Pharmacologically-induced stress has minimal impact on judgement and attention biases in sheep
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The emotional impact of exposure to stressors has not been well quantified in animals. We hypothesised that exogenous induction of stress in sheep would induce a pessimistic
judgement bias and increased attention towards a threatening stimulus, suggestive of a negative emotional state. Stress was induced pharmacologically by administering synthetic
adrenocorticotropic hormone. Judgement bias was assessed using a spatial go/no-go task after exposure to acute stress (one injection), chronic stress (21 daily injections) and
acute-on-chronic stress (2 min isolation after 28 daily injections). Attention bias was assessed during chronic stress only (22 daily injections). In contrast with our hypotheses, there was
no strong evidence that Synacthen administration altered judgement bias or attention bias at any stage of the experiment. Stressed sheep were more likely to approach ambiguous locations than
saline Control animals, however, statistical evidence for models fitting treatment group was very weak. Overall, our findings suggest that elevated levels of cortisol may not fully explain
changes to judgement bias observed in previous studies after environmentally-induced stress. Further studies are required to better understand which aspects of environmentally-induced stress
alter judgement bias and to further validate cognitive methods of assessing affect in sheep. SIMILAR CONTENT BEING VIEWED BY OTHERS IMPACT OF CANINE EPILEPSY ON JUDGEMENT AND ATTENTION
BIASES Article Open access 20 October 2020 EMOTIONALITY MODULATES THE IMPACT OF CHRONIC STRESS ON MEMORY AND NEUROGENESIS IN BIRDS Article Open access 03 September 2020 SELF-EXPERIENCE OF A
NEGATIVE EVENT ALTERS RESPONSES TO OTHERS IN SIMILAR STATES THROUGH PREFRONTAL CORTEX CRF MECHANISMS Article 03 December 2024 INTRODUCTION Domesticated animals regularly experience stressful
events and environments which may impact their physical and mental well-being. However, the impact of stress on the affective (or emotional) states of animals is not fully understood. The
stress response involves activation of the hypothalamic-pituitary-adrenal (HPA) axis, which leads to a series of highly conserved neuroendocrine reactions allowing animals to
physiologically, behaviourally and psychologically respond to homeostatic threats1,2. This response is typically considered to be adaptive, however when a stressor is prolonged or repeated,
the stress can become chronic and may lead to dysregulation of the HPA-axis and maladaptive stress responses3,4. In humans, this can lead to mood changes and has been implicated in the
development and maintenance of depressive and anxious emotional states3,5. In addition to the impact of chronic stress itself, an increased effort to maintain homeostasis (allostatic load)
can reduce the ability of animals to cope with and adapt to additional acute stressors, and may also lead to further shifts in affective state4,6,7,8,9,10. Thus, the potential impact of
stress, particularly chronic stress, on the mental well-being of animals and their ability to cope with additional stressors deserves further attention. A key limitation for the study of
affective states in animals has been a lack of quantitative assessment methods. To address this limitation, an increasing body of literature has assessed emotional states via their impact on
an individual’s cognitive processing of information, termed cognitive bias. Many of these studies have focused on sheep, which are farmed globally in large numbers and are used in
biomedical research11,12,13,14. The type of cognitive bias most widely studied in sheep is judgement bias, where affective state can alter an individual’s interpretation of ambiguous
situations15,16. Individuals in positive affective states typically make more positive judgements about ambiguous information (optimism), while individuals in negative affective states
typically make more negative judgements about ambiguous information (pessimism)14. Judgement biases can be assessed using a range of test paradigms. For example, in a go/no-go spatial
discrimination task, subjects are trained to discriminate two locations, such that they approach the rewarded location (go) and avoid the negative or non-rewarded location (no-go). Subjects
are then presented with an ambiguous, intermediate location and their go or no-go response is interpreted as the subject perceiving the ambiguous cue to be positive or negative respectively.
In sheep, animals treated with an anxiolytic drug or an opioid, to induce positive affective states, were more likely to approach ambiguous locations during a go/no-go spatial
discrimination task, suggesting they were more optimistic17,18. Sheep treated with a serotonin inhibitor to induce a depressed state were less likely to approach ambiguous locations19. These
studies show that judgement bias tests may be used as a measure of affective states in sheep. Another type of cognitive bias which has been more recently studied in animals is an attention
bias, where affective state can alter an individual’s allocation of attention towards different types of information20. For example, humans in anxious states pay more attention towards
threatening information than non-anxious individuals21,22,23. Tests for attention bias are potentially a more rapid and practical option for assessing affective states in animals, compared
to typical judgment bias test paradigms. In sheep and cattle, a novel attention bias test has been developed and pharmacologically validated as a potential measure of anxiety-like states,
where animals given an anxiogenic drug spent more time looking towards a threatening stimulus and displayed increased vigilance behaviour compared to control animals24,25,26. Further,
behavioural responses in a modified version of the test have also been shown to reflect pharmacologically-induced depression-like states in sheep27. Together, these studies show that
attention bias tests can also potentially be used to assess affective states in sheep. Examination of judgement and attention biases together may provide additional information on the
affective states of animals. Recently, studies in humans have also highlighted a need to better understand the way in which these biases interact and relate to one another, for a greater
understanding of their underlying mechanisms28. Examining the relationships between cognitive bias tests in animals may allow us to gain a greater understanding of the mechanisms driving
cognitive biases, which may include stress response pathways. A number of studies in sheep have examined the impacts of environmental manipulation to induce endogenous acute and chronic
stress on cognitive biases, however the results have not always been consistent between studies. Using a go/no-go spatial discrimination judgement bias task, pessimistic judgement biases
were observed in sheep after 3 weeks29 and 9 weeks30 of unpredictable, uncontrollable exposure to aversive husbandry procedures, expected to have induced chronic stress. Two further studies
found several months of unpredictable, stimulus-poor housing only had a weak effect on judgement bias, with one study finding a slight pessimistic bias31, while the other found a slight
optimistic bias32. In contrast, three days of restraint and isolation stress induced an optimistic bias in sheep33, as did an acute shearing challenge34. Most recently, Verbeek _et al_.35
examined the impact of chronic stress induced by 9 days of lying deprivation on both judgement and attention bias in sheep. Further, they examined the impact of an additional acute stressor
(shearing) on judgement bias. Prior to the shearing challenge, chronically stressed sheep appeared to be more optimistic during judgement bias testing and showed an attention bias away from
a threat when compared to control animals, consistent with a more positive emotional state. After shearing, no differences in optimism were evident between the groups. It has been proposed
that the unexpected optimistic judgement biases observed in many of these studies may reflect a positive emotional state caused by release from the imposed environmental stressors. This
highlights a key challenge in using environmental manipulations to alter the state of an animal, as it can be difficult to identify which state the manipulation has induced and to maintain
the state throughout the testing period33,34. Pharmacological treatments can alter the state of an animal in a more standardised and repeatable manner than environmental manipulations, while
remaining active for the duration of testing36,37. Further, pharmacological treatments can be readily matched with appropriate controls (e.g. saline injections). A number of studies have
examined the impact of pharmacological treatments on cognitive biases in animals, with most pharmacological agents aiming to alter the affective states of the subjects19,24,27,38,39. Few
studies have examined the impact of exogenous induction of stress on the affective states of animals through pharmacological manipulation. The link between stress and affective states is
well established in humans and the mechanism of action is well described, with exogenous glucocorticoids shown to readily cross the blood-brain barrier to access the emotion related parts of
the brain, including the amygdala. Adrenergic receptors have been found in the amygdala and play an important role in fear processing and memory for emotionally relevant information40. In
addition, the link between mood disturbances, such as psychosis, and glucocorticoid administration has been confirmed in humans41. In animals, two studies have investigated the impact of
pharmacologically-induced stress on judgement biases. In rats, injection of corticosterone and a noradrenaline reuptake inhibitor, to mimic the early stages of the acute stress response,
caused a pessimistic bias in an audio discrimination judgement bias task42. In chickens, seven days of corticosterone administration, expected to induce chronic stress, caused a pessimistic
bias during a spatial discrimination judgement bias test43. Conducting a similar study in sheep, stimulating the HPA axis via exogenous induction of stress, could help to better understand
the impact of stress on cognitive biases and affective state. Previous studies in sheep have developed a model using daily injections of Synacthen, a synthetic adrenocorticotropic hormone,
over a period of 4 weeks to chronically elevate plasma cortisol concentrations and induce a stress response44,45. Synacthen may therefore be a suitable candidate drug to pharmacologically
validate the effect of acute and chronic stress on the affective states of sheep. The aim of the current experiment was to determine the impact of stress on the emotional states of sheep,
examining the effects of acute, chronic and acute-on-chronic stress. The study followed a similar design to Verbeek _et al_.35, assessing emotional state using judgement bias and attention
bias tests, however acute and chronic stress states were induced pharmacologically using Synacthen, instead of using environmental manipulations. We hypothesised that sheep given one
injection of Synacthen (Stress; acute stress stage) would be more pessimistic than control animals given one saline injection (Control; acute stress stage) in a judgement bias test. Further,
we hypothesised that continued daily injections of Synacthen over a 3 week period (Stress; chronic stress stage) would induce a more pessimistic judgement bias and an increased attention
bias towards a threat compared to animals given daily saline injections (Control; chronic stress stage). Preliminary analyses were conducted to compare animal responses in the judgement bias
and attention bias tests on days 21 and 22. It was expected that more pessimistic sheep would show increased vigilance and attention to the dog, consistent with a negative emotional state.
Finally, we hypothesised that an additional acute stressor (2 min of isolation) would induce a more pessimistic judgement bias in the chronically stressed animals compared to the control
animals exposed to an acute stressor only (acute-on-chronic stage). It was expected that the Stress group would become increasingly more pessimistic between the acute, chronic and
acute-on-chronic stress stages. Plasma cortisol concentrations were assessed during the experiment to confirm the effect of Synacthen administration on HPA-axis responses. RESULTS JUDGEMENT
BIAS One animal from the Stress group failed to go to the Positive (P) location on day 1 of judgement bias testing, but approached the P location on the other test days. Data for that sheep
were retained for analyses. The other 26 sheep approached the P location on all test days. The maximal model fitted to the judgement bias data included fixed effects of location, treatment,
test day and all interactions. A subset of the best ranked models from the “dredge” function in R (Δ_i_ < 4) are presented in Table 1. The null model consisting of the intercept only had
a high Δ_i_ AICc of 173 and Δ_i_ BIC of 157. All models listed in Table 1 included location as a fixed effect. The best-ranked models by AICc also included treatment as a fixed effect,
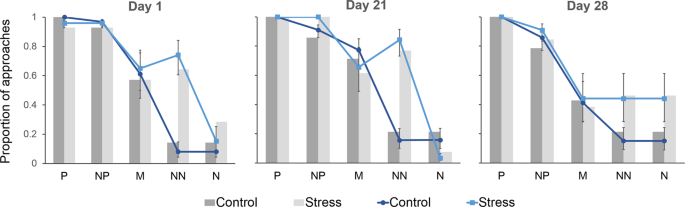
however support for a treatment effect was considerably weaker when considering BIC (Table 1). Model predictions from the maximum model are given in Fig. 1. Predicted values from this model
suggest that sheep from the Stress group were more likely to approach the ambiguous locations than Control animals. This is particularly demonstrated by the observation that the Stress group
were approximately 3 times more likely to approach the Near-Negative (NN) location than the Control group across all test days (raw proportion of combined go responses 0.63 and 0.19
respectively, Fig. 1). Evidence that test day had an effect on approach was negligible. One of the models fitting day as a fixed effect had a Δ_i_ AICc < 4, however the Δ_i_ BIC value for
this model was greater than 20 (Table 1). ATTENTION BIAS Control animals had a greater latency to eat than Stress animals (89.6 and 52.5 s respectively), however this difference was not
significant in the Cox proportional hazards model (Log likelihood ratio (1) = 1.83, P = 0.2; Fig. 2). All other variables examined were best described by a model fitting the intercept only,
as opposed to models fitting treatment group (Table 2). For amount of food eaten and time spent immobile, models fitting treatment group also had some evidence. CORTISOL RESPONSE There was
strong evidence for the model fitting timepoint, treatment and their interaction for cortisol response on day 1 (Table 3). For cortisol response on day 29, there was strong evidence for the
model fitting treatment only (Table 3). Animals treated with Synacthen showed a greater cortisol response than Control animals on both days of testing (Fig. 3). Control animals showed little
deviation from the baseline cortisol concentration on either test day (Fig. 3). RELATIONSHIP BETWEEN JUDGEMENT AND ATTENTION BIASES Figure 4 shows the raw number of go responses of sheep to
ambiguous locations on day 21 of judgement bias testing, plotted against key behavioural responses during attention bias testing on day 22. Jonckheere-Terpstra tests showed no significant
relationships between the number of go responses and behavioural responses during attention bias testing (P values ranging between 0.20 and 0.85). DISCUSSION In contrast with our hypotheses,
administration of an exogenous glucocorticoid (Synacthen) to induce stress had no strong effect on judgement bias or attention bias in sheep, relative to the control animals. Further, the
results do not support our hypothesis that the judgement biases of stressed sheep would change between the acute, chronic and acute-on-chronic stress stages. There was some evidence for an
optimistic bias in the stress animals at all time points, which is consistent with some33,34,35, but not all previous studies29,30,46. However, statistical evidence for this optimistic bias
was weak. The effect of Synacthen on the Stress animals was confirmed by an increased plasma cortisol concentration on days 1 and 29. This response is similar to that observed previously in
sheep administered Synacthen daily for 4 weeks44, and provides physiological evidence that the induced cortisol responses persisted for the duration of judgement bias and attention bias
testing. A lack of effect on cognitive bias therefore suggests that elevated levels of cortisol cannot fully explain changes to judgement biases observed in previous studies after
environmentally-induced stress35. Alternatively, these results may suggest that the cognitive bias tests used in this study could not detect changes to affective state caused by increased
circulating cortisol concentrations. Further studies are therefore required to better understand which aspects of environmentally-induced stress may be contributing to an altered judgement
bias in sheep, and to further validate cognitive bias tests as measures of affective state. It is possible that administration of exogenous glucocorticoids did not impact on the affective
states of sheep. A lack of effect could be due to the use of an exogenous hormone to induce stress, which may not equate to the stress response caused by an environmental manipulation, that
impacts on an array of neurophysiological pathways. Alternatively, it may suggest that glucocorticoids do not have a role in modulating affective state in sheep, regardless of their origin.
Previously, environmentally-induced elevations in cortisol have been observed without alterations to judgement bias in pigs47, and altered judgement bias has been observed without evidence
of changes to endogenous cortisol concentrations in sheep. However, studies in humans have established a clear link between administration of exogenous glucocorticoids and affective
states40. Further, direct relationships between exogenous corticosteroids and judgement bias have been demonstrated in chickens and rats42,43. We therefore suggest our inability to replicate
these results in sheep may be due to the limitations of the cognitive bias tests we used and our study design, as discussed below. It is possible that the cognitive bias tests were unable
to detect a change in affective state in the Stress group, due to the confounding effects of feeding motivation or sensitivity to reward, as discussed by Verbeek _et al_.35. Glucocorticoids
have been shown to increase the salience of pleasurable and compulsive activities in humans and rodents, such as ingestion of sugars, fats and drugs of abuse48,49,50,51. Increased
motivational behaviours for rewarding stimuli and ingestion of “comfort foods” are thought to reduce the negative consequences of stress by downregulating HPA-axis activity52,53. Further, it
has been proposed that animals may seek positive or rewarding experiences to counteract negative experiences54. In sheep, cortisol responsiveness has been linked to metabolic and
behavioural traits associated with obesity55. As such, an increased motivation to eat palatable food rewards in the Stress group may have masked a potential change in judgement bias and
attention bias, and may explain the observation of a weak optimistic bias during the current study. Another factor which may have limited the ability of the judgement bias test to detect a
response is the presence of olfactory cues from the dog and food during testing. Ideally, testing of ambiguous locations would occur in the absence of any cues56, however this is often
difficult to achieve in a practical setting. Instead, both positive and negative cues can be kept in place throughout all training and testing, as to not include an additional olfactory cue
which may signal a particular outcome during ambiguous trials. In the current study however, the scent of the positive location changed between the positive and negative training sessions
as, while some food was present next to the arena at all times, this scent may have been stronger during positive trials when there was food located inside the arena. This means the strength
of the scent of food during the ambiguous trials was the same during negative training trials, potentially providing an additional cue that the outcome would be negative. If this were the
case, we might expect a low proportion of go responses across all treatment groups to the ambiguous locations, however this was not observed, which suggests the presence of olfactory cues
had minimal impact on results. Nevertheless, it cannot be ruled out that elevated cortisol levels enhanced the animals’ perception of the scent of the food outside of the arena during
ambiguous trials, such that they were more likely to expect a positive outcome. Further studies should be aware of and control for this potential effect during judgement bias tests as
described by Verbeek _et al_.35. An effect of Synacthen on the Stress group may not have been detected if the Control animals had an equivalent stress response, due to the impact of the
treatment and testing procedures. This is not supported by the cortisol responses of the Control animals, that remained very low on days 1 and 29 during judgement bias testing and blood
sampling. Further, sheep often voluntarily moved into the testing areas during the experiment, being led by a familiar handler with a feed bucket. Therefore, it seems unlikely that the
Control animals found the treatment and testing procedures to be highly aversive. Nevertheless, the judgement bias and attention bias testing procedures involved isolation, novelty and
exposure to predators, which are innately stressful for sheep57,58,59. Thus, it cannot be ruled out that the treatment and testing procedures impacted on Control animal responses during
cognitive bias testing, so that they could not be distinguished from the Stress group. Regardless of an impact on affective state, it makes biological sense for an animal which has been
recently threatened and that is undergoing a physiological stress response to be more vigilant and pay more attention towards additional threats60,61. A lack of response during attention
bias testing in the chronically stressed animals may therefore suggest that the dog and novel environment during attention bias testing were not perceived to be threatening and therefore did
not elicit an additional response from the Stress group. Although as discussed previously, this seems unlikely given that isolation and predators are innately stressful for sheep. Another
possibility is that the Stress group perceived the dog as a threat more strongly than Control animals, but failed to respond accordingly with an appropriate behavioural response, potentially
demonstrating a maladaptive response associated with chronic stress and increased allostatic load4,6,7,8,9,10. Finally, a lack of response may again indicate that the pharmacological model
of chronic stress was not effective as a manipulation of affective state, or that the attention bias test was not sensitive enough to detect an effect of the drug on the animals’ behaviour.
Further studies examining the impact of stress on the attention bias test only, using naive animals, would provide further insight into these issues. No clear relationships were observed
between animal responses in the judgement and attention bias tests during the chronic stress stage of this study. This finding suggests that each test may provide different information on
the affective state of an animal. It has been suggested that judgement biases are largely influenced by longer term mood states, while attention biases are more situation specific and may
reflect shorter term emotional responses62. Nevertheless, recent studies in humans have demonstrated relationships between judgement and attention biases63,64. It is possible that no
relationships between cognitive biases were observed in the current study due to limited data availability and low statistical power. Additional studies using larger numbers of animals will
help to better understand the nature of the relationship between judgement and attention biases in animals, if one exists. Such studies may help to inform on the underlying mechanisms
driving cognitive biases in animals. It is important to consider the potential impact of repeated assessments on animal behaviour during testing, both between and within the two
experiments35. The attention bias test is still relatively new and the effects of repeated testing on animal behaviour have not yet been explored. Therefore, exposure to the attention bias
test during the previous study may have altered animal responses during the current study. Additionally, while a different dog was used in the attention bias test than for judgement bias
training and testing, repeated exposure to dogs may have reduced the animals’ responses to this potential threat during the attention bias test. Another potential limitation of the study was
that it only applied one test session at each stage of the experiment, with a single exposure to each ambiguous stimulus per session. As animal responses can vary considerably between
trials due to day to day variation, this may have limited our ability to detect an actual effect in the study. On the other hand, there are also limitations to repeated judgement bias
testing as animals learn the ambiguous cues are not rewarded upon repeated testing, and begin to approach ambiguous cues less often65,66,67,68. In the current study, we observed no effect of
day on judgement bias test responses. Further, the study design was balanced so that each treatment underwent the same number of tests. As such, we do not expect repeated testing within the
current study to have affected the differences in judgement bias between treatment groups. Animals allocated to a stress treatment during the previous study35 were balanced between the
Control and Stress groups in the current study, however, it is important to acknowledge the potential effect of earlier stress-inducing treatments on animal responses during the current
study. Early life stress or neglect has been shown to impact judgement biases later in life in rats and female goats69,70,71. Even prenatal stress has been found to influence judgement bias
in lambs72. Thus, it would not be surprising if the treatments applied to animals in the previous study impacted their responses in the current study. However, due to the small number of
animals used in the current study, we were unable to further investigate these potential effects. Using the same sheep between studies reduced the time taken for animals to reach the
necessary learning criteria for judgement bias testing. However, due to the potentially confounding effect of previous experiments on animal responses, through earlier treatments and
habituation to the test procedures, it is suggested that future studies use naive animals for cognitive bias testing, unless it relates to the hypotheses being tested. It has been proposed
that the differences in results between previous judgement bias studies may be due to the duration of the applied stressors, where chronic stress has more consistently caused a shift towards
pessimism, while acute stress has yielded mixed results14,73. This theory is not supported by the findings of the current study, which found no evidence of a pessimistic judgement bias
after chronic stress, or an optimistic bias after acute stress. Further, this is not supported by the results of Verbeek _et al_.35, which observed an optimistic bias after chronic stress.
However, it should be noted that the durations over which chronic stress have been induced vary greatly between studies29,30,31,32,35, and that studies intending to induce chronic stress may
not have done so successfully. Alternatively, it has been suggested that previous judgement bias studies may have incorrectly inferred the affective states induced by the given treatments,
by failing to consider mismatches between the animals’ perceived expectations and outcomes during affective state induction or testing74. However, this theory is also unsupported by the
current study, as well as a recent meta-analysis which aimed to determine whether such mismatches could predict judgement bias test outcomes more accurately than the inferred affective
states75. The differing effects of stress on judgement bias responses in previous studies could also potentially be explained by differences in assessment methods or the breeds of sheep used
in each study. Most studies in sheep have used a spatial discrimination task to assess judgement bias, however the types of positive and negative reinforcers have varied. Each of the
studies which found an optimistic bias in stressed animals tested Merino sheep using a dog to reinforce the negative location33,34,35. Studies which found a pessimistic bias tested Romane
sheep and used an air blower to reinforce the negative location29,30,46. Two further studies which found no effect on judgement bias tested Lacaune sheep using either an air blower31 or
presentation of straw instead of feed32 as a negative reinforcer. A range of fear studies have observed differences in behavioural reactivity between sheep breeds57,58,76. Further, sheep
have been shown to exhibit context specific patterns of behaviour, depending on the fear-eliciting stimuli presented57,58,76. Together these findings suggest the type of reinforcer and/or
breed of sheep used in the experiments may have impacted the direction of the bias observed during previous judgement bias studies. Further studies aiming to examine the differences in
judgement biases between sheep breeds or comparing different types of positive and negative reinforcers may shed light on the discrepancies observed between studies. Overall, the results
from this study do not support our hypotheses that pharmacologically-induced acute and chronic stress would cause pessimism and an attention bias towards threatening information in sheep.
Further, our results show that increased circulating cortisol concentrations may not fully explain the responses observed during previous chronic stress studies, although the observation of
a weak optimistic judgement bias is consistent with some previous results33,35. We suggest these findings should not be taken to say that chronic stress does not negatively impact on animal
affective states. Instead, we suggest researchers should be cautious in their interpretation of cognitive bias test results in sheep until the methods are further validated. The study of
affective states in livestock is a field of research in its infancy. Contradicting results between studies of judgement bias in particular show that tests for affective state require
continued refinement and validation, before they can be considered robust measures of affect as an aspect of animal welfare. METHODS ETHICAL STATEMENT The protocol and conduct of this study
were approved by the CSIRO McMaster Laboratory Animal Ethics Committee (Animal research authority #12–30), under the New South Wales Animal Research Act 1985. The protocol was carried out in
accordance with all relevant guidelines and regulations. All animals were closely monitored for health and welfare during and after the experiments. ANIMALS AND MANAGEMENT Thirty-two maiden
Merino ewes (18–19 months of age, average bodyweight 50.8 ± 3.9 kg) were used in this experiment, which was conducted in 2013 between April and May. All ewes were born on the same
experimental farm in Armidale, Australia and were reared as one group after weaning. During training and testing, animals were grazed on pasture located approximately 50 m away from the
handling and testing facilities. Sheep were walked between the pasture and testing facilities by a familiar handler. Sheep were fed a familiar supplementary ration of pelleted, lucerne-based
concentrate each afternoon following training. All of the ewes in the current study had previous experience with judgement bias and attention bias testing during the experiment conducted by
Verbeek _et al_.35. Sheep were returned to the farm flock between the previous and current studies, for a period of 10 months, during which the sheep were managed as per normal farm
practices. Readers are asked to refer to Verbeek _et al_.35 for further details of the previous experiment. Briefly, in the earlier study all ewes were trained to judgement bias testing over
a 2 month period, then 30 of the sheep were randomly allocated to control and chronic stress treatment groups (_n_ = 15 per group). The chronic stress group was subjected to social
isolation and 18hrs of lying deprivation per day over 9 days, while the control animals were housed as a group in a paddock over the same time period. On day 9, all sheep were exposed to an
additional acute shearing challenge. All sheep underwent judgement bias testing twice during the experimental period, before and after shearing. All sheep underwent attention bias testing
once prior to shearing. EXPERIMENTAL DESIGN A summary of the experimental protocol and timeline is given in Table 4. Details of each procedure are given in the following sections. As a
general overview, sheep were retrained over a period of one month to undertake a judgement bias test. Twenty-eight sheep which successfully reached the training criteria were then
pseudo-randomly split into two groups; Stress and Control treatments, balancing for positive location side and cue colour, as well as treatment allocation in Verbeek _et al_.35 (see below
for details). All sheep underwent once daily injections from days 1 to 29 with saline (Control) or synthetic ACTH (Stress). On day 1, the first injection was expected to induce an acute
stress state in the Stress group. By days 21 and 22, ongoing injections were expected to have induced a chronic stress state in the Stress group. On day 28, all animals underwent 2 min of
isolation to induce acute stress in the Control group and acute on top of chronic stress (acute-on-chronic) in the Stress group. Judgement bias was assessed at each of these stages.
Attention bias was assessed during the chronic stress stage as an additional assessment of affective state. Plasma cortisol response to the injection was assessed on days 1 and 29 of the
experiment. Due to unforeseen circumstances, one animal in the Stress treatment group was removed from the study due to injury between days 1 and 21. Data from this animal were removed from
the study. Only data for the remaining 27 animals were analysed. JUDGEMENT BIAS ARENA The current study used the same judgement bias arena described by Verbeek _et al_.35, adapted from
Verbeek _et al_.77. Testing occurred in a 3 × 3 m arena surrounded by 1.5 m high wooden walls (Fig. 5). Sheep entered the arena through a start box centred along the front wall of the arena
and exited through a door on the right (Fig. 5). The back wall of the arena was divided into five sections with wood panels 40 cm high (hereafter locations). Removable panels were located at
the front of each location. A single location was made accessible to the test sheep for each trial or training session, by removing one of the front panels prior to testing. Sliding
vertical doors were located behind the two most outer locations, which could be lifted to reveal a dog, lying down quietly (negative reinforcer). Dummy sliding doors identical in appearance
to the actual doors were placed behind the three middle locations (ambiguous locations). To facilitate discrimination between locations, five green coloured cue cards which differed in
colour density (0, 25, 50, 75 and 95%) were created by adjusting the transparency of the same colour green in Microsoft PowerPoint77,78. The cues, printed on A3 paper and laminated, could be
attached to the doors at each location in the judgement bias test. Therefore, a total of five cues and locations were used; positive (P, location of a bucket containing feed), near positive
(NP), middle (M), near negative (NN) and negative (N, location of the dog). Only one location was accessible (front panel removed), with only one corresponding cue card present at a time
during training and testing. Only the accessible location contained a bucket, which was placed in front of the closed sliding door, visible to the sheep. The bucket either contained a small
feed reward (approximately 100 g of pelleted lucerne-based concentrate, P location) or was empty (all other locations). The bucket was located inside the arena during the current study,
while the previous study positioned the bucket outside the arena behind the vertical sliding door at the P location35. This change in bucket location was made to better facilitate learning,
by providing an additional visual cue for the location being tested. JUDGEMENT BIAS TRAINING For two days before beginning judgement bias training, sheep were moved into the arena in groups
of two for 5 min to re-habituate to the arena, with no buckets or cue cards present. Sheep then underwent one judgement bias training session per weekday for 4 consecutive weeks, following
the procedure described by Verbeek _et al_.35. During retraining, each sheep was assigned the same Positive location (either the outer left or outer right location in the arena) and Positive
cue card (0 or 95% colour density) assigned to them in the previous study. Sheep were first trained to approach their assigned P location, containing a bucket with a small feed reward,
while all other locations were inaccessible. Once a sheep left the start box, they were given 3 min to step over the P location decision line with both front feet (go response) and begin
consuming feed (Fig. 5). The decision line was physically visible to the sheep. The sheep were let out of the arena after consuming the feed, or if 3 min had elapsed without the sheep
crossing the decision line (no-go response). Positive training was conducted over the first 2 training sessions. During the first session, sheep entered the test arena 3 consecutive times.
During the second session, and for all subsequent training and testing days, sheep entered the test arena 5 consecutive times per session. During the remaining training sessions, sheep were
exposed to the P location for 3 of the arena entries and the N location for 2 of the entries in each training session. The sequences in which P and N locations were presented were randomised
between sessions. During training to the N location, an empty bucket was placed in the corner location opposite the assigned P location (right or left) with an alternate cue card (95 or 0%
colour density). If a sheep crossed the N location decision line (go response), the sliding door was opened to reveal a live dog sitting quietly. Once the sheep retreated from the bucket,
the door was lowered and the sheep was let out of the arena. If the sheep did not approach a location within 30 s of exiting the starting box, the sheep was let out of the arena. A sheep was
considered to be trained when it correctly responded to 14 out of 15 consecutive positive locations and to 8 out of 10 consecutive negative locations. A ‘positive response’ was considered
correct when the sheep stepped over the P decision line (go response) within 10 s of exiting the starting box. A ‘negative response’ was considered to be correct when the sheep did not cross
the N decision line within 30 s of exiting the starting box (no-go response). During training, 4 sheep failed to reach these criterion and were removed from the study. PHARMACOLOGICAL
TREATMENTS All animals received an intramuscular (i.m.) injection once daily for 29 consecutive days (Table 4). Control animals were administered 0.5 ml of BP Saline while Stress animals
were administered 0.5 ml of Synacthen Depot (0.5 mg of tetracosactrin zinc phosphate complex, Mallinckrodt Pharmaceuticals, UK). Synacthen has been used in sheep previously at this dose rate
to chronically elevate plasma cortisol concentrations, mimicking the physiological response of the HPA axis to stress44. Injections for each animal were staggered by approximately 10 min on
days 1, 21, 22 and 28 to allow for judgement bias and attention bias testing, beginning and ending at approximately 8:00 AM and 1:00 PM respectively. On all other days, injections were
administered to all animals at 8:30 AM. The injection site was rotated each day between the rump, thigh and dorsal muscles on the left and then right sides of the animals. BLOOD SAMPLING AND
ANALYSIS Blood sampling occurred on days 1 and 29 for assessment of plasma cortisol concentration as an indicator of HPA axis response. Blood samples were collected via jugular venepuncture
into heparinised vacutainers on each of the sampling days at four consecutive time points. On day 1 (acute stress), samples were taken at time 0 (baseline prior to Synacthen injection) then
at 0.5, 1 and 1.5 hrs post injection to evaluate the acute cortisol response to the Synacthen injection. Judgement bias testing occurred immediately prior to collection of the 1 hr post
injection blood sample. On day 29 (chronic stress) samples were taken at time 0 (baseline prior to Synacthen injection) then at 1, 4 and 8 hrs post injection to evaluate the cortisol return
to baseline. Blood tubes were kept on ice until they could be processed. The blood samples were centrifuged at 2000 × _g_ for 15 min at 5 °C, then the plasma was distributed into 2 ml
aliquots that were stored at −80 °C until analysis for cortisol concentration. Plasma cortisol concentrations were measured using a commercial radioimmunoassay (Orion Diagnostica, Espo,
Finland), previously validated for ovine plasma cortisol79. The intra-assay and inter-assay coefficients of variance (CV) for quality controls containing 24.9, 51.6 and 104.9 nmol/L of
cortisol were 3.4, 5.7 and 7.7% and 6.5, 4.5 and 12.6% respectively. JUDGEMENT BIAS ASSESSMENT Judgement bias was assessed 3 times during the experiment, on days 1, 21 and 28, as described
by Verbeek _et al_.35. Testing occurred 1 hr post injection for each given day, where treatment groups were spread evenly throughout the day. During testing, each sheep was released into the
arena five consecutive times with each of the 5 locations presented once. The trained locations were reinforced with food (P) or exposure to the dog (N), however the ambiguous locations
were not reinforced. Presentation of the locations occurred in one of the following orders: P, NP, M, NN, N (P first) or N, NN, M, NP, P (N first). Half the animals were pseudo randomly
assigned to each start location on each day of testing, balancing between treatment groups, so that presentation order for each animal may have differed between test days. After leaving the
start box, sheep were given a maximum of 30 s to respond for each location before being let out of the arena. The go or no-go responses were recorded from a video screen in real time. All
tests were continuously recorded by two video cameras and a 16 channel Digital Video Recorder. Immediately prior to judgement bias testing on day 28, each sheep was moved into an isolation
box for 2 min to induce acute isolation stress80,81. The isolation box consisted of a 1.5 × 0.5 × 2 m wooden box with an open roof covered by shade cloth. ATTENTION BIAS ASSESSMENT Attention
bias was assessed as described by Verbeek _et al_.35, using a similar method to that validated by Lee _et al_.24. The test was conducted in a 4 × 4 m arena surrounded by a 1.5 m high fence
covered in opaque black cloth (Fig. 5). The concrete floor was divided into 12 rectangular zones (1 × 1.2 m) marked on the ground with white paint. A familiar bowl containing 200 g of
concentrate pellets was placed in the centre of the arena. A small window (77 × 58 cm) was located on one wall behind which a dog was sitting quietly (different from the dog used in
judgement bias training). Each sheep was tested individually for a total of 3 min. The dog was visible to the sheep for the first 30 s of testing, then the window was covered by a
retractable opaque cover and the dog was removed for the remainder of the test. All tests were continuously recorded by a video camera. The following behaviours were recorded in real time or
from video footage; time spent looking towards the dog or covered window with binocular vision (attention to dog24), duration with the head at or above shoulder height (vigilance24,82),
latency to eat the food, number of zones crossed with both front legs and duration spent immobile with no movements of the head or body for greater than 3 s. Latency to eat was recorded from
the beginning of the test. All other behaviours were recorded separately for the durations with and without the dog present. Only data without the dog present are presented, as this is the
period where responses are to a perceived threat, as opposed to an actual threat posed by the presence of a predator, and thus better reflect anxiety states assessed using attention
bias24,35. Between animals, all uneaten food was removed and the bowl refilled with new pellets so that the amount of food eaten by each sheep could be calculated. The arena was also cleaned
after eliminations to avoid the influence of odour cues on further subjects. Vocalisations, eliminations and foot stamps were also recorded, however these data were not further analysed due
a low number of occurrences during the test. STATISTICAL ANALYSIS The data were analyzed using R version 3.5.183. All model residuals were checked for normality and homoscedasticity using
Shapiro Wilks test for normality and visual assessment of Q-Q and residuals vs. fitted values plots where appropriate. An information criterion based approach was used for model selection to
determine which models most appropriately fit the data within a given set of models35,84,85. A maximal model was fitted for each analysis, then the parameters for models containing each
possible combination of the predictors and their interactions were calculated using the “dredge” function from the package “MuMIn”86. We examined both the Akaike Information criterion
adjusted for small samples sizes (AICc) and the Bayesian Information criterion (BIC), which more heavily penalizes model complexity. Due to this heavier penalisation, models selected by BIC
are simpler and emphasize the key predictors, whereas model selection by AICc is typically preferred for model predictions87. Thus, our interpretation of results relied more heavily on BIC,
however AICc is also presented. Models were selected based on Δ_i_ (AICc or BIC difference relative to the smallest AICc or BIC value in the given set of models) and _w__i_ (Akaike or
Schwarz weight), indicating the relative weight of evidence for model _i_ being the best fit for the data within the given set of models. Where multiple models had a Δ_i_ < 2, those
models could be considered equally likely to be the best fit for the data, however models with a Δ_i_ < 4 also have considerable empirical support88. JUDGEMENT BIAS TESTING The go/no-go
responses during judgement bias trials were analysed using generalized linear mixed effects models using the package “lme4”89, as described by Gygax _et al_.85. Fitted models included all
go/no-go responses across the 3 test days. The maximal model included fixed effects of location, treatment, day and all possible interactions between those variables. Location was fitted as
a factor rather than a continuous variable, due to evidence of a non-monotonous pattern in go responses across locations. The random effects for each model were day nested in animal (id) to
account for repeated measures across test days. ATTENTION BIAS TESTING Latency to eat data were analysed using a Cox’s proportional hazards model using survival analysis, as described by
Monk _et al_.25,90,91. Animals which failed to eat within 180 s were deemed as censored results. Attention to dog, vigilance, food eaten and time spent immobile were analysed using linear
models. Zones crossed data were analysed using a negative binomial general linear model, due to evidence of over-dispersion in the data. The maximal model for attention bias data fitted
treatment group as a fixed effect only. CORTISOL RESPONSE Cortisol data were analysed using linear mixed effects models. The maximum models for each day of sample collection included
treatment, timepoint and their interaction as fixed effects and sheep id as a random effect. Data from one animal in the Control group on day 1 and two animals from the Stress group on day
29 were removed from the analysis due to abnormally high baseline cortisol concentrations (>150 nmol/L). Thus, data for _n_ = 26 and _n_ = 25 animals were analysed for days 1 and 29
respectively. RELATION BETWEEN JUDGEMENT AND ATTENTION BIASES Analyses were conducted to determine the relationship between go responses during judgement bias testing on day 21 and key
behavioural responses during attention bias testing on day 22. Due to the low amount of data available, go responses to ambiguous bucket locations (NN, M and NP) were pooled for further
analyses. The number of go responses to any of the three ambiguous locations were summed for each animal. All sheep approached at least one location. Nine, seven and 11 sheep approached one,
two or three of the locations, respectively. Jonckheere-Terpstra tests were then used to determine whether vigilance, attention to the dog, latency to feed or zones crossed behaviours
during attention bias testing differed as the number of approaches to ambiguous locations increased. The Jonckheere-Terpstra test is a non-parametric, trend-based test, used due to
non-normality of the data. Permutation tests were used for P-value estimation, based on 5000 permutations, due to the presence of tied values in the attention bias data. DATA AVAILABILITY
The datasets generated during the current study are available in the CSIRO Data Access Portal (DAP) https://doi.org/10.25919/5c8725f046880. REFERENCES * Hermans, E. J., Henckens, M. J. A.
G., Joëls, M. & Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. _Trends Neurosci._ 37, 304–314 (2014). Article CAS PubMed Google Scholar
* Chrousos, G. P. Stress and disorders of the stress system. _Nat. Rev. Endocrinol._ 5, 374–381 (2009). Article CAS PubMed Google Scholar * Beauchaine, T. P., Neuhaus, E., Zalewski,
M., Crowell, S. E. & Potapova, N. The effects of allostatic load on neural systems subserving motivation, mood regulation, and social affiliation. _Dev. Psychopathol._ 23, 975–999
(2011). Article PubMed Google Scholar * McEwen, B. Allostasis and Allostatic Load: Implications for Neuropsychopharmacology. _Neuropsychopharmacology_ 22, 108–124 (2000). Article CAS
PubMed Google Scholar * Southwick, S. M., Vythilingam, M. & Charney, D. S. The Psychobiology of Depression and Resilience to Stress: Implications for Prevention and Treatment. _Annu.
Rev. Clin. Psychol._ 1, 255–291 (2005). Article PubMed Google Scholar * McEwen, B. S., Eiland, L., Hunter, R. G. & Miller, M. M. Stress and anxiety: Structural plasticity and
epigenetic regulation as a consequence of stress. _Neuropharmacology_ 62, 3–12 (2012). Article CAS PubMed Google Scholar * Destrez, A., Deiss, V., Leterrier, C., Boivin, X. & Boissy,
A. Long-term exposure to unpredictable and uncontrollable aversive events alters fearfulness in sheep. _animal_ 7, 476–484 (2013). Article CAS PubMed Google Scholar * Reefmann, N.,
Muehlemann, T., Wechsler, B. & Gygax, L. Housing induced mood modulates reactions to emotional stimuli in sheep. _Appl. Anim. Behav. Sci._ 136, 146–155 (2012). Article Google Scholar *
Novati, A. _et al_. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. _Sleep_ 31, 1579–85
(2008). Article PubMed PubMed Central Google Scholar * Verbeek, E. _et al_. Reduced cortisol and metabolic responses of thin ewes to an acute cold challenge in mid-pregnancy:
Implications for animal physiology and welfare. _PLoS One_ 7 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Dwyer, C. M. Welfare of sheep: Providing for welfare in an
extensive environment. _Small Rumin. Res._ 86, 14–21 (2009). Article Google Scholar * Holland, J. C. _et al_. Examination of osteoarthritis and subchondral bone alterations within the
stifle joint of an ovariectomised ovine model. _J. Anat._ 222, 588–597 (2013). Article CAS PubMed PubMed Central Google Scholar * Yates, D. T. _et al_. Technical note: Effects of rumen
passage on fluoxetine bioavailability in serum and effects of fluoxetine on serum prolactin concentration and demeanor in ewes. _J. Anim. Sci._ 88, 3611–3616 (2010). Article CAS PubMed
Google Scholar * Bethell, E. J. A. “How-To” Guide for Designing Judgment Bias Studies to Assess Captive Animal Welfare. _J. Appl. Anim. Welf. Sci._ 18, S18–S42 (2015). Article PubMed
Google Scholar * Harding, E. J., Paul, E. S. & Mendl, M. Animal behaviour: Cognitive bias and affective state. _Nature_ 427, 312–312 (2004). Article ADS CAS PubMed Google Scholar *
Marchant-Forde, J. N. The Science of Animal Behavior and Welfare: Challenges, Opportunities, and Global Perspective. _Front_. _Vet_. _Sci_. 2 (2015). * Destrez, A., Deiss, V., Belzung, C.,
Lee, C. & Boissy, A. Does reduction of fearfulness tend to reduce pessimistic-like judgment in lambs? _Appl. Anim. Behav. Sci._ 139, 233–241 (2012). Article Google Scholar * Verbeek,
E., Ferguson, D., Quinquet de Monjour, P. & Lee, C. Generating positive affective states in sheep: The influence of food rewards and opioid administration. _Appl. Anim. Behav. Sci._ 154,
39–47 (2014). Article Google Scholar * Doyle, R. E. _et al_. Administration of serotonin inhibitor p-Chlorophenylalanine induces pessimistic-like judgement bias in sheep.
_Psychoneuroendocrinology_ 36, 279–288 (2011). Article CAS PubMed Google Scholar * Crump, A., Arnott, G. & Bethell, E. Affect-Driven Attention Biases as Animal Welfare Indicators:
Review and Methods. _Animals_ 8, 136 (2018). Article PubMed Central Google Scholar * Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J. & van IJzendoorn, M. H.
Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. _Psychol. Bull._ 133, 1–24 (2007). Article PubMed Google Scholar * Bradley, B. P., Mogg, K.,
Millar, N. & White, J. Selective processing of negative information: effects of clinical anxiety, concurrent depression, and awareness. _J. Abnorm. Psychol._ 104, 532–536 (1995). Article
CAS PubMed Google Scholar * Bradley, B. P., Mogg, K. & Lee, S. C. Attentional biases for negative information in induced and naturally occurring dysphoria. _Behav. Res. Ther._ 35,
911–927 (1997). Article CAS PubMed Google Scholar * Lee, C., Verbeek, E., Doyle, R. & Bateson, M. Attention bias to threat indicates anxiety differences in sheep. _Biol. Lett._ 12,
20150977 (2016). Article PubMed PubMed Central CAS Google Scholar * Monk, J. E. _et al_. Towards a more practical attention bias test to assess affective state in sheep. _PLoS One_ 13,
e0190404 (2018). Article PubMed PubMed Central CAS Google Scholar * Lee, C. _et al_. Anxiety influences attention bias but not flight speed and crush score in beef cattle. _Appl_.
_Anim_. _Behav_. _Sci_, 0–1, https://doi.org/10.1016/j.applanim.2017.11.003 (2017). Article Google Scholar * Monk, J., Belson, S., Colditz, I. & Lee, C. Attention bias test
differentiates anxiety and depression in sheep. _Front. Behav. Neurosci._ 12, 246 (2018). Article PubMed PubMed Central Google Scholar * Kress, L. & Aue, T. The link between optimism
bias and attention bias: A neurocognitive perspective. _Neurosci. Biobehav. Rev._ 80, 688–702 (2017). Article PubMed Google Scholar * Doyle, R. E. _et al_. Measuring judgement bias and
emotional reactivity in sheep following long-term exposure to unpredictable and aversive events. _Physiol. Behav._ 102, 503–510 (2011). Article CAS PubMed Google Scholar * Destrez, A.
_et al_. Chronic stress induces pessimistic-like judgment and learning deficits in sheep. _Appl. Anim. Behav. Sci._ 148, 28–36 (2013). Article Google Scholar * Vögeli, S., Lutz, J., Wolf,
M., Wechsler, B. & Gygax, L. Valence of physical stimuli, not housing conditions, affects behaviour and frontal cortical brain activity in sheep. _Behav. Brain Res._ 267, 144–155 (2014).
Article PubMed Google Scholar * Guldimann, K., Vögeli, S., Wolf, M., Wechsler, B. & Gygax, L. Frontal brain deactivation during a non-verbal cognitive judgement bias test in sheep.
_Brain Cogn._ 93, 35–41 (2015). Article PubMed Google Scholar * Doyle, R. E., Fisher, A. D., Hinch, G. N., Boissy, A. & Lee, C. Release from restraint generates a positive judgement
bias in sheep. _Appl. Anim. Behav. Sci._ 122, 28–34 (2010). Article Google Scholar * Sanger, M. E., Doyle, R. E., Hinch, G. N. & Lee, C. Sheep exhibit a positive judgement bias and
stress-induced hyperthermia following shearing. _Appl. Anim. Behav. Sci._ 131, 94–103 (2011). Article Google Scholar * Verbeek, E., Colditz, I., Blache, D. & Lee, C. Chronic stress
influences attentional and judgement bias and the activity of the HPA axis in sheep. _PLoS One_ 14, e0211363 (2019). Article CAS PubMed PubMed Central Google Scholar * Mendl, M.,
Burman, O. H. P., Parker, R. M. A. & Paul, E. S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. _Appl. Anim. Behav. Sci._ 118,
161–181 (2009). Article Google Scholar * Doyle, R. E., Lee, C., McGill, D. M. & Mendl, M. Evaluating pharmacological models of high and low anxiety in sheep. _PeerJ_ 3, e1510 (2015).
Article PubMed PubMed Central CAS Google Scholar * Stracke, J., Otten, W., Tuchscherer, A., Puppe, B. & Düpjan, S. Serotonin depletion induces pessimistic-like behavior in a
cognitive bias paradigm in pigs. _Physiol. Behav._ 174, 18–26 (2017). Article CAS PubMed Google Scholar * Rygula, R., Papciak, J. & Popik, P. The effects of acute pharmacological
stimulation of the 5-HT, NA and DA systems on the cognitive judgement bias of rats in the ambiguous-cue interpretation paradigm. _Eur. Neuropsychopharmacol._ 24, 1103–1111 (2014). Article
CAS PubMed Google Scholar * Lupien, S. J., Maheu, F., Tu, M., Fiocco, A. & Schramek, T. E. The effects of stress and stress hormones on human cognition: Implications for the field of
brain and cognition. _Brain Cogn._ 65, 209–237 (2007). Article CAS PubMed Google Scholar * Conn, H. & Poynard, T. Corticosteroids and peptic ulcer: meta-analysis of adverse events
during steroid therapy. _J. Intern. Med._ 236, 619–632 (1994). Article CAS PubMed Google Scholar * Enkel, T. _et al_. Ambiguous-Cue Interpretation is Biased Under Stress- and
Depression-Like States in Rats. _Neuropsychopharmacology_ 35, 1008–1015 (2010). Article PubMed Google Scholar * Iyasere, O. S., Beard, A. P., Guy, J. H. & Bateson, M. Elevated levels
of the stress hormone, corticosterone, cause ‘pessimistic’ judgment bias in broiler chickens. _Sci. Rep._ 7, 6860 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Henry,
B. A., Blache, D., Dunshea, F. R. & Clarke, I. J. Altered “set-point” of the hypothalamus determines effects of cortisol on food intake, adiposity, and metabolic substrates in sheep.
_Domest. Anim. Endocrinol._ 38, 46–56 (2010). Article CAS PubMed Google Scholar * Clarke, I. J., Bartolini, D., Conductier, G. & Henry, B. A. Stress Increases Gonadotropin Inhibitory
Hormone Cell Activity and Input to GnRH Cells in Ewes. _Endocrinology_ 157, 4339–4350 (2016). Article CAS PubMed Google Scholar * Destrez, A., Deiss, V., Leterrier, C., Calandreau, L.
& Boissy, A. Repeated exposure to positive events induces optimistic-like judgment and enhances fearfulness in chronically stressed sheep. _Appl. Anim. Behav. Sci._ 154, 30–38 (2014).
Article Google Scholar * Carreras, R. _et al_. Housing conditions do not alter cognitive bias but affect serum cortisol, qualitative behaviour assessment and wounds on the carcass in pigs.
_Appl. Anim. Behav. Sci._ 185, 39–44 (2016). Article Google Scholar * la Fleur, S. E., Houshyar, H., Roy, M. & Dallman, M. F. Choice of Lard, But Not Total Lard Calories, Damps
Adrenocorticotropin Responses to Restraint. _Endocrinology_ 146, 2193–2199 (2005). Article PubMed CAS Google Scholar * Dallman, M. F. Stress-induced obesity and the emotional nervous
system. _Trends Endocrinol. Metab._ 21, 159–165 (2010). Article CAS PubMed Google Scholar * Pecoraro, N., Reyes, F., Gomez, F., Bhargava, A. & Dallman, M. F. Chronic Stress Promotes
Palatable Feeding, which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. _Endocrinology_ 145, 3754–3762 (2004). Article CAS PubMed Google Scholar * Dallman,
M. F. _et al_. Glucocorticoids, chronic stress, and obesity. in. _Progress in Brain Research_ 153, 75–105 (2006). Article CAS PubMed Google Scholar * Dallman, M. F. _et al_. Chronic
stress and obesity: A new view of ‘comfort food’. _Proc. Natl. Acad. Sci._ 100, 11696–11701 (2003). Article ADS CAS PubMed PubMed Central Google Scholar * Dallman, M. F., Pecoraro, N.
C. & La Fleur, S. E. Chronic stress and comfort foods: Self-medication and abdominal obesity. _Brain. Behav. Immun._ 19, 275–280 (2005). Article PubMed Google Scholar * Spruijt, B.
M., van den Bos, R. & Pijlman, F. T. A. A concept of welfare based on reward evaluating mechanisms in the brain: anticipatory behaviour as an indicator for the state of reward systems.
_Appl. Anim. Behav. Sci._ 72, 145–171 (2001). Article PubMed Google Scholar * Lee, T. K. _et al_. Stress-induced behavioral and metabolic adaptations lead to an obesity-prone phenotype in
ewes with elevated cortisol responses. _Psychoneuroendocrinology_ 47, 166–177 (2014). Article CAS PubMed Google Scholar * Roelofs, S., Boleij, H., Nordquist, R. E. & van der Staay,
F. J. Making Decisions under Ambiguity: Judgment Bias Tasks for Assessing Emotional State in Animals. _Front. Behav. Neurosci._ 10, 1–16 (2016). Article Google Scholar * Romeyer, A. &
Bouissou, M.-F. Assessment of fear reactions in domestic sheep, and influence of breed and rearing conditions. _Appl. Anim. Behav. Sci._ 34, 93–119 (1992). Article Google Scholar *
Forkman, B., Boissy, A., Meunier-Salaün, M. C., Canali, E. & Jones, R. B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. _Physiol. Behav._ 92, 340–374
(2007). Article CAS PubMed Google Scholar * Boissy, A. Fear and fearfulness in animals. _Q. Rev. Biol._ 70, 165–191 (1995). Article CAS PubMed Google Scholar * Mendl, M., Burman, O.
H. P. & Paul, E. S. An integrative and functional framework for the study of animal emotion and mood. _Proc. R. Soc. B Biol. Sci._ 277, 2895–2904 (2010). Article Google Scholar *
Beauchamp, G. What can vigilance tell us about fear? _Anim. Sentience_ 0.15, 1–31 (2017). Google Scholar * Paul, E. S., Harding, E. J. & Mendl, M. Measuring emotional processes in
animals: The utility of a cognitive approach. _Neurosci. Biobehav. Rev._ 29, 469–491 (2005). Article PubMed Google Scholar * Peters, M. L., Vieler, J. S. E. & Lautenbacher, S.
Dispositional and induced optimism lead to attentional preference for faces displaying positive emotions: An eye-tracker study. _J. Posit. Psychol._ 11, 258–269 (2016). Article Google
Scholar * Kress, L., Bristle, M. & Aue, T. Seeing through rose-colored glasses: How optimistic expectancies guide visual attention. _PLoS One_ 13, e0193311 (2018). Article PubMed
PubMed Central CAS Google Scholar * Bethell, E. J. & Koyama, N. F. Happy hamsters? Enrichment induces positive judgement bias for mildly (but not truly) ambiguous cues to reward and
punishment in Mesocricetus auratus. _R. Soc. Open Sci._ 2, 140399 (2015). Article ADS PubMed PubMed Central Google Scholar * Starling, M. J., Branson, N., Cody, D., Starling, T. R.
& McGreevy, P. D. Canine Sense and Sensibility: Tipping Points and Response Latency Variability as an Optimism Index in a Canine Judgement Bias Assessment. _PLoS One_ 9, e107794 (2014).
Article ADS PubMed PubMed Central CAS Google Scholar * Scollo, A., Gottardo, F., Contiero, B. & Edwards, S. A. Does stocking density modify affective state in pigs as assessed by
cognitive bias, behavioural and physiological parameters? _Appl. Anim. Behav. Sci._ 153, 26–35 (2014). Article Google Scholar * Doyle, R. E. _et al_. The effect of repeated testing on
judgement biases in sheep. _Behav. Processes_ 83, 349–352 (2010). Article PubMed Google Scholar * Brydges, N. M., Hall, L., Nicolson, R., Holmes, M. C. & Hall, J. The Effects of
Juvenile Stress on Anxiety, Cognitive Bias and Decision Making in Adulthood: A Rat Model. _PLoS One_ 7, e48143 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Chaby, L.
E., Cavigelli, S. A., White, A., Wang, K. & Braithwaite, V. A. Long-term changes in cognitive bias and coping response as a result of chronic unpredictable stress during adolescence.
_Front. Hum. Neurosci._ 7, 1–10 (2013). Article Google Scholar * Briefer, E. F. & McElligott, A. G. Rescued goats at a sanctuary display positive mood after former neglect. _Appl.
Anim. Behav. Sci._ 146, 45–55 (2013). Article Google Scholar * Coulon, M. _et al_. Effects of prenatal stress and emotional reactivity of the mother on emotional and cognitive abilities in
lambs. _Dev. Psychobiol._ 57, 626–636 (2015). Article PubMed Google Scholar * Doyle, R. E. _Sheep cognition and its implications for welfare_. _Advances in Sheep Welfare_ (Elsevier Ltd.,
2017), https://doi.org/10.1016/B978-0-08-100718-1.00004-2. Chapter Google Scholar * Eldar, E., Rutledge, R. B., Dolan, R. J. & Niv, Y. Mood as Representation of Momentum. _Trends
Cogn. Sci._ 20, 15–24 (2016). Article PubMed PubMed Central Google Scholar * Raoult, C. M. C., Moser, J. & Gygax, L. Mood As Cumulative Expectation Mismatch: A Test of Theory Based
on Data from Non-verbal Cognitive Bias Tests. _Front. Psychol._ 8, 1–10 (2017). Article Google Scholar * Beausoleil, N. J., Blache, D., Stafford, K. J., Mellor, D. J. & Noble, A. D. L.
Selection for temperament in sheep: Domain-general and context-specific traits. _Appl. Anim. Behav. Sci._ 139, 74–85 (2012). Article Google Scholar * Verbeek, E., Ferguson, D. & Lee,
C. Are hungry sheep more pessimistic? The effects of food restriction on cognitive bias and the involvement of ghrelin in its regulation. _Physiol. Behav._ 123, 67–75 (2014). Article CAS
PubMed Google Scholar * Bazely, D. R. & Ensor, C. V. Discrimination learning in sheep with cues varying in brightness and hue. _Appl. Anim. Behav. Sci._ 23, 293–299 (1989). Article
Google Scholar * Paull, D., Lee, C., Colditz, I., Atkinson, S. & Fisher, A. The effect of a topical anaesthetic formulation, systemic flunixin and carprofen, singly or in combination,
on cortisol and behavioural responses of Merino lambs to mulesing. _Aust. Vet. J._ 85, 98–106 (2007). Article CAS PubMed Google Scholar * Murphy, P. M., Purvis, J. W., Lindsay, D. R.
& Le Neindre, P. Measures of temperament are highly repeatable in Merino sheep and some are related to maternal behaviour. in _Proceedings - Australian Society of Animal Production_ 20,
247–250 (Australian Society of Animal Production, 1994). * Bickell, S. L. _et al_. Temperament does not affect the overall establishment of mutual preference between the mother and her young
in sheep measured in a choice test. _Dev. Psychobiol._ 51, 429–438 (2009). Article CAS PubMed Google Scholar * Frid, A. Vigilance by female Dall’s sheep: interactions between predation
risk factors. _Anim. Behav._ 53, 799–808 (1997). Article Google Scholar * R Core Team. R: a language and environment for statistical computing. (2018). * Symonds, M. R. E. & Moussalli,
A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. _Behav. Ecol. Sociobiol._ 65, 13–21 (2011).
Article Google Scholar * Gygax, L. The A to Z of statistics for testing cognitive judgement bias. _Anim. Behav._ 95, 59–69 (2014). Article Google Scholar * Barton, K. MuMIn: Multi-Model
Inference (2018). * Jae Myung, I. The importance of complexity in model selection. _J. Math. Psychol._ 44, 190–204 (2000). Article MATH Google Scholar * Burnham, K. P. & Anderson, D.
R. _Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach_. (Springer-Verlag New York, 2002). * Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting
Linear Mixed-Effects Models Using lme4. _J. Stat. Softw._ 67, 1–48 (2015). Article Google Scholar * Therneau, T. A Package for Survival Analysis in S. version 2.38. (2015). * Therneau, T.
M. & Grambsch, P. M. _Modeling Survival Data: Extending the Cox Model_. (Springer New York, 2000), https://doi.org/10.1007/978-1-4757-3294-8. Book MATH Google Scholar Download
references ACKNOWLEDGEMENTS The authors would like to thank Else Verbeek for her contributions to generating the hypotheses, designing the experiment and coordinating the conduct of the
experiment, Deborah Rufo for her contributions to the coordination and conduct of the experiment, Belinda Henry for advising on the ACTH injection model for chronic stress, Ian Colditz for
contributions to the revision of the manuscript and Lorenz Gygax for revision of the manuscript and statistical advice. The authors would also like to thank the staff and students at CSIRO
who assisted with the training and testing during this experiment; Jim Lea, Tim Dyall, Dom Niemeyer, Danila Marini, Alison Small, Carlos Hernandez, Ian Colditz, Clemence Laude, Guillaume
Havard and Benedicte Cazergue. The first author would like to thank the Sheep CRC and Commonwealth Government for supporting her postgraduate studies with scholarships and training. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * CSIRO, Agriculture and Food, Armidale, 2350, Australia Jessica E. Monk, Sue Belson & Caroline Lee * University of New England, School of
Environmental and Rural Science, Armidale, 2350, Australia Jessica E. Monk * Sheep CRC, University of New England, Armidale, 2350, Australia Jessica E. Monk Authors * Jessica E. Monk View
author publications You can also search for this author inPubMed Google Scholar * Sue Belson View author publications You can also search for this author inPubMed Google Scholar * Caroline
Lee View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS C.L. contributed to the generation of hypotheses, design and conduct of the experiment
and revision of the manuscript. C.L. attained funding for the study. S.B. contributed to the coordination and conduct of the experiment. J.M. conducted the statistical analyses, wrote the
main manuscript text and prepared the figures and tables. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Jessica E. Monk. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Monk, J.E., Belson, S. & Lee, C. Pharmacologically-induced stress has minimal impact on judgement and attention biases in sheep. _Sci Rep_ 9, 11446 (2019).
https://doi.org/10.1038/s41598-019-47691-7 Download citation * Received: 03 April 2019 * Accepted: 22 July 2019 * Published: 07 August 2019 * DOI: https://doi.org/10.1038/s41598-019-47691-7
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative