of an adsorbed protective layer
of BTC inhibitor on the metal surfaces, which retards the attack of the Cl− species The wide scan spectrum (Fig. 9) and the high resolution XPS spectra (Fig. 10) are obtained after immersing
the reinforcing steel for 24 h in SCP including 3.5 wt.% NaCl in the existence of 50 μmol L−1 of BTC corrosion inhibitor at pH 12.5. The Cl 2p spectrum is deconvoluted into two components
at BE of 199.1 and 200.7 eV which are attributed to Cl− and FeClx, respectively41, see Fig. 10a. It is noteworthy that the peak intensity of Cl− species is low; indicating that the adsorbed
BTC inhibitor lowers the adsorption affinity of the Cl− ions to the metal surface which subsequently lessens the corrosion rate. The adsorption of the BTC inhibitor on the reinforcing steel
surface is further confirmed by the analysis of C spectra, which showed the presence of CN+-R3 and (C-C & C-H) at BE of 284.5 and 287.7 eV, respectively42,43, see Fig. 10b. However, Ca
2p spectrum shown in Fig. 10c is decomposed into two bands at 347.8 eV and 351.5 eV that are credited to Ca 2P3/2 and 2P1/2 respectively, of CaO/Ca(OH)28. Figure 10d shows the appearance of
N 1 s peaks at 399.4 and 402.5 eV, characteristics of N-CH2 and N+ quaternary nitrogen, respectively43,44,45,46. This approves the adsorption of the BTC inhibitor on the reinforcing steel
surface. There is no peak for C–N–Fe bonding was observed at 397.7–398.6 eV, suggesting that BTC inhibitor was adsorbed on the reinforcing steel through physisorption47. The XPS spectrum of
O 1 s in Fig. 10e, exhibits three peaks at 530.3, 531.7 and 534.2 eV, that are credited to O2− of iron oxides, OH− of hydrous iron oxides (FeOOH), and H2O, respectively43,46. On the other
hand, Fe 2p spectra in Fig. 10f is deconvoluted into six peaks. In fact, the interpretation of Fe 2p spectra is a complex owing to the existence of iron (Fe), in variable oxidation states of
Fe°, Fe2+, Fe3+, and satellites of Fe3+ species. The (Fe 2p3/2), XPS spectra at high resolution involves four bands at 707.1 eV that is related to the metallic iron, 710.9 eV for Fe3+ of
Fe2O3/ FeOOH and 713.9 eV, which could be attributed to a mixture of (Fe2+ & Fe3+), in different forms of iron (II) oxide (FeO), iron (II) hydroxide Fe(OH)2, iron (III) hydroxide
Fe(OH)3, FeOOH, iron (III) oxide (Fe2O3), and magnetite (Fe3O4)48. The shake up phenomenon found at 716.6 and 719.8 eV is ascribed to Fe2+ and Fe3+, respectively. The spectra of the Fe 2p1/2
peaks at BE of 722.7 and 725.3 eV can be ascribed to Fe2O3 and FeO(OH), respectively49. MECHANICAL PROPERTIES Although corrosion inhibitors can protect steel against corrosion, however it
can badly affect its mechanical features50. Therefore, the mechanical characteristics of cured mortars are investigated in the presence and absence of a 20 ppm (50 μmol L−1), of the BTC
inhibitor. This is achieved through measuring the flexural and compressive strength of cement, see Table 5. It can be observed that there is almost no change in the mechanical properties of
the concrete after the addition of the inhibitor, which is attributed to the low concentration of the BTC. CONCLUSIONS A new BTC corrosion inhibitor for reinforcing steel is investigated in
3.5 wt.% NaCl at different pH values. Taflel plots indicated that BTC is a mixed type inhibitor. The BTC inhibitor showed a corrosion inhibition efficiency (IE%) of 88, 78 and 71% in 3.5
wt.% NaCl using 50 μmol L−1 at pH values of 12.5, 10 and 7, respectively, which is effective if carbonation of the concrete happens and the pH of the concrete is lowered. Based on the
adsorption isotherm calculations, BTC inhibitor showed the best fitting with Temkin isotherm. XPS results illustrate that BTC inhibitor is physisorbed on the reinforcing steel surface, which
is matching with the _∆G_°ads calculations. The surface roughness of the metal surface is significantly decreased upon the addition of BTC inhibitor as shown in the AFM results confirming a
high corrosion inhibition efficiency of BTC. Additionally, no change in the mechanical features of concrete is observed upon the addition of BTC inhibitor, which allows using it in concrete
without any reservation. DATA AVAILABILITY The raw/processed data required to reproduce these findings could not be shared at this time due to time limitations. However, it will be
available on request. REFERENCES * Ormellese, M., Lazzari, L., Goidanich, S., Fumagalli, G. & Brenna, A. A study of organic substances as inhibitors for chloride-induced corrosion in
concrete. _Corrosion Science_ 51, 2959–2968, https://doi.org/10.1016/j.corsci.2009.08.018 (2009). Article CAS Google Scholar * Diamond, S. Chloride concentrations in concrete pore
solutions resulting from calcium and sodium chloride admixtures. _Cement, concrete and aggregates_ 8, 97–102 (1986). Article CAS Google Scholar * Enevoldsen, J. N., Hansson, C. M. &
Hope, B. B. The influence of internal relative humidity on the rate of corrosion of steel embedded in concrete and mortar. _Cement and concrete research_ 24, 1373–1382 (1994). Article CAS
Google Scholar * Hussain, S. E., Al-Musallam, A. & Al-Gahtani, A. S. Factors affecting threshold chloride for reinforcement corrosion in concrete. _Cement and Concrete Research_ 25,
1543–1555 (1995). Article CAS Google Scholar * Moreno, M., Morris, W., Alvarez, M. G. & Duffó, G. S. Corrosion of reinforcing steel in simulated concrete pore solutions: effect of
carbonation and chloride content. _Corrosion Science_ 46, 2681–2699 (2004). Article CAS Google Scholar * Ghods, P., Isgor, O. B., McRae, G. & Miller, T. The effect of concrete pore
solution composition on the quality of passive oxide films on black steel reinforcement. _Cement and Concrete Composites_ 31, 2–11 (2009). Article CAS Google Scholar * Liu, Y. _et al_.
Effect of ginger extract as green inhibitor on chloride-induced corrosion of carbon steel in simulated concrete pore solutions. _Journal of Cleaner Production_ 214, 298–307,
https://doi.org/10.1016/j.jclepro.2018.12.299 (2019). Article CAS Google Scholar * Wang, Y., Zuo, Y., Zhao, X. & Zha, S. The adsorption and inhibition effect of calcium lignosulfonate
on Q235 carbon steel in simulated concrete pore solution. _Applied Surface Science_ 379, 98–110, https://doi.org/10.1016/j.apsusc.2016.04.013 (2016). Article ADS CAS Google Scholar * El
Haleem, S. M. A., El Wanees, S. A., El Aal, E. E. A. & Diab, A. Environmental factors affecting the corrosion behavior of reinforcing steel. IV. Variation in the pitting corrosion
current in relation to the concentration of the aggressive and the inhibitive anions. _Corrosion Science_ 52, 1675–1683 (2010). Article Google Scholar * El Wanees, S. A., Radwan, A. B.,
Alsharif, M. A. & El Haleem, S. M. A. Initiation and inhibition of pitting corrosion on reinforcing steel under natural corrosion conditions. _Materials Chemistry and Physics_ 190, 79–95
(2017). Article Google Scholar * Yang, D. _et al_. Functionalization of citric acid-based carbon dots by imidazole toward novel green corrosion inhibitor for carbon steel. _Journal of
Cleaner Production_ 229, 180–192, https://doi.org/10.1016/j.jclepro.2019.05.030 (2019). Article CAS Google Scholar * Goñi, S. & Andrade, C. Synthetic concrete pore solution chemistry
and rebar corrosion rate in the presence of chlorides. _Cement and Concrete Research_ 20, 525–539, https://doi.org/10.1016/0008-8846(90)90097-H (1990). Article Google Scholar * Andrade,
C., Alonso, M. & Gonzalez, J. In _An Initial Effort to Use the Corrosion Rate Measurements for Estimating Rebar Durability_ (1990). * Andrade, C. & Alonso, M. In _Values of Corrosion
Rate of Steel in Concrete to Predict Service Life of Concrete Structures_ (1994). * El Haleem, S. M. A., El Wanees, S. A. & Bahgat, A. Environmental factors affecting the corrosion
behaviour of reinforcing steel. VI. Benzotriazole and its derivatives as corrosion inhibitors of steel. _Corrosion Science_ 87, 321–333 (2014). Article Google Scholar * Oranowska, H. &
Szklarska-Smialowska, Z. An electrochemical and ellipsometric investigation of surface films grown on iron in saturated calcium hydroxide solutions with or without chloride ions. _Corrosion
Science_ 21, 735–747 (1981). Article CAS Google Scholar * Eyu, D. G., Esah, H., Chukwuekezie, C., Idris, J. & Mohammad, I. Effect of green inhibitor on the corrosion behaviour of
reinforced carbon steel in concrete. _ARON Journal of engineering and Applied sciences_ 8, 326–332 (2013). CAS Google Scholar * Jiang, S. _et al_. Deoxyribonucleic acid as an inhibitor for
chloride-induced corrosion of reinforcing steel in simulated concrete pore solutions. _Construction and Building Materials_ 150, 238–247 (2017). Article CAS Google Scholar * Zhang, Z.,
Ba, H., Wu, Z. & Zhu, Y. The inhibition mechanism of maize gluten meal extract as green inhibitor for steel in concrete via experimental and theoretical elucidation. _Construction and
Building Materials_ 198, 288–298, https://doi.org/10.1016/j.conbuildmat.2018.11.216 (2019). Article CAS Google Scholar * Basha Nusrath Unnisa, C. _et al_. Linear polyesters as effective
corrosion inhibitors for steel rebars in chloride induced alkaline medium – An electrochemical approach. _Construction and Building Materials_ 165, 866–876,
https://doi.org/10.1016/j.conbuildmat.2018.01.080 (2018). Article CAS Google Scholar * Fazayel, A. S., Khorasani, M. & Sarabi, A. A. The effect of functionalized polycarboxylate
structures as corrosion inhibitors in a simulated concrete pore solution. _Applied Surface Science_ 441, 895–913, https://doi.org/10.1016/j.apsusc.2018.02.012 (2018). Article ADS CAS
Google Scholar * Shanmugapriya, S., Prabhakar, P. & Rajendran, S. Corrosion Resistance Property of Mild Steel in Simulated Concrete Pore Solution Prepared in Well Water by Using an
Aqueous Extract of Turmeric. _Materials Today: Proceedings_ 5, 8789–8795, https://doi.org/10.1016/j.matpr.2017.12.307 (2018). Article CAS Google Scholar * Anitha, R. _et al_. Implications
of eco-addition inhibitor to mitigate corrosion in reinforced steel embedded in concrete. _Construction and Building Materials_ 213, 246–256,
https://doi.org/10.1016/j.conbuildmat.2019.04.046 (2019). Article CAS Google Scholar * Wang, Y. & Zuo, Y. The adsorption and inhibition behavior of two organic inhibitors for carbon
steel in simulated concrete pore solution. _Corrosion Science_ 118, 24–30, https://doi.org/10.1016/j.corsci.2017.01.008 (2017). Article CAS Google Scholar * Cao, F., Wei, J., Dong, J.
& Ke, W. The corrosion inhibition effect of phytic acid on 20SiMn steel in simulated carbonated concrete pore solution. _Corrosion Science_ 100, 365–376,
https://doi.org/10.1016/j.corsci.2015.08.020 (2015). Article CAS Google Scholar * Asaad, M. A. _et al_. Enhanced corrosion resistance of reinforced concrete: Role of emerging eco-friendly
Elaeis guineensis/silver nanoparticles inhibitor. _Construction and Building Materials_ 188, 555–568, https://doi.org/10.1016/j.conbuildmat.2018.08.140 (2018). Article CAS Google Scholar
* Cameron, D. M. _et al_. Confirmation of _in vitro_ and clinical safety assessment of behentrimonium chloride-containing leave-on body lotions using post-marketing adverse event data.
_Toxicology in Vitro_ 27, 2203–2212, https://doi.org/10.1016/j.tiv.2013.09.016 (2013). Article CAS PubMed Google Scholar * Ai, Z. _et al_. Passive behaviour of alloy corrosion-resistant
steel Cr10Mo1 in simulating concrete pore solutions with different pH. _Applied Surface Science_ 389, 1126–1136, https://doi.org/10.1016/j.apsusc.2016.07.142 (2016). Article ADS CAS
Google Scholar * ASTM C348-18, Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars, _ASTM International, West Conshohocken, PA_, doi:www.astm.org (2018). * ASTM
C109/C109M-16a, Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in or [50-mm] Cube Specimens), _ASTM International, West Conshohocken, PA_,
doi:www.astm.org (2018). * Poursaee, A. & Hansson, C. M. Reinforcing steel passivation in mortar and pore solution. _Cement and Concrete Research_ 37, 1127–1133,
https://doi.org/10.1016/j.cemconres.2007.04.005 (2007). Article CAS Google Scholar * Ghantous, R. M., Poyet, S., L’Hostis, V., Tran, N.-C. & François, R. Effect of crack openings on
carbonation-induced corrosion. _Cement and Concrete Research_ 95, 257–269, https://doi.org/10.1016/j.cemconres.2017.02.014 (2017). Article CAS Google Scholar * Wan, K., Li, L. & Sun,
W. Solid–liquid equilibrium curve of calcium in 6mol/L ammonium nitrate solution. _Cement and Concrete Research_ 53, 44–50, https://doi.org/10.1016/j.cemconres.2013.06.003 (2013). Article
CAS Google Scholar * Macdonald, J. R. Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. _Journal of Electroanalytical Chemistry
and Interfacial Electrochemistry_ 223, 25–50, https://doi.org/10.1016/0022-0728(87)85249-X (1987). Article CAS Google Scholar * J. R. & MacDonald. Impedance Spectroscopy Emphasizing
Solid Materials and Systems. _John Wiley & Sons, New York_ (1987). * Frankel, G. S. Pitting Corrosion of Metals: A Review of the Critical Factors. _Journal of The Electrochemical
Society_ 145, 2186–2198, https://doi.org/10.1149/1.1838615 (1998). Article CAS Google Scholar * Ahamad, I., Prasad, R. & Quraishi, M. A. Adsorption and inhibitive properties of some
new Mannich bases of Isatin derivatives on corrosion of mild steel in acidic media. _Corrosion Science_ 52, 1472–1481, https://doi.org/10.1016/j.corsci.2010.01.015 (2010). Article CAS
Google Scholar * Martin, F. & Olek, J. The Nature of Passivity of Reinforcing Steel. _ASCE Materials Congress_, Washington, D.C (1996). * Stefanoni, M., Angst, U. & Elsener, B.
Corrosion rate of carbon steel in carbonated concrete – A critical review. _Cement and Concrete Research_ 103, 35–48, https://doi.org/10.1016/j.cemconres.2017.10.007 (2018). Article CAS
Google Scholar * Shi, X., Xie, N., Fortune, K. & Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. _Construction and Building Materials_ 30,
125–138, https://doi.org/10.1016/j.conbuildmat.2011.12.038 (2012). Article Google Scholar * Zhou, X., Yang, H. & Wang, F. Investigation on the inhibition behavior of a pentaerythritol
glycoside for carbon steel in 3.5% NaCl saturated Ca(OH)2 solution. _Corrosion Science_ 54, 193–200, https://doi.org/10.1016/j.corsci.2011.09.018 (2012). Article CAS Google Scholar * Cao,
W., Wang, Z., Zeng, Q. & Shen, C. 13C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse.
_Applied Surface Science_ 389, 404–410, https://doi.org/10.1016/j.apsusc.2016.07.095 (2016). Article ADS CAS Google Scholar * Swift, A., Paul, A. J. & Vickerman, J. C. Investigation
of the surface activity of corrosion inhibitors by XPS and time-of-flight SIMS. _Surface and Interface Analysis_ 20, 27–35, https://doi.org/10.1002/sia.740200106 (1993). Article CAS Google
Scholar * Santos, A. R., Blundell, R. K. & Licence, P. XPS of guanidinium ionic liquids: a comparison of charge distribution in nitrogenous cations. _Physical Chemistry Chemical
Physics_ 17, 11839–11847, https://doi.org/10.1039/C5CP01069A (2015). Article CAS PubMed Google Scholar * Park, J. S. _et al_. A ZnO/N-doped carbon nanotube nanocomposite charge transport
layer for high performance optoelectronics. _Journal of Materials Chemistry_ 22, 12695–12700, https://doi.org/10.1039/C2JM30710C (2012). Article CAS Google Scholar * Tourabi, M., Nohair,
K., Traisnel, M., Jama, C. & Bentiss, F. Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by
3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. _Corrosion Science_ 75, 123–133, https://doi.org/10.1016/j.corsci.2013.05.023 (2013). Article CAS Google Scholar * Finšgar, M.,
Fassbender, S., Hirth, S. & Milošev, I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in
near-neutral chloride media. _Materials Chemistry and Physics_ 116, 198–206, https://doi.org/10.1016/j.matchemphys.2009.03.010 (2009). Article CAS Google Scholar * Solomon, M. M., Umoren,
S. A., Obot, I. B., Sorour, A. A. & Gerengi, H. Exploration of Dextran for Application as Corrosion Inhibitor for Steel in Strong Acid Environment: Effect of Molecular Weight,
Modification, and Temperature on Efficiency. _ACS Applied Materials & Interfaces_ 10, 28112–28129, https://doi.org/10.1021/acsami.8b09487 (2018). Article CAS Google Scholar *
Grosvenor, A. P., Kobe, B. A., Biesinger, M. C. & McIntyre, N. S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. _Surface and Interface
Analysis_ 36, 1564–1574, https://doi.org/10.1002/sia.1984 (2004). Article CAS Google Scholar * De Schutter, G. & Luo, L. Effect of corrosion inhibiting admixtures on concrete
properties. _Construction and Building Materials_ 18, 483–489, https://doi.org/10.1016/j.conbuildmat.2004.04.001 (2004). Article Google Scholar Download references ACKNOWLEDGEMENTS The
publication of this article was funded by the Qatar National Library. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Advanced Materials, Qatar University, Doha, P.O. Box 2713,
Qatar A. Bahgat Radwan, Mostafa H. Sliem, Noor S. Yusuf, Nasser A. Alnuaimi & Aboubakr M. Abdullah * Department of Civil and Architectural Engineering, Qatar University, Doha, P.O. Box
2713, Qatar Nasser A. Alnuaimi Authors * A. Bahgat Radwan View author publications You can also search for this author inPubMed Google Scholar * Mostafa H. Sliem View author publications You
can also search for this author inPubMed Google Scholar * Noor S. Yusuf View author publications You can also search for this author inPubMed Google Scholar * Nasser A. Alnuaimi View author
publications You can also search for this author inPubMed Google Scholar * Aboubakr M. Abdullah View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Ahmed Bahgat Radwan, Mostafa Sliem and Noor Yusuf were responsible for the experimental work including preparation and characterizataion. Dr. Nasser Alnuaimi supervised the
concrete preparation and mechanical testing section. Dr. Aboubakr Abdullah was resposnsible for the corrosion section and the overall manuscript gathering, reviewing and submission.
CORRESPONDING AUTHOR Correspondence to Aboubakr M. Abdullah. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Radwan, A.B., Sliem, M.H., Yusuf, N.S. _et al._ Enhancing the
corrosion resistance of reinforcing steel under aggressive operational conditions using behentrimonium chloride. _Sci Rep_ 9, 18115 (2019). https://doi.org/10.1038/s41598-019-54669-y
Download citation * Received: 31 July 2019 * Accepted: 30 October 2019 * Published: 02 December 2019 * DOI: https://doi.org/10.1038/s41598-019-54669-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative