Nfatc3 controls tumour growth by regulating proliferation and migration of human astroglioma cells
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Calcium/Calcineurin/Nuclear Factor of Activated T cells (Ca/CN/NFAT) signalling pathway is the main calcium (Ca2+) dependent signalling pathway involved in the homeostasis of brain
tissue. Here, we study the presence of NFATc members in human glioma by using U251 cells and a collection of primary human glioblastoma (hGB) cell lines. We show that NFATc3 member is the
predominant member. Furthermore, by using constitutive active NFATc3 mutant and shRNA lentiviral vectors to achieve specific silencing of this NFATc member, we describe cytokines and
molecules regulated by this pathway which are required for the normal biology of cancer cells. Implanting U251 in an orthotopic intracranial assay, we show that specific NFATc3 silencing has
a role in tumour growth. In addition NFATc3 knock-down affects both the proliferation and migration capacities of glioma cells _in vitro_. Our data open the possibility of NFATc3 as a
target for the treatment of glioma. SIMILAR CONTENT BEING VIEWED BY OTHERS REGNASE-2 INHIBITS GLIOBLASTOMA CELL PROLIFERATION Article Open access 18 January 2024 MAP4 KINASE-REGULATED
REDUCED CLSTN1 EXPRESSION IN MEDULLOBLASTOMA IS ASSOCIATED WITH INCREASED INVASIVENESS Article Open access 06 January 2025 KIDINS220 SETS THE THRESHOLD FOR SURVIVAL OF NEURAL STEM CELLS AND
PROGENITORS TO SUSTAIN ADULT NEUROGENESIS Article Open access 04 August 2023 INTRODUCTION Gliomas are among the most severe forms of cancer. Gliomas originate from glia cells (astrocytes,
oligodendrocytes, ependymal cells) or cancer stem cells, and are classified by the World Health Organization (WHO) into four grades based on malignancy (I to IV). The most common of these
fatal tumours is grade IV astrocytoma or glioblastoma (GBM) and the standard treatment is surgical resection followed by radiation and chemotherapy, which does not dramatically improve
clinical outcome. Calcium (Ca2+) dependent signalling pathways are pivotal in the homeostasis of brain tissue. A key element of the cellular response to Ca2+ signals is the action of
calcineurin (CN), a Ca2+- and calmodulin-dependent phosphatase firstly discovered in brain tissue, where is highly abundant1 and described later in many other tissues (reviewed in2,3). CN
activation regulates the activation of the Nuclear Factor of Activated T cells (NFAT) family of transcription factors4,5. NFAT family includes four classic members: c1, c2, c3 and c4 which
were first described in immune cells4,5 and have been associated with malignancies and tumour progression (reviewed in6,7). NFATc members expression has been shown in astrocytoma8, C69 and
U251 glioma cell lines10. Inhibition of the Ca/CN/NFATc pathway by cyclosporine A (CsA) and FK506 has a negative impact on the growth/survival rate of rat and human GBM cells and on glioma
invasion capacity11,12. Accordingly, CsA infusion inhibits tumour growth of implanted glioma _in vivo_11. Although the relevance of the Ca/CN/NFATc pathway in glioma biology has been already
described by using pharmacological intervention, the mechanism and contribution of the different individual NFATc members, and their transcriptional program, remain to be fully assessed.
Cytokines have been proven to be potent mediators of antitumor immunity, and overall, their use as treatment for glioma has produced mixed results. The initial preclinical successes using
systemic or local delivery of recombinant cytokines failed to adequately translate into clinical benefit13, but the search for possible cytokine or other immune based therapies remains to be
fully explored. Cytokines and cytokine receptors expressed by glioma cells have been shown to modulate cell invasiveness, survival, proliferation and angiogenesis14, and many of these
cytokines are over-expressed in high grade human glioma15. Cytokines and chemokines prevalently produced in glioma tissue include Tumour Necrosis Factor alpha (TNF-α)16, IL-8
(CXCL8)17,18,19, macrophage chemo attractant protein-1 MCP-1/CCL220, granulocyte-macrophage colony-stimulating factor (GM-CSF/CSF2)21 and Chemokine Receptor 3 (CXCR3). In other cellular
systems, the expression of these cytokines has demonstrated to be sensitive to the inhibitors of the Ca/CN signalling pathway such as CsA22,23,24,25. The source of these molecules,
attributable to glioma cell, surrounding glial cells, tumour-associated macrophages (TAMS), neuronal or lymphocytes, remains to be fully elucidated. In this work, we evaluated the expression
of the individual NFATc members and investigated the role of the most abundant, NFATc3, by exogenous activation and knock-down strategies. We showed that NFATc3 has a positive role in
tumour growth, since NFATc3 knock-down inhibits both proliferation and migration of U251 glioma cells as a model. Our results suggest that NFATc3 alone is able to modulate a cytokine network
required for the normal growth of glioma. Tools limiting NFATc3 might be considered useful to explore future therapeutic approaches against glioma. RESULTS ANALYSIS OF NFATC MEMBER
EXPRESSION IN U251 CN/NFATc pathway has been previously implicated in the regulation of glioma growth by using pharmacological inhibitors9,26. Nevertheless, the expression and the
contribution of the different NFATc members in glioma cells have not been completely assessed. U251 is a cell line widely established as a model for human glioma of astrocytic nature27.
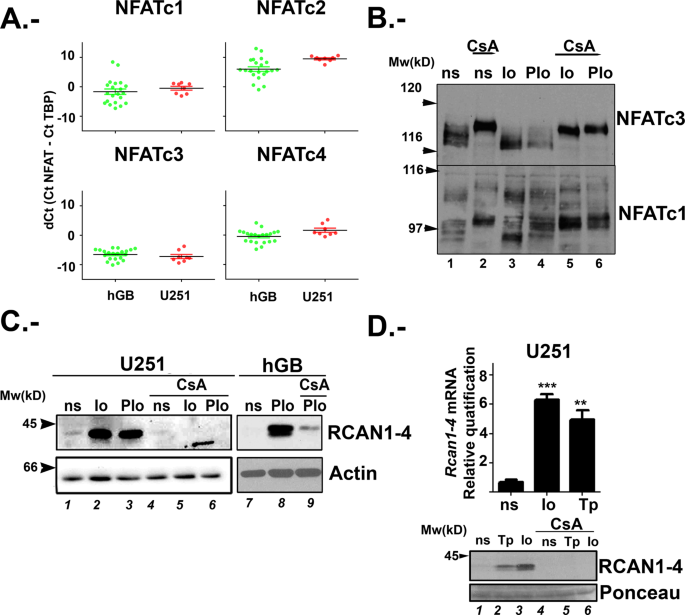
First, we evaluated the expression of the individual NFATc members (c1 to c4) by quantitative PCR (qPCR) in U251 cells, together with a collection of human glioblastoma (hGB) cell lines
obtained from xenografts. We found that _NFATc3_ has the highest expression levels in both hGB cell lines and U251. _NFATc1_ is also clearly expressed, but in relative less amount. _NFATc2_
transcript was observed only in some hGB cell lines but hardly detected in U251, and _NFATc4_ expression varied among hGB cell lines (Fig. 1A), while it was detectable only in starved U251
(data not shown). In summary, U251 cells have a NFATc expression pattern similar to most of the primary hGB tested, and _NFATc3_ and _NFATc1_ are consistently expressed in both models.
Figure 1A shows mRNA expression data presented as delta Ct (dCt). dCt corresponds to the difference between the Ct (cycle threshold) of target gene and the Ct of the reference gene, i.e. TBP
(TATA binding protein). By using TaqMan probes with similar efficiency, NFATc3 dCt negative values imply that this member is the most abundant in glioma samples. Our results are consistent
with data generated by the TCGA Research Network showing that _NFATc1_ and _NFATc3_ are expressed in higher amount in human glioblastoma samples when compared to normal brain tissue. On the
other hand, _NFATc2_ and _NFATc4_ expression was comparable between normal and tumour samples (Supplemental Fig. S1A), although with low abundance (Fig. 1A). Furthermore, we interrogated the
RNAseq data of already published results (TCGA-GBM study), and found that there was a significant increase of _NFATc_ expression across glioblastoma tumour grade (Supplemental Fig. S1B).
Then, we evaluated individual NFATc1-c4 members expression at the protein level using specific antibodies previously validated28,29. U251 total protein extracts showed clear NFATc3 and
NFATc1 protein expression (Fig. 1B); NFATc2 and NFATc4 immunoblots did not reveal specific signal of the expected molecular weight. NFATc proteins have a complex electrophoretic mobility
since there is different phosphorylation/ dephosphorylation status of NFATc proteins in response to changes in intracellular calcium concentration ([Ca2+]i) by ionophore A23187 (Io)30. Cells
were stimulated with Io (1 μM) for 30 minutes and, as expected, faster migrating bands were detected, corresponding to dephosphorylated forms of NFATc3 and NFATc1 (Fig. 1B, lane 3).
Noteworthy, NFATc1 antibody recognized different NFATc1 isoforms, as previously described29. As additional control, CsA pre-treatment, known to inhibit the CN dependent NFATc
dephosphorylation, retarded the gel mobility of NFATc3 and NFATc1 members, confirming antibody specificity (Fig. 1B, lanes 5 and 6). Therefore, we consider that U251 is a valuable glioma
model since the expression pattern of NFATc members is comparable to other hGB cell lines and clinical samples (Fig. 1A and Supplemental Fig. S1) and confirmed the specificity of the
antibodies used. RCAN1-4 GENE IS INDUCED IN U251 GLIOMA CELLS IN A CA/CN/NFATC DEPENDENT MANNER RCAN1-4 is a member of the calcipresin family, endogenous modulators of CN activity (reviewed
in31), known to be involved in tumour progression32. We and others have previously shown that RCAN1-4 expression is highly sensitive to NFATc activation. Therefore, it has been used as a
sensor for the Ca/CN/NFAT pathway activation in different biological setting30,33,34. Thus, we tested if an increase of [Ca2+]i by Io alone was able to induce the RCAN1-4 expression in U251
glioma cells. We also included, as stimulus, the MAPK inducer Phorbol 12-myristate-acetate (PMA) in combination with Io (PIo) to activate other cofactors known to partner with the NFATc
proteins to regulate a variety of genes29,30. We observed that Io alone or in combination with PMA (PIo), increased RCAN1-4 protein levels. This increase was inhibited by CsA pre-treatment
(Fig. 1C). Alternative glioma cell lines, such as primary hGB and U87 showed similar results (Fig. 1C and data not shown). Accordingly, analysis by qPCR showed that Io treatment induced the
accumulation of _RCAN1-4_ mRNA in a CsA sensitive manner (Fig. 1D). Similar results were confirmed by using other means of increasing [Ca2+]i such as thapsigargin A (Tp) (Fig. 1D). When U251
glioma cells were stimulated with PMA alone, there was no detectable induction of RCAN1-4 mRNA or protein, in accordance to the results previously observed in primary astrocytes30
(Supplemental Fig. S2). NFATC3 PROTEIN IS THE MAIN MODULATOR OF RCAN1-4 EXPRESSION IN GLIOMA Since NFATc members have been associated with malignancies and tumour progression (reviewed in6),
we investigated the individual contribution of NFATc members expressed in U251 glioma cell line. Therefore, we used lentiviral vectors expressing short hairpin RNA (shRNA) to specifically
silence each of the two NFATc members mostly expressed in these cells. Lentiviral shRNA vectors targeting NFATc1 and NFATc3, defined as NFATc1 shRNA (KD-c1) and NFATc3 shRNA (KD-c3), and
lentiviral vectors encoding for a scramble shRNA (KD-Sc) as control, were previously described and validated29. More than 95% infection efficiency was monitored by flow cytometry of Green
Fluorescent Protein (GFP) reporter gene (Fig. 2A). Immunoblot analyses showed that KD-c3 yielded a very efficient silencing of NFATc3 protein, although a minimal effect on NFATc1 expression
was also observed. When KD-c1 was used, NFATc1 knockdown was also efficiently achieved with minimal inhibition of the NFATc3 expression. This slight cross-downregulation between specific
shRNA and non- targeted NFATc member might be due to either mechanisms of transcriptional cross regulation among members or, giving the high efficiency of infections, to shRNA unspecificity
(Fig. 2B). This effect have not been observed in other cell types29. Besides the minimal cross inhibition between shRNA and NFATc members, considering the strong knockdown effect on the
target member, we assumed that most of the experimental effects observed were consequence of the target member silencing. Next, we analysed the effect of NFATc1 and NFATc3 knock-down on
RCAN1-4 protein expression. PIo-induced RCAN1-4 protein expression was inhibited by 80% in KD-c3 cells when compared to KD-Sc control and the effect was comparable to the CsA pre-treatment
(Fig. 2C,D). A lesser, but significant, inhibition was also observed in KD-c1 cells (Fig. 2C,D). In this case, seen the small reduction, we did not discard the possibility that this effect
might be associated to the slight NFATc3 inhibition by KD-c1 and not strictly to NFATc1, but further analyses will be needed. In summary, our data suggest that NFATc3 is highly expressed in
human glioma and silencing of this member is critical for RCAN1-4 expression in U251. ANALYSIS OF GENES REGULATED BY NFATC3 IN U251 GLIOMA CELLS To define cancer related genes depending on
NFATc3 activity in glioma cells, we infected U251 with an adenovirus encoding nuclear constitutively active (CA) NFATc3 (Ad NFATc3 CA)35, or with an empty adenovirus vector (Ad Control) as a
mock control. We have shown in astrocytes that RCAN1-4 depends on NFATc activity alone, while COX-2 (Cyclooxygenase 2) transcription depends on a cooperation between NFATc and cofactors
activated by stimulation with PMA30. COX-2 and RCAN1-4 protein expression was used as read out to validate the constitutive activation of this signalling pathway with the Ad NFATc3 CA (Fig.
3A). Cells were left non stimulated (ns) or stimulated with PMA alone. Since the Ad NFATc3 CA is a constitutively nuclear localized mutant, calcium signal is not required to activate NFATc3.
As expected, the presence of only nuclear NFATc3 was sufficient for maximal RCAN1-4 protein and mRNA expression (Fig. 3A,B); whereas COX-2 expression was maximally induced after stimulation
with PMA of U251 infected with Ad NFATc3 CA (Fig. 3A,C), coherent to published data on astrocytes30. We have noticed that in some of the experiments the adenoviral vectors alone increased
slightly the amount of RCAN1-4 protein and mRNA, mainly in PMA treated cells (Fig. 3A,B). Adenoviral gene transfer could result in alteration in membrane structure and in turn can affect
intracellular [Ca2+]i36. In fact, PMA-stimulated induction of RCAN1-4 was not statistically different between experiments and, most importantly, this increase was not observed in cells
treated with PMA but not transduced with adenoviral vectors, as shown in supplemental Fig. 2S. To understand if NFATc3 is a critical factor for cytokines and chemokines known to be involved
in tumour growth, we evaluated the impact of nuclear NFATc3 on _TNF-α_, _CXCR-3_, _GM-CSF_, _IL-2 and CCL-2 mRNA_ expression. As shown in Fig. 4A–E, the Ad NFATc3 CA expression alone
increased _TNF-α_, _CXCR-3_, _GM-CSF_, _IL-2 and CCL-2_ mRNA levels. PMA stimulation was needed to achieve maximal induction of these genes, indicating the requirement of cofactors that are
elicited by other signalling pathways, similarly to _COX-2_ (Fig. 3). IL-6 and IL-8 were included as controls, representing genes expected to be mainly regulated by PMA-dependent signals. In
fact, IL-6 promoter is known to contain binding sites for several transcription factors which contribute to the complex regulation of this gene37. For IL8 promoter, participation of a
number of different transcription factors has also been described: nuclear factor-κB (NF-κB), activating protein (AP-1), CAAT/enhancer-binding protein β (C/EBPβ, also known as NF-IL-6),
C/EBP homologous protein (CHOP) and cAMP response element binding protein (CREB)38. In our experiments, the presence of PMA alone was sufficient to obtain maximal expression of _IL-6_ (Fig.
4F) and _IL-8_ (Fig. 4G) mRNA. However, a relevant role for NFATc in IL-8 expression has been described in some other type of cancerous cells39. In our results, the fold induction of _IL-8_
mRNA in presence of PMA was of 17.15 ± 1.89 over the control cells, while in presence of NFATc3 CA the fold induction was only mildly increased to 20.51 ± 1.13, a difference that was not
statistically significant. _IL-1β_ and _VEGF_ were included as negative control for all the stimuli tested, as shown in Fig. 4H,I. To further explore the impact of NFATc3 in the regulation
of these genes, we compared control KD-Sc cells with silenced KD-c3 cells. PIo-treated KD-Sc cells showed an increase in the levels of _TNF-α_, _CXCR-3_, _GM-CSF_, _IL-2_, _CCL-2; IL-8_
(Fig. 5C–H) similar to PIo-treated non transduced cells (data not shown). NFATc3 silencing significantly impaired the expression of PIo dependent genes _RCAN1-4_, _COX-2; TNF-α; CXCR-3_,
_GM-CSF and IL-2_ (Fig. 5A–F). Unexpectedly, PIo-induced _CCL-2_ expression was not affected (Fig. 5G) and we observed a mild but consistent inhibition of _IL-8_ mRNA induction in KD-c3
cells (Fig. 5H), that might suggest some NFATc role as previously described in other tumour cells39. _VEGF_ was not induced by PIo treatment nor inhibited in KD-c3 cells (Fig. 5I). NFATc
members are known to bind the same sequence on target genes promoters. Although we found that NFATc3 is the major member expressed in glioma cells we wanted to evaluate if NFATc1 has a role
for the expression of these cytokines (Fig. 5). We found that NFATc1 silencing had no impact on _CXCR-3_, _GM-CSF_, _IL-2 and IL-8_ mRNA expression and concluded that these genes are NFATc3
dependent in this cellular setting. Nevertheless, our data indicated that NFATc1 could participate in the _RCAN1-4_ (as shown in Fig. 2C,D) and _TNF-α_ regulation (Fig. 5). Finally, NFATc1
seems to be relevant for the regulation of COX-2 but as negative regulator, in contrast to NFATc3. NFATc1 as a negative regulator has been previously described in lymphoid cells29 and muscle
cells40. NFATC3 CONTROLS U251 TUMOUR GROWTH IN AN ORTHOTOPIC MOUSE MODEL Our results indicate that NFATc3 is the most expressed NFATc member in U251 and other hGB cell lines and in an array
of human glioma samples according to published databases (Fig. 1 and Supplemental Fig. 1S). In addition, we suggest here that NFATc3 is a key positive regulator of _RCAN1-4_, _COX-2; TNF-α;
CXCR-3_, _GM-CSF_ and _IL-2_, known to be implicated in glioma growth15,16,17,18,19,20,21. Therefore, we evaluated the specific contribution of this NFATc member in tumour formation _in
vivo_. We established a reporter U251 cell line expressing the fluorescence marker mCherry and the luciferase gene, selected based on puromycin resistance (U251 mCherry-Luc). Next,
puromycin-selected mCherry-Luc U251 glioma cells were transduced with KD-Sc (Sc) or KD-NFATc3 (c3) lentiviral vector. Cells were analysed by flow cytometry confirming that 100% of the cells
co-expressed mCherry and GFP proteins (Fig. 6A). Before injection, NFATc3 knockdown was validated by immunoblotting (Fig. 6B). In order to evaluate if the knockdown of NFATc3 has an impact
on the viability of the glioma, we performed an orthotopic glioma mouse model, previously described in41. Briefly, 1 × 105 of U251 Sc or c3 were injected in the striatum of nude mice.
Luciferase expression was assessed as a proxy of tumour growth every 10 days, starting from day 35, until the end of the experiment (55 days), when control Sc animals started to show
physical distress. Luminescence signal showed that implanted Sc tumour cells were surviving and growing. In contrast, implanted c3 tumour cells was under the detection limit. As a further
confirmation that c3 cells were not able to grow _in vivo_, we visualized by immunofluorescence GFP expression in brains isolated from the two animal groups (Fig. 6D). The results confirmed
the luciferase measures: animals injected with c3 cells did not show glioma growth. Interestingly, the surviving c3 cells were observed sitting around the injection area, although they did
not appear to be able to grow or invade the surrounding tissue (insert in Fig. 6D). NFATC3 IS REQUIRED FOR U251 PROLIFERATION AND MIGRATION There are two main cellular pathways involved in
the establishment of tumour mass: proliferation and migration. Migration and invasion of glioma cells is a very complex and multistep process that comprises the coordinated action of
different cytokines and enzymes42. As mentioned above, we have found that NFATc3 is required for the expression of several mediators known to be involved in tumour growth (Figs 3–5).
Additionally, it has been previously demonstrated that the inhibition of the whole NFATc signalling pathway by CsA is able to inhibit the invasion capacity and growth of glioma cells12.
Therefore, we questioned the specific contribution of NFATc3 to this process by analysing the effect of NFATc3 silencing in the U251 glioma growth _in vitro_. The proliferation capacity of
KD-Sc and KD-c3 cells was measured by flow cytometry during three days. As shown in Fig. 7A, the NFATc3 knockdown markedly reduced the proliferation capacity of U251 cells in presence of low
serum (2%), although KD-Sc cells, in the same conditions, were able to proliferate (Fig. 7A). Next, considering that the cells inoculated in the mouse model appeared to have remained in the
same site of injection, we decided to investigate the effect of NFATc3 knockdown on the migration capacity of U251 glioma cells _in vitro_. U251 were grown to confluence and a wound-healing
assay was performed by linear disruption of the monolayer. As additional control, KD-c1 cells were also included. 24 h after the monolayer disruption, KD-Sc and KD-c1 U251 cells efficiently
reoccupied the denuded area, while KD-c3 cells were unable to produce the closure, indicating impaired proliferation and migration capacity (Fig. 7B). The migratory activity of U251 cells
was further assessed by transwell assay in response to a serum gradient. After 4 hours, the number of KD-c3 cells migrating through the membrane of the transwell chamber was reduced when
compared with KD-Sc cells, indicating the strong participation of NFATc3 in the glioma cells migration (Fig. 7C). Therefore, our results show that NFATc3 expression is required for both
proliferation and migration and are in line with the data obtained in the _in vivo_ model. DISCUSSION Glioma therapy without damaging the affected brain parenchyma is still difficult due to
the infiltrative growth pattern of these tumours and the rapidly growing rate of this type of cancer cells. The Ca/CN/NFATc pathway has been used as a target for various inhibitors, with
discrete success and large side effects. If it would be possible to block this route more specifically, better therapies could be developed without the inconveniences presented by the
current ones. Our data suggest that _NFATc1_ and especially _NFATc3_ are overexpressed in tumour samples compared with normal tissue, while _NFATc2_ is minimally expressed. This result is
not in line with the study from Tie _et al_.43 where _NFATc2_ is described as highly expressed. The reason behind this apparent contradiction might be related to the selection of high grade
glioma samples in the referred study. TCGA data show that all members are overexpressed in grade IV glioma samples, and our data suggest a role of _NFATc3_ in earlier phases, during the
establishment of the tumour. The U251 glioma model used in this study meets a number of requirements: these cells are of astrocytic nature, which account for 60–70% of total malignant brain
tumours and has been used widely to study the effect of different signalling pathways involved in the orthotopic intracranial assays. Here, in the context of _NFATc_ expression, we have
validated this model by showing that U251 reproduced a pattern of _NFATc_ expression similar to primary hGBs cell lines and glioma samples (TCGA-GBM data). Nonetheless, we found that the
expression of the individual _NFATc_ members varies in the different human xenografts lines tested, being always _NFATc3_ the most abundant (Fig. 1). This indicates that to interfere
efficiently with the NFATc-signalling, an analysis of the different members in the distinct type of glioma cell should be carefully performed. Analysis of NFATc proteins is a difficult task,
as mentioned above. Here we describe the appearance of slower migrating bands for NFATc3 in response to 1 h PIo treatment in line with previously reported by our group in other
cells29,30,44. Other NFATc members, such as NFATc2, have a similar behaviour. Pre-treatment of these cells with CsA, retards the gel mobility of all NFAT members analysed, as previously
described28,29, and is shown here in Fig. 1B. RCAN1-4 has been previously shown to be a NFATc-target gene in a variety of cellular systems30,34,45, sensitive to calcium stress in human
glioma cell lines46 and described as a sensor of the Ca/CN/NFAT signalling pathway activation in different biological setting30,33,34. In this study we have used pharmacological activation
with Io or Tp alone to achieve a robust increase of [Ca2+]i. The physiological stimuli that produce [Ca2+]i increments has not been interrogated in this work, although there is a great
variety of stimuli and receptors that have been involved in [Ca2+]i in glioma cells (reviewed in47 and references therein). The impact of CsA and FK506 as inhibitors of the Ca/CN/NFAT
signalling pathway on glioma growth has been published before9,26. High doses of CsA (40 μM) has been reported to induces apoptosis of rat C6 glioma cells48. However, other authors have not
found morphological changes indicative of apoptosis in human glioma cells line such as U251 when using a lower dose, 1 μM49. We have used 1 μM CsA which do not produce significant cell death
but inhibit efficiently the activation of Ca/CN/NFAT pathway. Furthermore, KD-c3 and KD-Sc U251 cells present similar cell cycle profiles at 24 hours before injection in the orthotopic
mouse model (Fig. 6 and data not shown). Gene deletion of single NFATc members on immune cells has shown a mild impact, and this fact has been interpreted as the existence of functional
redundancy (reviewed in50). Our experiments show that even in condition in which NFATc1 and NFATc3 knockdown were efficiently achieved, the majority of the NFATc-directed signalling
correlates with NFATc3 presence. Combining the results obtained by silencing or over-expressing NFATc3, this study indicates that this member is involved in _RCAN1-4_, _COX-2_, _TNF-α_,
_IL-2_, _GM-CSF_ and _CXCR-3_ gene regulation in U251 glioma cells. Although the KD-c3 reduced partially the expression of PIo-inducible _IL-8_, NFATc3 overexpression did not enhance _IL-8_
induction above PMA values. It has been shown that IL-8 promoter can be activated by PMA-elicited signals alone in cancer cells38 although, in breast cancer, requires NFATc participation39.
Therefore, it is quite plausible that the inhibition of the abundant NFATc3 in U251 might be able to partially compromise the IL-8 transcription in glioma cells. Migration and invasion
capacity of glioma cells are main features which account for the aggressive behaviour of brain tumours. Many processed are involved in tumour migration, from cytokine and chemokine
expression, regulation of adhesion capacity and changes in the extracellular matrix. A role of NFATc signalling in migration has been described for different cancer cells7 (reviewed in6 and
references therein) and, in other cell systems, has been related to the response to VEGF34, to SFRP (secreted frizzled-related protein)51 and activated Peroxisome Proliferator Activated
Receptor gamma (PPARγ)52. Treatment of glioma cells with CsA inhibited growth of EGFP-transfected glioblastoma cells in organotypic brain slices12. CsA is an inhibitor of the CN, which has
different cellular substrates besides NFATc53; if the CsA mediated inhibition of glioma proliferation and migration is NFATc dependent has not been clearly assessed _in vivo_. Here we show
that NFATc3 is required for the normal proliferation and migration capacity of the U251 glioma cells, and for tumour establishment in xenografts. We have identified NFATc3-dependent genes
that are known to be involved in tumour migration: _COX-2_ and _CXCR-3_. NFATc dependent _COX-2_ induction has been observed in different cellular systems such as endothelial cells and
breast epithelial cells and is required to promote invasive migration29,54. High COX-2 expression in tumour cells is associated with clinically more aggressive gliomas and is a strong
predictor of poor survival55. More recently, epidemiologic studies have highlighted associations between the regular use of non-steroidal anti-inflammatory drugs (NSAID) and reduced glioma
risks in humans and it has been shown that COX-2 pathway promotes gliomagenesis by directly supporting systemic development of myeloid derived suppressor cells (MDSCs) and their accumulation
in the tumour microenvironment56. Our results support a specific role for NFATc3 in the positive _COX-2_ regulation in U251 glioma cells, with clear therapeutic implication for the glioma
growth. Interestingly, the opposite role of NFATc1 on _COX2_ regulation will require to be further explored. The CXCL10/CXCR-3 axis has been shown to be expressed in glioma cells, and NFATc3
dependent regulation of CXCR3 is on line with the role of this receptor in migration and site specific dissemination in many cancerous cells15,57,58,59. Migration is a complex multistep
process and we expect that many other factors, besides the genes explored in this study, contribute to cell migration. If these factors are NFATc3 dependent will need further analyses.
Preliminary tests by using individual blocking antibodies against target genes were not sufficient to inhibit the migration of control glioma cells (data not shown). Inhibition of
NFATc-signalling using small molecule inhibitors is predicted to suppress tumorigenesis, hopefully without the secondary effects of the immunosuppressant regimes. Therefore, future cancer
therapy targeting NFATc must take into account the different cellular types which participate in glioma microenvironment such as cancer cells, microglia and macrophages, and tools for
specific delivery of this regulators will be required. MATERIAL AND METHODS CELL LINES _U87 and U251_ human glioblastoma cell lines were purchased from ATCC and maintained in DMEM (LONZA),
containing 1X Glutamine (LONZA), 10% Fetal Bovine Serum (FBS) (HiMedia), and 100 U/mL PenStrep (LONZA). Cells were genotyped using StemElite ID genotyping system (Promega). _Human
glioblastoma_ (_hGB_) cell lines obtained from xenografts were obtained after injection of small pieces of human tumours (embedded in Matrigel, Corning) in the flank of immunodeficient mice,
as described in60. Samples were obtained from patients treated at Hospital Universitario 12 de Octubre (H12O, Madrid, Spain). Informed consent from patients was obtained. None of them was
under the age of 18th. The study was performed with the approval and following the guidelines of the Research Ethics Committee of Hospital 12 de Octubre (CEI 14/023). U251-MCHERRY-LUC CELLS
U251 cells were transduced with lentiviral vector (mCherry-Luc) as below. After 48 hours, cultures were monitored for cherry fluorescence. Cherry positive cells were selected with 1 μg/mL
puromycin in media and analysed by flow cytometry. CELL LYSIS AND IMMUNOBLOT ANALYSIS For whole-cell extracts, cells grown and stimulated were washed twice with cold PBS and lysed for 30 min
on ice in 100 μL hypertonic buffer as described30. Total extracts were resolved by 10% SDS-PAGE and proteins transferred to nitrocellulose membranes. The following antibodies were used:
anti-NFATc1 [7A6, Alexis Biochemicals], NFATc3 [M75, Santa Cruz], NFATc2 [25A10.D6.D2, Abcam], NFATc4 [H-74, Santa Cruz] α-tubulin (Sigma), β-actin (Sigma), mouse anti RCAN1-4 (as described
in30). Antibody was detected with enhanced chemiluminescence (ECL) detection reagent (GE Healthcare Lifesciences). RNA ISOLATION AND REAL-TIME PCR Total RNA was extracted with Tripure
(Roche), and 2 μg were reverse transcribed to cDNA with MMLV-RT (Invitrogen). Real-time quantitative PCR (qPCR) in duplicate was carried out on 100 ng cDNA, using TaqMan probes and SYBR
Green system (Applied Biosystems) in ABI Prism 7900 or 7500 Fast Real Time PCR System (Applied Biosystems). Taqman probes used were NFATc1 (cat # Hs 00542678_m1), NFATc2 (cat # Hs
00234855_m1), NFATc3 (cat # Hs 00190046_m1), NFATc4 (cat # Hs 00190037_m1), COX2 (cat #Hs00153133_m1), IL2 (cat #Hs00174117_m1) RCAN1.4 (custom assay hDSCR1.4-E4_5 cat#4331348), Rcan1 (cat#
Hs01120954_m1), IL8 (cat# Hs00174103_m1), VEGF (cat#Hs00173626_m1), TNFα (Hs00174128_m1). The following Taqman probes were used as endogenous controls: TFRC (cat #4333770 F), GAPDH (cat
Hs99999905_m1) and 18 S rRNA (cat#4319413E). Relative quantification was calculated according to manufacturer’s instructions. Primers used for SYBR Green assays were as follow, TFRC was used
as endogenous control. CCL2: Forward: CAGCCAGATGCAATCAATGCC Reverse: TGGAATCCTGAACCCACTTCT CxCR3: Forward: TTTGACCGCTACCTGAACATAGT Reverse: GGGAAGTTGTATTGGCAGTGG GM CSF: Forward:
GTGGCATTCAAGGAGTACCTC Reverse: TGATGGCCTTCGATTCTGGATT TFRC: Forward: GCAAGTAGATGGCGATAACAGT Reverse: CAATAGCCCAAGTAGCCAATCAT LENTIVIRAL VECTORS Knockdown (KD) sequences were designed cloned
into an EcoRI-NotI LV-Ubq-GFP lentiviral vector (LV) as described previously in29. The KD sequences employed were as in29,61. KD-Sc (5′-3′):
GATCCGGCAACAAGATGAAGAGCACCTCGAGTTGGTGCTCTTCATCTTGTTG TTTTTGTTTAAACGC. KD-c1 (5′-3′): GATCCCCGCCAGTACCAGCGTTTCACTTCAAGAGAGTGCGCTGGTACTGGCTTTTTGGAAC. KD-c3 (5′-3′):
GATCCCCGACAGTCGCTACTGCAAGCTTCAAGAGAGCTTGCAGTAGCGACTGTCTTTTTGGAAC Lentiviruses expressing mCherry and luciferase genes (mCherry-Luc) were produced using PLKO-mcherry-luc-puro-Renilla cDNA
(Addgene). LENTIVIRAL PRODUCTION, TITRATION AND INFECTION Lentiviruses expressing mCherry-Luc and KD sequences were obtained by transient calcium phosphate transfection of HEK-293 cells with
a three plasmid HIV-derived and VSV pseudotyped lentiviral system kindly provided by M. K. Collins (University College London, UK). Supernatant containing lentiviral particles was collected
48 h after removal of the calcium phosphate precipitate and was then ultracentrifuged for 2 h at 121,986xg at 4 °C (Ultraclear Tubes, SW28 rotor and Optima L-100 XP Ultracentrifuge,
Beckman). Viruses were collected by adding cold sterile DMEM and were titrated by qPCR, as described by Scherr _et al_.62. U251 glioma cells were infected by adding virus (MOI = 6) and
incubating for ON at 37 °C in complete medium DMEM 10% FBS and antibiotics. Infection efficiency (GFP or mCherry expression) and cell death (propidium iodide staining) were monitored by flow
cytometry. ADENOVIRUS INFECTION OF U251 GLIOMA CELLS The adenovirus encoding constitutively active NFATc3 (Ad NFATc3 CA) and the control adenovirus (Ad Control) were as described34.
Adenoviruses were generated and purified following the standard protocols30. Adenovirus infection was performed on subconfluent U251 glioma cells, and expression of the encoded protein was
monitored by immunoblotting. FLOW CITOMETRY Single cell suspensions were prepared in staining buffer (2% FBS) in PBS. Analysis was performed in a LRS Fortessa X-20 (BD Biosciences)
cytometer, using the FlowJo v6.3.4 (TreeStar) and DIVA v8.0 software packages. Proliferation of infected U251 cells was analysed with the Violet Proliferation Dye 450 (BD Horizon). Infected
cells were labelled according to the manufacturer’s protocol and stimulated with 2% FBS for three days. Violet staining was determined by flow cytometry in viable cells at day 0 and day 3
after stimulation. _Division Index_: the average number of cell divisions that a cell in the original population has undergone. _Proliferation Index_: the total number of divisions divided
by the number of cells that went into division. Only takes into account the cells that underwent at least one division. Data was analysed with FlowJo 7.6.3 software (Tree Star, Inc.). MOUSE
BRAIN XENOGRAFT ASSAYS Housing and animal experiments were performed according to European Union 86/609/EEC guidelines and approved by the Animal Committee of Instituto de Salud Carlos III.
All surgical procedures were carried out under general anaesthesia with 8 μL/g intraperitoneal (IP) anaesthetic solution composed of 10% ketamine (Imalgene) and 9.3% xylazine (Xilagesic)
with subcutaneously administration of 10% analgesic solution (4 μL/g, Metacam). After surgery, mice were woken from anaesthesia with 7.7% reverting solution (4 μL/g, Antisedan). All of them
prepared in PBS. ORTHOTOPIC XENOGRAFTS gGuided intracranial injections using stereotactic frame (Leica) in athymic nude Foxn1nu mice (Harlan Iberica) were performed by administering 1 × 105
of U251 transduced with lentiviral vector as indicated. Cells were resuspended in 2 μL PBS and injection were made into the striatum (coordinates: A-P, −0.5 mm; M-L, +2 mm; D-V, −3 mm;
related to Bregma) using a Hamilton syringe. Animals were sacrificed at the onset of distress or when specify if symptoms were not apparent. _IN VIVO_ IMAGING Mice were injected with 100 μL
of luciferin (12,5 mg/mL) in PBS (Becton Dickinson), anesthetized with isoflurane and placed in an IVIS Lumina XR Serie III (Caliper, BD), being kept with the same solution of 2% isoflurane
gas in 100% oxygen at a flow rate of 0.5 mL/min during the process. Mice were placed in the IVIS and 5 images were taken every 30 seconds to get the signal peak. Imaging data are reported as
(photons/sec/cm2/steradian). TISSUE PROCESSING For confocal studies, mice were deeply anesthetized by intraperitoneal (ip) injection of a mixture of ketamine and xylazine and transcardially
perfused with 25–30 mL of saline solution for 5 min, followed by 10 min with 4% PFA (Sigma), pH 7.4, in 0.1 M phosphate buffer (PB, Sigma). Brains were obtained and post fixed with 4% PFA
for 18–20 h at 4 °C, rinsed in 0.1 M PB and placed in 15% glucose at 4 °C until they sank followed by immersion in 30% sucrose in PB at 4 °C for 72 h. Finally, brains were embedded in tissue
freezing medium (Tissue-Tek O.C.TTM, Sakura), by submerging brains in increasing concentrations of OCT, snap frozen in dry-ice-cooled 2-methylbutane (Sigma) and stored at −80 °C. Coronal
sections (30 μm) were obtained with a CM1950 cryostat (Leica Microsystems) and stored at −20 °C until use. To perform qPCR on brain samples, brains were dissected and tumours were extracted
and snap-freeze. Samples were liquid-nitrogen-cooled pestle and mortar fragmented for subsequent analysis. CONFOCAL MICROSCOPY AND ANALYSIS Images were acquired on a Leica confocal
microscope SP5 (Leica Microsystems). Brain maps were imaged using a 20X immersion objective and a 3.0 optic zoom (2.0 μm step size, 512 × 512 pixel resolutions). Map images are presented as
maximum projections of z-stacks. Co-localization was measured on one z using the Leica LAS AF program to obtain Pearson coefficient. MIGRATION ASSAYS For wound healing assay, infected U251
cells were plated at 90% confluence in p35 dishes. After 24 h, monolayers were scratched with a p200 pipette tip. Cells were then washed and stimulated with medium containing 2% serum. Wound
healing was monitored by time lapse microscopy with a NIKON Ti-Eclipse inverted microscope fitted with a 4X objective lens. Images were acquired with Nikon Nd2 Viewer and cell-free area was
calculated with ImageJ software. For transmigration assay, infected U251 cells (10 × 105/well) were seeded onto 8 µm-pore transwell 24-well inserts (Costar) in DMEM 0.1% BSA (DMEM-BSA). 1
mL DMEM-BSA with or without 2% FBS was added in the lower chamber of the transwell, after 4 hours non migrating cells were removed and the filter was fixed in 4% paraformaldehyde and stained
with Hoechst 33342 (Invitrogen). Migrated cells were counted with Image J software in four random fields in 4X pictures using a Nikon Eclipse TE2000-U Microscope in duplicates. STATISTICAL
ANALYSIS Presented data is shown as means ± SD of three or more independent experiments. Statistical significance was estimated by Student’s t using GraphPad Prism 5.0 (GraphPad Software).
Differences were considered significant at *p < 0.05. For immunoblots, a linear correlation was observed between increasing amounts of input protein and signal intensity. DATABASE
ANALYSIS TCGA expression data (http://cancergenome.nih.gov/) were analysed using GlioVis (http://gliovis.bioinfo.cnio.es/) data portal for visualization and analysis of brain tumour
expression datasets63. Data downloaded were analysed using GraphPad Prims 5.0. REFERENCES * Klee, C. B., Crouch, T. H. & Krinks, M. H. Calcineurin: a calcium- and calmodulin-binding
protein of the nervous system. _Proc. Natl. Acad. Sci. USA_ 76, 6270–6273 (1979). Article ADS CAS Google Scholar * Mansuy, I. M. Calcineurin in memory and bidirectional plasticity.
_Biochem. Biophys. Res. Commun._ 311, 1195–1208 (2003). Article CAS Google Scholar * Martinez-Martinez, S. & Redondo, J. M. Inhibitors of the calcineurin/NFAT pathway. _Curr. Med.
Chem._ 11, 997–1007 (2004). Article CAS Google Scholar * Jain, J., Miner, Z. & Rao, A. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. _J
Immunol._ 151, 837–848 (1993). CAS PubMed Google Scholar * McCaffrey, P. G., Perrino, B. A., Soderling, T. R. & Rao, A. NF-ATp, a T lymphocyte DNA-binding protein that is a target for
calcineurin and immunosuppressive drugs. _J Biol. Chem._ 268, 3747–3752 (1993). CAS PubMed Google Scholar * Mancini, M. & Toker, A. NFAT proteins: emerging roles in cancer
progression. _Nat. Rev Cancer_ 9, 810–820 (2009). Article CAS Google Scholar * Jauliac, S. _et al_. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. _Nat.
Cell Biol._ 4, 540–544 (2002). Article CAS Google Scholar * Jones, E. A., Sun, D., Kobierski, L. & Symes, A. J. NFAT4 is expressed in primary astrocytes and activated by glutamate. _J
Neurosci. Res._ 72, 191–197 (2003). Article CAS Google Scholar * Mosieniak, G., Pyrzynska, B. & Kaminska, B. Nuclear factor of activated T cells (NFAT) as a new component of the
signal transduction pathway in glioma cells. _J Neurochem._ 71, 134–141 (1998). Article CAS Google Scholar * Wang, L. _et al_. NFATc1 activation promotes the invasion of U251 human
glioblastoma multiforme cells through COX-2. _Int J Mol. Med._ 35, 1333–1340 (2015). Article Google Scholar * Gabrusiewicz, K. _et al_. Characteristics of the alternative phenotype of
microglia/macrophages and its modulation in experimental gliomas. _PLoS One_ 6, e23902 (2011). Article ADS CAS Google Scholar * Sliwa, M. _et al_. The invasion promoting effect of
microglia on glioblastoma cells is inhibited by cyclosporin A. _Brain_ 130, 476–489 (2007). Article Google Scholar * Hofman, F. M., Stathopoulos, A., Kruse, C. A., Chen, T. C. &
Schijns, V. E. Immunotherapy of malignant gliomas using autologous and allogeneic tissue cells. _Anticancer Agents Med. Chem._ 10, 462–470 (2010). Article CAS Google Scholar * Proudfoot,
A. E., Power, C. A. & Schwarz, M. K. Anti-chemokine small molecule drugs: a promising future? _Expert. Opin. Investig. Drugs_ 19, 345–355 (2010). Article CAS Google Scholar * Liu, C.
_et al_. Chemokine receptor CXCR3 promotes growth of glioma. _Carcinogenesis_ 32, 129–137 (2011). Article Google Scholar * Maruno, M., Kovach, J. S., Kelly, P. J. & Yanagihara, T.
Distribution of endogenous tumour necrosis factor alpha in gliomas. _J Clin Pathol._ 50, 559–562 (1997). Article CAS Google Scholar * Samaras, V. _et al_. Analysis of interleukin (IL)-8
expression in human astrocytomas: associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. _Hum. Immunol._ 70, 391–397 (2009). Article CAS
Google Scholar * Desbaillets, I., Diserens, A. C., de, T. N., Hamou, M. F. & Van Meir, E. G. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma.
_Oncogene_ 18, 1447–1456 (1999). Article CAS Google Scholar * Brat, D. J., Bellail, A. C. & Van Meir, E. G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral
angiogenesis. _Neuro. Oncol._ 7, 122–133 (2005). Article CAS Google Scholar * Kielian, T., van, R. N. & Hickey, W. F. MCP-1 expression in CNS-1 astrocytoma cells: implications for
macrophage infiltration into tumors _in vivo_. _J Neurooncol._ 56, 1–12 (2002). Article Google Scholar * Curran, C. S., Evans, M. D. & Bertics, P. J. GM-CSF production by glioblastoma
cells has a functional role in eosinophil survival, activation, and growth factor production for enhanced tumor cell proliferation. _J Immunol._ 187, 1254–1263 (2011). Article CAS Google
Scholar * Cockerill, P. N. _et al_. Human granulocyte-macrophage colony-stimulating factor enhancer function is associated with cooperative interactions between AP-1 and NFATp/c. _Mol. Cell
Biol._ 15, 2071–2079 (1995). Article CAS Google Scholar * Venkatesha, R. T., Ahamed, J., Nuesch, C., Zaidi, A. K. & Ali, H. Platelet-activating factor-induced chemokine gene
expression requires NF-kappaB activation and Ca2+/calcineurin signaling pathways. Inhibition by receptor phosphorylation and beta-arrestin recruitment. _J Biol. Chem._ 279, 44606–44612
(2004). Article CAS Google Scholar * Kaplan, A. _et al_. The effects of cyclosporin A and FK506 on proliferation and IL-8 production of cultured human keratinocytes. _J Dermatol. Sci._
10, 130–138 (1995). Article CAS Google Scholar * McCaffrey, P. G., Goldfeld, A. E. & Rao, A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-alpha gene
transcription. _J Biol. Chem._ 269, 30445–30450 (1994). CAS PubMed Google Scholar * Zupanska, A., Dziembowska, M., Ellert-Miklaszewska, A., Gaweda-Walerych, K. & Kaminska, B.
Cyclosporine a induces growth arrest or programmed cell death of human glioma cells. _Neurochem. Int_ 47, 430–441 (2005). Article CAS Google Scholar * Xie, Y. _et al_. The Human
Glioblastoma Cell Culture Resource: Validated Cell Models Representing All Molecular Subtypes. _EBioMedicine._ 2, 1351–1363 (2015). Article Google Scholar * Lyakh, L., Ghosh, P. &
Rice, N. R. Expression of NFAT-family proteins in normal human T cells. _Mol. Cell Biol._ 17, 2475–2484 (1997). Article CAS Google Scholar * Urso, K. _et al_. NFATc3 regulates the
transcription of genes involved in T-cell activation and angiogenesis. _Blood_ 118, 795–803 (2011). Article CAS Google Scholar * Canellada, A., Ramirez, B. G., Minami, T., Redondo, J. M.
& Cano, E. Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1-4 and cyclooxygenase-2 as NFAT target genes. _Glia_ 56, 709–722 (2008). Article Google Scholar *
Davies, K. J. _et al_. Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin. _FASEB J_ 21, 3023–3028 (2007). Article CAS Google Scholar * Ryeom, S. _et al_. Targeted
deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. _Cancer Cell_ 13, 420–431 (2008). Article CAS Google Scholar * Cano, E., Canellada, A., Minami, T., Iglesias, T. &
Redondo, J. M. Depolarization of neural cells induces transcription of the Down syndrome critical region 1 isoform 4 via a calcineurin/nuclear factor of activated T cells-dependent pathway.
_J Biol. Chem._ 280, 29435–29443 (2005). Article CAS Google Scholar * Minami, T. _et al_. The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific
gene expression during inflammation in mice. _J Clin Invest_ 119, 2257–2270 (2009). CAS PubMed PubMed Central Google Scholar * Minami, T. _et al_. Vascular endothelial growth factor- and
thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. _J Biol. Chem._ 279, 50537–50554 (2004). Article CAS
Google Scholar * O’Donnell, J. M. _et al_. Tight control of exogenous SERCA expression is required to obtain acceleration of calcium transients with minimal cytotoxic effects in cardiac
myocytes. _Circ. Res._ 88, 415–421 (2001). Article Google Scholar * Mori, N. _et al_. Transcriptional regulation of the human interleukin-6 gene promoter in human T-cell leukemia virus
type I-infected T-cell lines: evidence for the involvement of NF-kappa B. _Blood_ 84, 2904–2911 (1994). CAS PubMed Google Scholar * Xie, K. Interleukin-8 and human cancer biology.
_Cytokine Growth Factor Rev._ 12, 375–391 (2001). Article CAS Google Scholar * Kaunisto, A. _et al_. NFAT1 promotes intratumoral neutrophil infiltration by regulating IL8 expression in
breast cancer. _Mol. Oncol._ 9, 1140–1154 (2015). Article CAS Google Scholar * Ehlers, M. L., Celona, B. & Black, B. L. NFATc1 controls skeletal muscle fiber type and is a negative
regulator of MyoD activity. _Cell Rep._ 8, 1639–1648 (2014). Article CAS Google Scholar * El, M. R. _et al_. A preclinical orthotopic model for glioblastoma recapitulates key features of
human tumors and demonstrates sensitivity to a combination of MEK and PI3K pathway inhibitors. _Dis. Model. Mech._ 8, 45–56 (2015). Article Google Scholar * Lee, M. & Rhee, I. Cytokine
Signaling in Tumor Progression. _Immune. Netw._ 17, 214–227 (2017). Article Google Scholar * Tie, X., Han, S., Meng, L., Wang, Y. & Wu, A. NFAT1 is highly expressed in, and regulates
the invasion of, glioblastoma multiforme cells. _PLoS One_ 8, e66008 (2013). Article ADS CAS Google Scholar * Sobrado, M. _et al_. Regulator of calcineurin 1 (Rcan1) has a protective
role in brain ischemia/reperfusion injury. _J Neuroinflammation._ 9, 48 (2012). Article CAS Google Scholar * Esteban, V. _et al_. Regulator of calcineurin 1 mediates pathological vascular
wall remodeling. _J Exp. Med._ 208, 2125–2139 (2011). Article CAS Google Scholar * Lin, H. Y. _et al_. Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein. _Free Radic.
Biol. Med._ 35, 528–539 (2003). Article CAS Google Scholar * Maklad,A., Sharma,A. & Azimi,I. Calcium Signaling in Brain Cancers: Roles and Therapeutic Targeting. _Cancers_. (_Basel_)
11 (2019). * Pyrzynska, B., Lis, A., Mosieniak, G. & Kaminska, B. Cyclosporin A-sensitive signaling pathway involving calcineurin regulates survival of reactive astrocytes. _Neurochem.
Int_ 38, 409–415 (2001). Article CAS Google Scholar * Wakabayashi, K. _et al_. Inhibitory effects of cyclosporin A on calcium mobilization-dependent interleukin-8 expression and invasive
potential of human glioblastoma U251MG cells. _Oncogene_ 23, 6924–6932 (2004). Article CAS Google Scholar * Macian, F. NFAT proteins: key regulators of T-cell development and function.
_Nat. Rev Immunol._ 5, 472–484 (2005). Article CAS Google Scholar * Courtwright, A. _et al_. Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/NFAT signaling
pathway. _Cancer Res._ 69, 4621–4628 (2009). Article CAS Google Scholar * Bao, Y. _et al_. Activation of peroxisome proliferator-activated receptor gamma inhibits endothelin-1-induced
cardiac hypertrophy via the calcineurin/NFAT signaling pathway. _Mol. Cell Biochem._ 317, 189–196 (2008). Article CAS Google Scholar * Liu, J. _et al_. Inhibition of T cell signaling by
immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. _Biochemistry_ 31, 3896–3901 (1992). Article CAS Google Scholar * Yiu, G. K. & Toker, A. NFAT
induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. _J Biol. Chem._ 281, 12210–12217 (2006). Article CAS Google Scholar * Shono, T., Tofilon, P. J.,
Bruner, J. M., Owolabi, O. & Lang, F. F. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. _Cancer Res._ 61, 4375–4381 (2001). CAS PubMed
Google Scholar * Fujita, M. _et al_. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. _Cancer Res._ 71, 2664–2674 (2011). Article CAS Google
Scholar * Sciume, G. _et al_. CX3CR1/CX3CL1 axis negatively controls glioma cell invasion and is modulated by transforming growth factor-beta1. _Neuro. Oncol._ 12, 701–710 (2010). Article
CAS Google Scholar * Giuliani, N. _et al_. CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and
survival. _Haematologica_ 91, 1489–1497 (2006). CAS PubMed Google Scholar * Pradelli, E. _et al_. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. _Int J
Cancer_ 125, 2586–2594 (2009). Article CAS Google Scholar * Zahonero, C. _et al_. Preclinical Test of Dacomitinib, an Irreversible EGFR Inhibitor, Confirms Its Effectiveness for
Glioblastoma. _Mol. Cancer Ther._ 14, 1548–1558 (2015). Article CAS Google Scholar * Markovic, D. S. _et al_. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion.
_Proc. Natl. Acad. Sci. USA_ 106, 12530–12535 (2009). Article ADS CAS Google Scholar * Scherr, M., Battmer, K., Blomer, U., Ganser, A. & Grez, M. Quantitative determination of
lentiviral vector particle numbers by real-time PCR. _Biotechniques_ 31, 520, 522, 524, passim (2001). * Bowman, R. L., Wang, Q., Carro, A., Verhaak, R. G. & Squatrito, M. GlioVis data
portal for visualization and analysis of brain tumor expression datasets. _Neuro. Oncol._ 19, 139–141 (2017). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Dra
Isabel Liste for helpful discussions and critical reading of the manuscript; Dr Fernando González-Camacho from the confocal microscopy unit; Dr Ricardo Gargini for database analysis support
and Dr Javier García Castro for kindly providing mCherry-Luc lentiviral vector. This work was supported by grants from the Fondo de Investigaciones Sanitarias (FIS) Spain (PI09/0218) and Red
Temática Investigación Cooperativa en Cáncer (RTICC. (RD12/0036/0027) Grants from the Spanish Ministry (MINECO) (SAF2016-76451) to E.C. AUTHOR INFORMATION Author notes * Katia Urso and
Andrés Fernández contributed equally. AUTHORS AND AFFILIATIONS * Chronic Disease Programme, Madrid, Spain Andrés Fernández, Patricia Velasco, Javier Cotrina, Pilar Sánchez-Gómez & Eva
Cano * Centro Nacional de Microbiología, Madrid, Spain Belén de Andrés * Instituto de Enfermedades Raras, Instituto de Salud Carlos III, Madrid, Spain Sonsoles Hortelano * Department of
Vascular Biology and Inflammation, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain Katia Urso & Juan Miguel Redondo * Hospital Doce de Octubre, Madrid, Spain
Aurelio Hernández-Laín Authors * Katia Urso View author publications You can also search for this author inPubMed Google Scholar * Andrés Fernández View author publications You can also
search for this author inPubMed Google Scholar * Patricia Velasco View author publications You can also search for this author inPubMed Google Scholar * Javier Cotrina View author
publications You can also search for this author inPubMed Google Scholar * Belén de Andrés View author publications You can also search for this author inPubMed Google Scholar * Pilar
Sánchez-Gómez View author publications You can also search for this author inPubMed Google Scholar * Aurelio Hernández-Laín View author publications You can also search for this author
inPubMed Google Scholar * Sonsoles Hortelano View author publications You can also search for this author inPubMed Google Scholar * Juan Miguel Redondo View author publications You can also
search for this author inPubMed Google Scholar * Eva Cano View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS E.C. conceived the presented
idea. J.M.R., S.H. and B.d.A. contributed to the interpretation of the results. E.C., K.U., A.F., P.V. and J.C. carried out the experiments. A.H.-L. and P.S.-G. contributed to clinical
samples preparation. E.C., K.U. and A.F. wrote the manuscript with contribution from all authors. E.C., A.F. and K.U. helped supervised the project. Project administration and funding by
E.C. CORRESPONDING AUTHORS Correspondence to Andrés Fernández or Eva Cano. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION
PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPLEMENTAL MATERIAL RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Urso, K.,
Fernández, A., Velasco, P. _et al._ NFATc3 controls tumour growth by regulating proliferation and migration of human astroglioma cells. _Sci Rep_ 9, 9361 (2019).
https://doi.org/10.1038/s41598-019-45731-w Download citation * Received: 14 November 2018 * Accepted: 12 June 2019 * Published: 27 June 2019 * DOI: https://doi.org/10.1038/s41598-019-45731-w
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative