Linkage mapping of quantitative trait loci for fiber yield and its related traits in the population derived from cultivated ramie and wild b. Nivea var. Tenacissima
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Ramie is an important natural fiber crop, and the fiber yield and its related traits are the most valuable traits in ramie production. However, the genetic basis for these traits is
still poorly understood, which has dramatically hindered the breeding of high yield in this fiber crop. Herein, a high-density genetic map with 6,433 markers spanning 2476.5 cM was
constructed using a population derived from two parents, cultivated ramie Zhongsizhu 1 (ZSZ1) and its wild progenitor _B. nivea_ var. _tenacissima_ (BNT). The fiber yield (FY) and its four
related traits—stem diameter (SD) and length (SL), stem bark weight (BW) and thickness (BT)—were performed for quantitative trait locus (QTL) analysis, resulting in a total of 47 QTLs
identified. Forty QTLs were mapped into 12 genomic regions, thus forming 12 QTL clusters. Among 47 QTLs, there were 14 QTLs whose wild allele from BNT was beneficial. Interestingly, all QTLs
in Cluster 10 displayed overdominance, indicating that the region of this cluster was likely heterotic loci. In addition, four fiber yield-related genes underwent positive selection were
found either to fall into the FY-related QTL regions or to be near to the identified QTLs. The dissection of FY and FY-related traits not only improved our understanding to the genetic basis
of these traits, but also provided new insights into the domestication of FY in ramie. The identification of many QTLs and the discovery of beneficial alleles from wild species provided a
basis for the improvement of yield traits in ramie breeding. SIMILAR CONTENT BEING VIEWED BY OTHERS THE RECOMBINATION LANDSCAPE AND MULTIPLE QTL MAPPING IN A _SOLANUM TUBEROSUM_ CV.
‘ATLANTIC’-DERIVED F1 POPULATION Article Open access 22 March 2021 GENOMIC INTERROGATION OF A MAGIC POPULATION HIGHLIGHTS GENETIC FACTORS CONTROLLING FIBER QUALITY TRAITS IN COTTON Article
Open access 17 January 2022 DISCOVERY AND VALIDATION OF SSR MARKER-BASED QTL GOVERNING FRESH POD YIELD IN DOLICHOS BEAN (_LABLAB PURPUREUS_ L. SWEET) Article Open access 12 March 2025
INTRODUCTION Fibers are widespread among vascular plant species and are present in many different organs including roots, stems, and leaves. It plays important roles in the growth and
development of plant, including establishing plant architecture, defending from herbivory, storing ergastic carbon resources and water1. In addition, plant fibers are one of the most
important renewable resources, and are used as raw material in the paper industry, and for various textiles and for composites. Because the remarkable valuable of fiber for human and plant
itself, the fiber traits have been paid wide attention in research, especially in _Populus_ and cotton. Ramie (_B. nivea_ var. _nivea_), a diploid (2n = 28) perennial herbaceous plant, is
one of the most important natural fiber crops in China, and has been cultivated with more than 4,700 years2. Ramie fibers are one of the longest fibers in plant kingdoms, and its length can
reach to 55 cm3. Moreover, unlike _Populus_ and cotton fibers that are xylem and seed epidermal fibers, respectively, ramie fiber are extracted from stem barks and are bast fibers. Although
these interesting characteristics, there is limited information about the genetic and molecular basis of ramie fiber yield (FY) and its related traits (including stem length (SL) and
diameter (SD), bark weight (BW) and thickness (BT)4,5,6. Only one expanded SSR markers linkage map and one SNP markers linkage map have been developed in ramie, resulting in limited
quantitative trait loci (QTLs) identified for FY and its related traits6,7. One BT QTL, _qBT4a_, has been ascertained its candidate gene that encodes a MYB transcription factor, based on the
identification of an insertion of large-fragment that resulted in premature termination in this candidate gene in one parent6. However, the function of _qBT4a_ has not been validated by
further research. Cultivated ramie is deemed to be domesticated from the wild progenitor _B. nivea_ var. _tenacissima_ (BNT) according to morphological, genetic and molecular evidences8,9.
There are remarkable morphological differences between cultivated ramie and wild BNT, especially stem length and diameter10. The traits of stem length and diameter are two important
components for determining the fiber yield of ramie, and their changes likely result from the domestication to meet human need. Recently, transcriptome comparison between ramie and BNT had
identified several positively selected genes involved in the fiber yield8. Therefore, the yield-related traits have undergone principally selection in the ramie domestication by human.
However, the genetic basis about the domestication of yield-related traits is still uncharacterized. In the present study, to understand the genetic basis of yield-related traits and their
domestication, we developed an F2 population derived from two parents, the cultivar Zhongsizhu 1 (ZSZ1) and wild progenitor BNT; and then, a high-density genetic map was constructed, and
QTLs for yield-related traits were identified. This study provided insights into the genetic basis of fiber yield-related traits in ramie, and the identification of many QTLs presented a
basis for improving the yield traits in ramie breeding. RESULTS VARIATION OF FY AND ITS RELATED TRAITS IN THE POPULATION AND TWO PARENTS The two parents, ZSZ1 and wild BNT exhibited highly
significant difference in five fiber yield-related traits investigated (Table 1; Supplementary Fig. S1). ZSZ1 showed longer and thicker stems (130.5 cm in length and 9.11 mm in diameter)
than BNT (only 60.5 cm in length and 4.58 mm in diameter; Table 1). In addition, for stem barks, the thickness and weight were 0.69 mm and 19.9 g in ZSZ1, which were thicker and more weight
than BNT (0.42 mm in thickness and 2.6 g in weight; Table 1). Because all four traits above had important influence on of FY, the difference of them resulted in significant difference in FY
in two parents (2.07 g and 0.25 g for ZSZ1 and BNT, respectively; Table 1). There were wide variations observed in the population for five investigated traits in the two environments (Table
1). ANOVA revealed that the genetic factor (δg2) explained most observed phenotypic variance for all five traits in the population; except the BT trait, all traits had a high heritability in
the population, ranged from 80.4% to 87.7% (Table 2). In addition, the five traits showed significant positive correlations (_P_ < 0.01; Table 3). HIGH-DENSITY GENETIC MAP A total of
26,450 high-quality polymorphic SNPs, with the segregation pattern of aa × bb between two parents, have been identified. After filtering, 6,811 SNPs were performed for grouping, resulting in
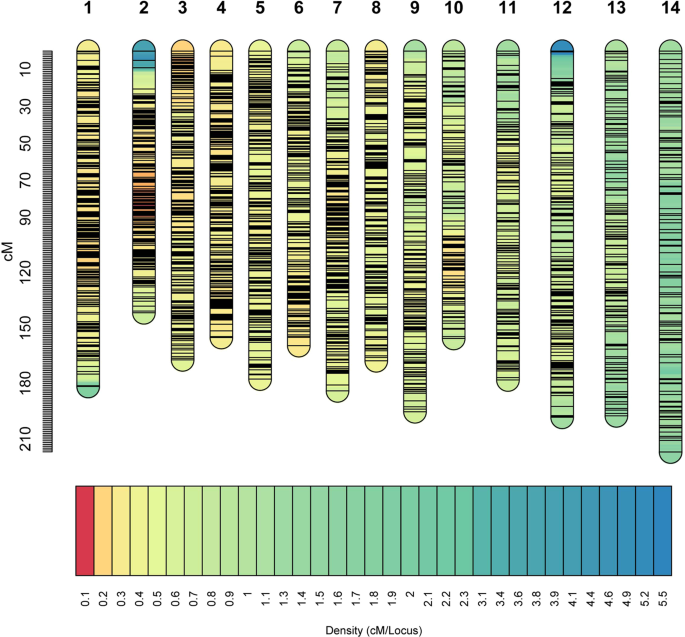
14 linkage groups. Thereafter, the SNPs unassigned into linkage groups were filtered, resulting in a total of 6,433 SNPs used to develop a high-density genetic map (including 2,175 binned
markers), with a total length of 2476.5 cM (Fig. 1). The length of individual linkage groups ranged from 141.7 to 217.1 cM. These 6,433 SNPs were distributed evenly in the genetic map; there
were 108–196 SNPs contained in per linkage group, and the average and maximum distance of interval was 0.38 cM and a 14.35 cM, respectively (Table 4). Only 23 intervals had genetic
distances of >5 cM (Table 4). QTL MAPPING FOR FY AND ITS RELATED TRAITS QTL analysis for five traits was performed by the program of Windows QTL CARTOGRAPHER. LOD thresholds (1000
permutations) were estimated for five investigated traits in two environments, and the result indicated that the values ranged from 3.3 to 3.8 (Table S1). Based on these thresholds, a total
of 29 and 26 QTLs were identified for these five traits in Environment 1 and Environment 2, respectively, nine and twelve of which had an overdominance in corresponding environment (Table 5,
Fig. 2). There were 16 QTLs detected in both two environments. Finally, in all 39 QTLs were identified, i.e., nine, eight, nine, two, and eleven QTLs for SD, SL, BW, BD and FY traits,
respectively (Table 5, Fig. 2). Among these 39 QTLs, two SD QTLs (_SD2-1_, and _SD13_), three SL QTLs (_SL2-1_, _SL9_ and _SL13_), two BW QTL (_BW2-1_ and _BW13_), one BT QTL (_BT1_), and
three FY QTLs (_FY2-1 FY9_ and _FY13_) improve the trait by the wild BNT allele, whereas the others increase the phenotype by ZSZ1’s. Additionally, QTLs for these five traits were also
detected by the QTL.gCIMapping.GUI program. There were six, four, four, and eight QTLs identified for SD, SL, BW, and FY trait, respectively; however, no QTL of BT trait was detected (Table
6). Among these 22 QTLs, 14 have been mapped by Windows QTL CARTOGRAPHER, and 9 improve the traits by the wild BNT allele (Table 6). Finally, a total of 47 QTLs were identified for five
traits by two programs of QTL analysis, of which 14 increase the trait phenotype by wild BNT allele. QTL CLUSTER FOR FIBER YIELD-RELATED TRAITS Among 47 QTLs, 40 were located in 12
pleiotropic genomic regions, thus constituting 12 QTL clusters (Table 7). Except Cluster 5, all clusters were made up of one FY QTL and one/several QTLs of FY-related traits (Table 7).
Because the FY is determined by its related component traits, the FY QTL and fiber yield-related QTLs of each cluster potentially were one same pleiotropic QTL. Interestingly, in each
cluster, all QTLs increased the traits phenotype by the alleles from the same parent (Table 7). Therefore, among 12 clusters, eight improve the FY and its related traits by ZSZ1 allele, and
the other four by the allele of wild BNT. All QTLs of Cluster 10 displayed overdominance, indicating that this cluster is a genomic region with overdominance (Table 7). DISCUSSION GENETIC
CHARACTERIZATION OF FY AND ITS RELATED TRAITS Ramie is an important industrial fiber crop, and the fiber yield and its four related traits are five of the most valuable traits in ramie
production. To characterize the genetic basis of these traits, this study developed a high-density genetic map using 6,433 SNP marker. Based on this genetic map, there were 47 QTLs
identified for FY and its related traits, 40 of which were mapped into the 12 genomic regions resulting in 12 QTL clusters. It is notable that significant positive correlations among FY and
four its related traits were observed in the population. Therefore, we deduced that the existence of many QTL clusters was the genetic basis of the correlation of fiver traits investigated,
which is accordance with the previous studies7,11,12. Overdominance was deemed to be one of the most important reasons caused the heterosis for FY-related traits in ramie7. In this study,
among 29 and 26 QTLs identified in Environment 1 and Environment 2 by Windows QTL CARTOGRAPHER, nine and twelve were found to have overdominance in corresponding environment, respectively.
Interestingly, all three QTLs in Cluster 10 showed overdominance, suggesting that this pleiotropic regions might be the heterotic loci. In addition, there were seven QTLs displayed
overdominance in single environment, which indicated that the overdominance effect can be affected by environment. Therefore, this study improved our understanding into the genetic basis of
FY and FY-related traits. INSIGHTS INTO THE GENETIC BASIS OF THE DOMESTICATION OF FY-RELATED TRAITS Cultivated ramie is domesticated from the wild progenitor BNT9. Unlike cultivated ramie
which shows outstanding performances in FY and FY-related traits, the BNT displayed inferior FY-related traits, and thus few fibers can be harvested from its stem barks. FY and FY-related
traits were domesticated potentially by human in ramie. However, the genetic basis of the domestication for these traits has uncovered. In this study, we used a population derived from
parent cultivated ZSZ1 and wild progenitor BNT to dissect the genetic basis of FY and FY-related traits, resulting in 47 QTLs. There were 13 genes underwent positive selection identified by
transcriptomic comparison between cultivated ramie and wild BNT8, of which two (CL10581Contig1 and T3_Unigene_BMK.28528) fell into the QTL region, and two (CL16310Contig1 and CL12943Contig1)
were mapped into the regions near to the QTLs by aligning these transcripts to genome (Table S2). Among these four genes, CL12943Contig1 and T3_Unigene_BMK.28528 are two transcripts near to
the _BT1_ and Cluster 8, respectively, and their encoding-proteins WAT1-related protein and homeobox-leucine zipper protein have important roles in fiber differentiation and secondary wall
thickening13,14,15. In addition, CL16310Contig1 encodes an ankyrin repeat-containing protein which is involved in the regulation of cell differentiation and development16, suggesting that
this gene have a potential role in the stem growth; and CL16310Contig1 was found in the regions near to the Cluster 9. Therefore, these genes underwent positive selection potentially have
important roles in the regulation of fiber yield. In this study, there were 10 QTLs (including nine QTLs from three clusters) identified to be near to these four genes. The adjacency between
identified QTLs and positively selected genes provided new insights into the domestication of FY and FY-related traits. QTLS FOR RAMIE HIGH-YIELD BREEDING Improving the FY is one of the
most important objectives in ramie breeding program. Understanding the genetic basis of FY and its related traits will be helpful for the improvement of these traits. In this study, a total
of 47 QTLs were identified, and 40 QTLs were found to clustered distribute in 12 pleiotropic loci, including one heterotic loci. Thus, the application of these QTL clusters can improve the
performance of several FY-related traits simultaneously by marker assisted selection. Among 47 QTLs, there were still 14 QTLs (including 12 QTL from 4 clusters) which increased phenotype of
FY and its related traits by the alleles of wild BNT, although the wild BNT displayed inferior performance in these traits. In crop domestication, there are some alleles lost in some loci,
including excellent alleles, which causes that the genetic diversity of traits dramatically drops17,18,19. Discovery and application of excellent gene resources from wild species were
frequently performed by crop breeder. Therefore, the identification of 14 QTLs with excellent alleles from wild species was invaluable gene resources for the ramie high-yield breeding.
MATERIALS AND METHODS EXPERIMENTAL POPULATIONS AND INVESTIGATION OF TRAITS A population consisting of 111 F2 progenies was developed using two parents, the cultivar Zhongsizhu 1 (ZSZ1) and
wild species _B. nivea_ var. _tenacissima_ (BNT), according to a previously described strategy7. Then, all 111 F2 progenies and two parents was reproduced by cutting propagation and
generated an agamous line, which had the identical genotype to the corresponding F2 individual. All these agamous family lines and parents were grown in the experimental farm of the
Institute of Bast Fiber Crops (IBFC) in June 2016. Two replications were grown in a randomized complete block design. For each family line, ten seedlings were grown into a plot of two rows,
with a distance of 45 cm between rows and 70 cm between plants within a row. The area around the population was planted with two-row ramie which was used to eliminate the boundary effect in
the population. Ramie is perennial, and the overground part of plants can grow again when harvested. In this study, the population was collected phenotype in two growing environments, i.e.
Environment 1 from Apr. 10, 2017 to June 14, 2017 and Environment 2 from June. 15, 2017 to Aug. 4, 2017., Population phenotype was collected when more than two-thirds leaves of ramie from
the population had shattered. The stem length (SL) was measured from the stem bottom to the shoot. The stem diameter (SD) and bark thickness (BT) were measured in the middle of stem using a
Vernier caliper. The stem bark was harvested individually and weighted, and bark weight (BW) was calculated as the mean bark weight per stem; and then, the fibers were extracted from these
stem bark, and were dried, and then, the trait of fiber yield (FY) was estimated as the mean weight of fiber per stem. GENOTYPING OF POPULATION Young leaves which were individually collected
from each line and parent were frozen in liquid nitrogen immediately, and stored at −80 °C until used. Total RNAs of each sample was extracted by the EZNA. Plant RNA Kit (OMEGA Bio-Tek,
Norcross, GA, USA). Thereafter, these RNAs were used to construct a cDNA library with fragment lengths of ~250 bp. Paired-end sequencing was performed by the Illumina HiSeq. 2500 sequencing
platform (Illumina, San Diego, CA, USA), at Shanghai OE Biotech. Co., Ltd (Shanghai, China). Finally, the raw reads were trimmed the adapter sequences and filtered low-quality reads, result
in high quality clean reads (SRA accession no. SRP182925). To genotype the population, the clean reads from the transcriptome of 111 F2 families and two parents were aligned into the ramie
genome (NCBI accession no: PHNS00000000)20, using the software Burrows-Wheeler Aligner (BWA) with the default parameters21. The software SAMtools22 was used to convert the alignment files as
bam files. If there are multiple read pairs with identical external coordinates, we retained the pair with the highest mapping quality. Thereafter, SNPs were identified from the two parents
and 111 families using the software SAMtools22, only high quality SNPs (coverage depth > 10, quality value > 50, minor allele frequency >_0.05, and missing genotype rate <_0.1)
were retained. Because both the parent ZSZ1 and BNT were heterozygous, the polymorphic markers between them were detected according to the following steps: firstly, all polymorphic markers
classified into eight segregation patterns, i.e., ef × eg, nn × np, ab × cc, aa × bb, ab × cd, lm × ll, hk × hk, and cc × ab23,24, according to the CP model in JoinMap 4.025; Then, the SNPs
with more than two genotypes were filtered out, finally retaining only polymorphic SNPs with the segregation pattern of ‘aa × bb’. CONSTRUCTION OF GENETIC MAP The genetic map was constructed
according to the description of Liu _et al_.6. Firstly, Markers that contained abnormal bases or exhibited significantly distorted segregation (P < 0.001) or non-integrity (missing data
in > 30% progenies) were filtered out using LepMap326. Thereafter, the regression algorithm, three times circulation sequence, and Kosambi mapping function were used for marker distance
calculation27, and the linkage map was constructed by the software LepMap326, and was drawn using the mapchart 2.3228, with the default parameters. DATA ANALYSIS QTL analysis was performed
according to the description of Liu _et al_.6. QTLs for fiber yield-related traits in two environments were detected using composite interval mapping (CIM) with Windows QTL
CARTOGRAPHER2.529. Window size was set at 10 cM, and forward stepwise regression was used to identify significant markers as cofactors. The experiment-wise LOD threshold significance level
was determined by computing 1000 permutations (P < 0.05) using a permutation test program in Windows QTL CARTOGRAPHER2.529. These permutations can account for non-normality in marker
distribution and trait values. By comparing with the CIM method, the genome-wide composite interval mapping (GCIM) based on the multi-locus mixed model has high power in the detection of
small and linked QTLs30, and thus, the QTL mapping for five traits was also performed by the QTL.gCIMapping.GUI program31, and an LOD threshold of 2.5 was set. For one same trait, if QTLs
detected from different environments or by different programs are mapped within a 10-cM region, these QTLs were considered as a same QTL. If there were several QTLs of different traits
identified into a 10-cM region, this region was deemed to be a QTL cluster. ANOVA was performed by the program STATISTICA 8.0. Heritability was estimated according to the formula as follows:
$${{\rm{H}}}^{{\rm{2}}}=\frac{{{\sigma }_{g}}^{2}}{{{\sigma }_{g}}^{2}+{{\rm{n}}}^{-{\rm{1}}}{{\sigma }_{ge}}^{2}+{(\mathrm{nr})}^{-{\rm{1}}}{{\sigma }_{e}}^{2}},$$ where σg2, σge2 and σe2
were the estimates of genetic, genetic by environment interaction and error variances derived from the mean square expectations of the analysis of variance, with n = 2 being the number of
environments and r = 2 being the number of replicates. Expected genotypic variance and expected genetic by environment interaction variance were estimated according to the model as the
description of Liu _et al_.32. DATA AVAILABILITY The datasets generated for this study can be found in the NCBI SRA database under the accession number SRP182925. REFERENCES * Gorshkova, T.
_et al_. Plant Fiber Formation: State of the Art, Recent and Expected Progress, and Open Questions. _Critical Reviews in Plant Sciences_ 31, 201–228 (2012). Article CAS Google Scholar *
Liao, L. _et al_. The domestication and dispersal of the cultivated ramie (_Boehmeria nivea_ (L.) Gaud. in Freyc.) determined by nuclear SSR marker analysis. _Genet. Resour. Crop Evol._ 61,
55–67 (2014). Article Google Scholar * Aldaba, V. C. The structure and development of the cell wall in plants I. Bast fibers of _Boehmeria_ and _Linum_. _Amer. J. Bot._ 14, 16–22 (1927).
Article Google Scholar * Chen, J., Liu, F., Tang, Y., Yuan, Y. & Guo, Q. Transcriptome sequencing and profiling of expressed genes in phloem and xylem of ramie (_Boehmeria nivea_ L.
Gaud). _PLoS ONE_ 9(10), e110623 (2014). Article ADS Google Scholar * Chen, J. _et al_. Transcriptome profiling using pyrosequencing shows genes associated with bast fiber development in
ramie (_Boehmeria nivea_ L.). _BMC Genomics_ 15, 919 (2014). Article Google Scholar * Liu, C. _et al_. QTL analysis of four main stem bark traits using a GBS-SNP-based high-density genetic
map in ramie. _Scientific Reports_ 7, 13458 (2017). Article ADS Google Scholar * Liu, T., Zhu, S., Tang, Q. & Tang, S. QTL mapping for fiber yield-related traits by constructing the
first genetic linkage map in ramie (_Boehmeria nivea_ L. Gaud). _Mol. Breeding_ 34, 883–892 (2014). Article Google Scholar * Liu, T., Tang, S., Zhu, S., Tang, Q. & Zheng, X.
Transcriptome comparison reveals the patterns of selection in domesticated and wild ramie (_Boehmeria nivea_ L. Gaud). _Plant Mol. Biol._ 86, 85–92 (2014). Article CAS Google Scholar *
Jiang, Y. & Jie, Y. Advances in research on the genetic relationships of _Boehmeria_ in China. _J. Plant Genet. Res._ 6, 114–118 (2005). Google Scholar * Wu, Z. Y., Raven, P. H., Hong,
D. Y. Flora of China. Volume 5: ulmaceae through Basellaceae. _Science Press, Beijing_ (2003). * Liu, T. _et al_. Comparison of quantitative trait loci for 1,000-grain weight and spikelets
per panicle across three connected rice populations. _Euphytica_ 175, 383–394 (2010). Article Google Scholar * Liu, T. _et al_. Comparison of quantitative trait loci for rice yield,
panicle length and spikelet density across three connected populations. _J. Genet._ 90, 377–382 (2011). Article Google Scholar * Zhong, R. & Ye, Z. Secondary cell walls: biosynthesis,
patterned deposition and transcriptional regulation. _Plant Cell Physiol._ 56(2), 195–214 (2015). Article CAS Google Scholar * Zhong, R. & Ye, Z. _IFL1_, a gene regulating
interfascicular fiber differentiation in _Arabidopsis_, encodes a homeodomain-leucine zipper protein. _Plant Cell_ 11, 2139–2152 (1999). Article CAS Google Scholar * Ranocha, P. _et al_.
Arabidopsis _WAT1_ is a vacuolar auxin transport facilitator required for auxin homoeostasis. _Nat. Commun._ 4, 2625 (2013). Article ADS Google Scholar * Zhang, H., Scheirer, D. C.,
Fowle, W. H. & Goodman, H. M. Expression of antisense or sense RNA of an ankyrin repeat-containing gene blocks chloroplast differentiation in _Arabidopsis_. _Plant Cell_ 4, 1575–1588
(1992). CAS PubMed PubMed Central Google Scholar * Qi, J. _et al_. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. _Nat. Genet._
45, 1510–1515 (2013). Article CAS Google Scholar * Mao, D. _et al_. Multiple cold resistance loci confer the high cold tolerance adaptation of Dongxiang wild rice (_Oryza rufipogon_) to
its high-latitude habitat. _Theor. Appl. Genet._ 128, 1359–1371 (2015). Article CAS Google Scholar * Lin, T. _et al_. Genomic analyses provide insights into the history of tomato
breeding. _Nat. Genet._ 46, 1220–1226 (2014). Article CAS Google Scholar * Luan, M. _et al_. Draft genome sequence of ramie, _Boehmerianivea_ (L.) Gaudich. _Mol. Ecol. Resour._ 18,
639–645 (2018). Article CAS Google Scholar * Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009). Article
CAS Google Scholar * Kosambi, D. D. The estimation of map distance from recombination values. _Ann Eugenics_ 12, 172–175 (1943). Article Google Scholar * Li, H. _et al_. and 1000
Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079 (2009). Article Google Scholar * Zhang, Z., Wei, T., Zhong, Y., Li,
X. & Huang, J. Construction of a high-density genetic map of _Ziziphus jujube_ Mill. using genotyping by sequencing technology. _Tree Genetics & Genomes_ 12, 76 (2016). Article
Google Scholar * Zhou, Z. _et al_. Genetic dissection of maize plant architecture with an ultra-high density bin map based on recombinant inbred lines. _BMC Genomics_ 17, 178 (2016).
Article Google Scholar * van Ooijen, J. W. Multi point maximum likelihood mapping in a full sib family of an out breeding species. _Genet. Res. (Camb)_ 93, 343–349 (2011). Article Google
Scholar * Rastas, P. Lep-MAP3: robust linkage mapping even for low-coverage whole genome sequencing data. _Bioinformatics_ 33, 3726–3732 (2017). Article CAS Google Scholar * Voorrips, R.
MapChart: software for the graphical presentation of linkage maps and QTLs. _J Hered._ 93, 77–8 (2002). Article CAS Google Scholar * Wang, S., Basten, C., Zeng, Z. Windows QTL
Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC (2012). * Wen, Y. _et al_. An efficient multi-locus mixed model framework for the detection of small
and linked QTLs in F2. _Briefings in Bioinformatics_, https://doi.org/10.1093/bib/bby058 (2018) * Wang, S. _et al_. Mapping small-effect and linked quantitative trait loci for complex traits
in backcross or DH populations via a multi-locus GWAS methodology. _Sci. Rep._ 6, 29951 (2016). Article ADS CAS Google Scholar * Liu, T. _et al_. Comparison of quantitative trait loci
for 1,000-grain weight and spikelets per panicle across three connected rice populations. _Euphytica_ 175, 383–394 (2010). Article Google Scholar Download references ACKNOWLEDGEMENTS This
work was supported by grants from the National Natural Science Foundation of China (31571725, 31871678), the Agricultural Science and Technology Innovation Program of China
(CAAS-ASTIP-IBFC), and Central Public-interest Scientific Institution Basal Research Fund (1610242019001, 1610242019005). AUTHOR INFORMATION Author notes * These authors contributed equally:
Zheng Zeng and Yanzhou Wang. AUTHORS AND AFFILIATIONS * Institute of Bast Fiber Crops and Center of Southern Economic Crops, Chinese Academy of Agricultural Sciences, Changsha, China Zheng
Zeng, Yanzhou Wang, Chan Liu, Fu Li & Touming Liu * Shanghai OE Biotech. Co., Ltd, Shanghai, China Xiufeng Yang & Hengyun Wang Authors * Zheng Zeng View author publications You can
also search for this author inPubMed Google Scholar * Yanzhou Wang View author publications You can also search for this author inPubMed Google Scholar * Chan Liu View author publications
You can also search for this author inPubMed Google Scholar * Xiufeng Yang View author publications You can also search for this author inPubMed Google Scholar * Hengyun Wang View author
publications You can also search for this author inPubMed Google Scholar * Fu Li View author publications You can also search for this author inPubMed Google Scholar * Touming Liu View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Z.Z. developed the genetic map. Y.W. conducted the fieldwork and investigated the trait
phenotype. C.L. carried out the ANOVA and heritability analysis, and X.Y. performed the other analysis, H.W. and F.L. prepared the reagents used in the experiments. T.L. designed the
research and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Touming Liu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION FIG. S1, TABLE
S1, TABLE S2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution
and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Zeng, Z., Wang, Y., Liu, C. _et al._ Linkage mapping of quantitative trait loci for fiber yield and its related traits in the population derived from cultivated ramie and wild _B.
nivea_ var. _tenacissima_. _Sci Rep_ 9, 16855 (2019). https://doi.org/10.1038/s41598-019-53399-5 Download citation * Received: 28 May 2019 * Accepted: 29 October 2019 * Published: 14
November 2019 * DOI: https://doi.org/10.1038/s41598-019-53399-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative