All-male groups in asian elephants: a novel, adaptive social strategy in increasingly anthropogenic landscapes of southern india
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Male Asian elephants are known to adopt a high-risk high-gain foraging strategy by venturing into agricultural areas and feeding on nutritious crops in order to improve their
reproductive fitness. We hypothesised that the high risks to survival posed by increasingly urbanising and often unpredictable production landscapes may necessitate the emergence of
behavioural strategies that allow male elephants to persist in such landscapes. Using 1445 photographic records of 248 uniquely identified male Asian elephants over a 23-month period, we
show that male Asian elephants display striking emergent behaviour, particularly the formation of stable, long-term all-male groups, typically in non-forested or human-modified and highly
fragmented areas. They remained solitary or associated in mixed-sex groups, however, within forested habitats. These novel, large all-male associations, may constitute a unique life history
strategy for male elephants in the high-risk but resource-rich production landscapes of southern India. This may be especially true for the adolescent males, which seemed to effectively
improve their body condition by increasingly exploiting anthropogenic resources when in all-male groups. This observation further supports our hypothesis that such emergent behaviours are
likely to constitute an adaptive strategy for male Asian elephants that may be forced to increasingly confront anthropogenically intrusive environments. SIMILAR CONTENT BEING VIEWED BY
OTHERS STRATEGIES OF PROTECTED AREA USE BY ASIAN ELEPHANTS IN RELATION TO MOTIVATIONAL STATE AND SOCIAL AFFILIATIONS Article Open access 02 November 2022 UNDERSTANDING THE SPATIAL
DISTRIBUTION AND HOT SPOTS OF COLLARED BORNEAN ELEPHANTS IN A MULTI-USE LANDSCAPE Article Open access 27 July 2022 AFRICAN FOREST ELEPHANT MOVEMENTS DEPEND ON TIME SCALE AND INDIVIDUAL
BEHAVIOR Article Open access 16 June 2021 INTRODUCTION Social organization in mammals is defined by their group size and demographic composition and is known to be influenced by certain
biological characteristics, including species-specific phylogenetic inertia and intrinsic individual-level attributes, such as age, sex, physiology or genetic traits. Additionally,
environmental factors such as resources, human-driven-threats and other stochastic events are known to affect social organisation1,2,3,4,5,6,7,8. Increasingly, a number of mammalian species
seem to be coping with large-scale changes in their environment mainly driven by anthropogenic factors through behavioural flexibility rather than commonly discussed genetic
adaptations5,6,7,9,10. Such developmental and social flexibility in behaviour allow for the expression of alternative strategies that may enable certain mammalian populations to successfully
survive and reproduce in changing environments2,6. This is especially true in the case of elephants, which have well-defined growth phases11,12 and sex-specific life-history strategies
(reviewed in13). Elephants live in mixed-sex social units termed as families, bond-groups or clans13,14,15,16. Changes in the sociality of male Asian elephants is pronounced between 11 and
20 years, as adolescent males disperse from their natal herds transitioning into socially mature adults. An important physiological change that occurs in male elephants during this phase is
the onset of musth17,18, characterized by enhanced testosterone level and increased sexual activity13,19. When in musth, male African and Asian elephants are known to associate with females
to mate with them13,20,21. Dynamic changes in social associations and behavioural strategies seem to be appearing amongst both female and male elephants, in populations that are living in
rapidly changing ecological and anthropogenic environments21,22,23. For instance, male African elephants are known to associate in large all-male groups of highly related individuals of
similar age24 in areas with high primary productivity and anthropogenic mortality risk21. Adult Asian elephant males are typically solitary but could also form small, short-term, all-male
groups, especially during crop-foraging18. Male Asian elephants are known to forage on agricultural crops nearly six times more, on average, than do female-led family groups in certain
populations of southern India25. It has been proposed that such male elephants may be adopting a high-risk high-gain foraging strategy25,26 to improve their body condition and come into
musth, thereby increasing their reproductive success27. As compared to protected forests, foraging in human-production habitats not only improves body condition, but also lowers nutritional
stress in male elephants28. Such males, however, are highly prone to human-related stressors including injuries and deaths29,30. Human-elephant conflict-related deaths of elephants due to
electrocution, poisoning, shooting and accidental deaths is high. In India alone, nearly 150 elephants succumb to human-elephant conflict every year31. The faecal glucocorticoid metabolite
levels in solitary adult male elephants are known to increase significantly following antagonistic interactions with humans, such as elephant drives32. Such physiological stress levels are
higher than that of adult females in herds in the same human-dominated landscape32, suggesting that social buffering (associating in groups) may effectively lower acute stress in elephants.
In this paper, we assess the relative influence of biological variables such as age and musth, and of environmental factors such as forest- or natural habitat contiguity as well as
anthropogenic influences, such as changing landuse patterns and other human activities on the sociality of male Asian elephants in an agricultural landscape of southern India. With
well-established body size and predictable musth periods, we expect very little flexibility in social decision-making in mature adults that mostly remain solitary. On the other hand,
sexually mature but socially immature males in the adolescent stage are likely to display high inter-individual variability in sociality as they disperse from their natal herds to explore
new environments and make important foraging- and social decisions that aid in their successful transitioning into socially mature adults. For mature male elephants that have dispersed from
their natal groups, therefore, remaining solitary in areas with high human-activity may make them highly vulnerable to human-induced stress and prone to direct threats such as poaching and
retaliatory killings13. Additionally, young dispersing males individually conduct trial-and-error exploratory forays to new habitats or resources33. Such explorations are occasionally
maladaptive and may be costly, especially if they select high-risk landscapes of low productivity (reviewed in8). Hence, grouping together of males may evolve as a behavioural strategy to
exploit resources while minimizing the per-capita risks in male elephants. We, therefore, firstly hypothesised that the decision by male Asian elephants to associate in all-male groups in
highly human-dominated areas may be driven primarily by environmental factors and anthropogenic influences rather than biological factors. Secondly, we hypothesised that the group size of
all-male groups in high-risk agricultural habitats such as the crop-growing regions of Tumkur, Ramanagara and Krishnagiri districts will be larger than those in low-risk, forested habitats
such as the Protected Areas of Bannerghatta National Park, Cauvery Wildlife Sanctuary and Cauvery North Wildlife Sanctuary (Karnataka and Tamil Nadu states, India). Finally, we hypothesised
that associating in all-male groups may be an adaptive social behaviour, especially for the adolescent males, as foraging in resource-rich agricultural landscapes would enable them to
improve body condition and become reproductively successful, large dominant bulls in the society. RESULTS During the study period (February 2016–December 2017) we sampled for a total of
10,705 days and obtained 20,124 photo-captures of elephants. From these elephant photographs, excluding calves, we were able to identify a total of 248 distinct males. Of these, 25 males
were classified as Sexually and Socially Mature (SSM, above 20 years of age), 113 as Sexually Mature but Socially Immature (SM, from 10 to 20 years of age) and 110 as Sexually Immature (SIM,
<10 years of age). Based on the frequencies of photographic records (n = 1445), SSM comprised nearly 20%, SM 43% and SIM 37% of the male elephant population in the study site. We thus
observed relatively fewer old and mature bulls in the study population. Male elephants in the intensive study area primarily occurred in mixed-sex groups (43.36%, n = 620), followed by
solitary (33.57%, n = 480) and in all-male groups (23.08%, n = 330). These associations ranged in size from one (solitary) to 25 individuals. The mean (±SE) size of the mixed-sex groups was
8.53 (±0.17, range 1 to 25, n = 620) and that of all-male groups 3.59 (±0.12, range 2 to 12, n = 330). While SIM males were sighted mostly in mixed-sex groups, SM males were observed to be
solitary or in all-male groups in equal proportions and the SSM males were seen to be mostly solitary (Supplementary Table 1). A significant difference in the occurrence of the three
maturity classes across the three different social units was thus observed (G-test of independence, G = 500.21, df = 4, p < 0.001). Hence, mixed-sex groups consisted mostly of juvenile
and all-male groups mostly of adolescent males while matures males remained mostly solitary (Supplementary Table 2). ALL-MALE ASSOCIATIONS The primary factor determining the association of
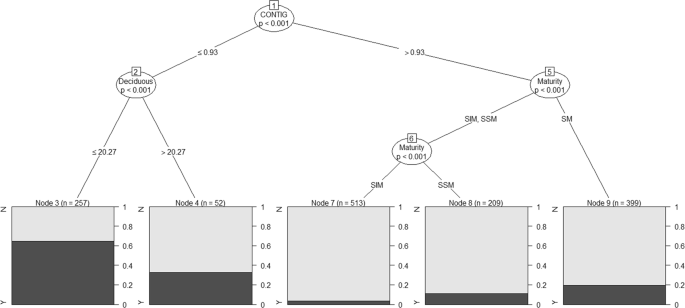
males in all-male groups was Forest Contiguity (G = 108.08, df = 1, p < 0.001, Fig. 1; Node 1). Nearly 65% (166 of 257, Fig. 1; Node 3) of male elephant sightings in all-male groups were
in areas with Forest Contiguity (CONTIG) ≤ 0.93 and Deciduous Forest ≤ 20.27%. This was not significantly different from their propensity to occur in all-male groups in CONTIG ≤ 0.93 areas
with Deciduous Forest > 20.27% (G = 2.00, df = 1, p = 0.157). A significant difference in the association of male elephants across the three maturity classes was also observed in areas
with CONITG > 0.93 (G = 26.22, df = 2, p < 0.001). The SM males showed the highest propensity of association in all-male groups (19.54%, Node 9) followed by SSM males (11.50%, Node 8)
while the SIM males displayed the lowest propensity at 3.31% (Node 7). Thus forest contiguity was the most significant factor determining all-male group formation in Asian elephants with SM
males showing the highest propensity to associate in such groups. MIXED-SEX ASSOCIATIONS Maturity was the first and the most significant factor determining the association of male elephants
in mixed-sex groups (G = 117.45, df = 2, p < 0.001, Supplementary Fig. 1; Node 1). A relatively high proportion (nearly 85%) of SIM male sightings were in areas with Crop ≤ 39.17% and
Human Use Index (HUI) ≤ 3.1 (Supplementary Fig. 1; Node 4). The SM males showed highest propensity to associate in mixed-sex groups (56%) in areas with CONTIG > 0.93 and Degraded Forest
> 2.95. The SSM males in musth showed a significantly higher propensity to associate with mixed-sex groups (72.72%) than when not in musth (G = 4.55, df = 1, p = 0.03; Supplementary Fig.
1). The maturity of the individual was thus the primary factor influencing decision-making in male elephants that associated in mixed-sex groups, with SIM males showing highest propensity to
associate in such groups followed by SSM males in _musth_. SOLITARY MALES Maturity was once again the primary determinant of the occurrence of male elephants as solitary individuals in the
intensive study area (G = 70.27, df = 2, p < 0.001, Supplementary Fig. 2; Node 1). SSM males were observed to be solitary at a relatively high proportion of 62.06% (Node 10) in Deciduous
Forest areas. Their occurrence in areas with Deciduous Forest >37.55% was not significantly different than observed in areas with less Deciduous Forest (G = 1,20, df = 1, p = 0.273, Node
11). For SM males, the propensity to remain solitary was significantly higher (53.15%) in areas with Crop ≤ 9.28 than in areas with more Crop (G = 4.27, df = 1, p = 0.04). SIM males on the
other hand remained mostly solitary in areas with Crop > 44.85% (n = 13, Supplementary Fig. 2; Node 6). The maturity of the individual was thus the primary factor influencing
decision-making in male elephants that occurred solitarily, with male elephants tending to become increasingly solitary with age, but mainly in Deciduous Forest. SOCIAL GROUP SIZE IN
ALL-MALE GROUPS There was a significant variability in the size of all-male groups in the intensive study area (Fig. 2). In cells with Crop > 40.8%, the group size was the highest, with a
mean (±SE) of 4.64 (±0.27, range 2 to 9, Node 5). In areas with Crop < 40.8% and Deciduous Forest ≤ 20.3%, group size reduced to a mean of 3.13 (±0.12, range 2 to 6, Node 4). The size of
all-male groups, however, was the least in areas with Crop ≤ 40.8% and Deciduous Forest > 20.3%, with a mean of 2.29 (±0.06, range 2 to 4, Node 3). The size of all-male groups was thus
the highest in areas under intensive cultivation and low deciduous forest. BODY CONDITION OF MALES IN ALL-MALE GROUPS There was a significant difference between the Body Condition (scored on
a scale of I to V) of males in all-male groups (G = 16.68, df = 3, p < 0.001), mixed-sex groups (G = 35.91, df = 3, p < 0.001) and those that remained solitary (G = 13.23, df = 3, p
< 0.004). Nearly 58% of males in all-male groups, 54% males ranging solitary and only 27% males in mixed-sex groups were scored V on Body Condition (Supplementary Table 3). None of the
males in all-male groups were assigned a Body Condition score below IV. On examining the Body Condition of males in all-male groups specifically, we found that only the Body Condition of SM
males was influenced by availability of Crop and Deciduous Forest (Fig. 3), while both SSM and SIM males were not influenced by any of the select biological and environmental factors
(Supplementary Figs 1 and 4). A significant difference in the Body Condition of SM males in areas with Crop > 15.39% was observed when compared to areas with less Crop (G = 11.70, df = 1,
p < 0.001, Fig. 3; Node 1). DISCUSSION In this study, we report for the first time, the occurrence of large and stable all-male groups and the processes that drive the formation of these
novel and long-term associations in male Asian elephants. We discuss these findings with respect to our current understanding of elephant sociality and life-history strategies with a
special emphasis on the evolving behavioural adaptations in male Asian elephants that are increasingly using human-dominated landscapes. We further discuss the implications of these
adaptations with respect to the management and conservation of Asian elephant populations. Male elephants are typically born into a matriarchal natal group and continue to stay in it at
least until adolescence18. Accordingly, we too found sexually and socially immature (SIM) males to predominantly occur in mixed-sex groups in our study area. In Asian elephant society, it is
well established that male elephants on reaching adolescence disperse from their natal groups in search of unrelated females to mate with and forage-rich areas in which to establish
themselves18. In the study population SM males and on very few occasions even older SIM males showed a tendency towards such behaviour by ranging in cropfields solitarily, however, only for
a short-duration (<12 h). For a number of sexually mature but socially immature (SM) dispersing males, movement into unknown habitats in search of food, water and other elephants, may
become maladaptive, due to a lack of knowledge of the risks that the newly occupied areas pose34. This is especially true in fragmented human-modified production landscapes, which typically
characterized our study region. In our study, we observed that adolescent male elephants associated in large bull groups almost exclusively in human-modified landscapes, predominantly with
croplands but interspersed by fragmented and isolated forest patches, supporting our first hypothesis that the recent all-male group formations in Asian elephants may be environmentally
rather than biologically influenced. Our study elephants occurred in a region close to major towns and cities, such as Bangalore, also known as the Silicon Valley of India. This region has
undergone major landuse changes, especially between the years 1973 and 1992, with rapid increase in agriculture, human densities, major and minor roads and a concomitant burgeoning of the
urban sprawl, all at the expense of forest cover and natural elephant habitats35,36. Reforestation, in the form of monoculture of exotic tree species such as _Acacia auriculiformis_ and
_Eucalyptus_ spp., in the years between 1992 and 2007, following deforestation, mainly outside Protected Areas36 (PAs) has resulted in the reduction in cultivation of subsistence crops
bordering PAs, which may have increased tree cover for elephants, but may not have helped them nutritionally. More recently, quarrying activity in hillocks adjoining the PAs has resulted in
the further loss of natural habitats and caused increased disturbance to elephants. These rapid and large-scale changes in landuse, within a lifetime of an individual elephant, may have
provided a perfect setting in which elephants need to be behaviourally flexible and adapt to these dynamic and potentially risky production landscapes. Conflict-related injuries and
mortality were recorded in our study area too with eight adolescent and two mature adult males succumbing to such injuries or captures in 15 months within the study period. Given such
environmental stochasticity, associations of male elephants may have emerged as a behavioural necessity for young male elephants in high-risk, high-resource landscapes, especially in recent
years (see also33,37,38). The all-male groups increased in size with increasing crop availability, supporting our second hypothesis that the group size of such associations would increase in
resource-rich, high-risk areas. These findings thus suggest that available environmental resources and certain anthropogenic factors may play a more significant role, than would intrinsic
factors, in shaping the social decisions of males associating in all-male groups, similar to their African counterparts21. The relatively high intraspecific variability in social
organization displayed by SM males and their increased propensity to associate with other males of the same or older age classes is reminiscent of what has been observed in African elephant
populations as well12,24. It is noteworthy that studies conducted on elephants in our study region, more than two decades ago, do not mention the occurrence of these large and stable
all-male groups35. One of us (RS) has been observing social groupings of elephants in the larger study landscape since the 1980s and began to observe such large all-male associations in the
human-use areas approximately over the last two decades. This suggests that this type of association of elephants may be of a rather recent origin in this landscape. We found the male
elephants in our study population, especially the SM males that used production landscapes, to have significantly better body condition than those inhabiting areas with relatively more
deciduous forest, supporting our third and final hypothesis that all-male group formation could be adaptive. Foraging on crops may, therefore, be an effective strategy for these young
dispersing males to increase their body size relatively rapidly. For the sexually and socially mature (SSM) males too this strategy may serve to maintain good body condition enabling them to
stay in musth for longer periods of time (Srinivasaiah N. M., Sinha, A., Vaidyanathan, S. & Sukumar, R. unpublished data). When in musth, these males either moved solitarily in search
of estrus females or associated with mixed-sex groups in deciduous forest areas, possibly to increase their chances of mating. We also found SSM males when not in _musth_ to remain largely
solitary in forested habitats, which conforms with previous studies on Asian elephants13. Hence, biological rather than environmental attributes influenced group formation in the case of SSM
males. Small- and medium-sized all-male groups, with a high turn-over between particularly associating individuals, were observed either within the large forested habitats or in cropfields
adjacent to such habitats alone. These short-term associations (for a few weeks in a year) of male elephants, were those of either peer group members or a cohort, usually of older SIM (5 to
10 years of age) or SM individuals, formed in response to a _musth_ bull, which may have associated with their natal herd and chased the younger males away. Such a social trigger may also be
the first step towards dispersal in young male elephants. The medium-sized all-male groups were again short-term associations (for one cropping season in a year) of males of the SM and SSM
class. Such associations, were observed mostly along the fringes of forests and nearby cropfields, and has been well-documented in previous studies18. Finally, the large all-male groups that
were observed in highly-fragmented, predominantly agricultural areas were long-term (for a few years or more) associations of males mostly belonging to the SM class and which is of
particular interest to this study. It is important to note that we have observed some of these large all-male associations of SM males to consist of particular individuals, who have been
stably associating with one another for over ten years now, since our long-term study was initiated. While the variation in group size in mixed-sex groups of elephants have been well
established in the study area (in press39), our observations on intraspecific variation in social organisation of male Asian elephants indicate that the emergence of large, stable all-male
groups in response to extrinsic environmental factors is a rather novel phenomenon. We would also like to point out that such a social strategy adopted by our study male elephants may
represent a specific example of more general risk-management strategies increasingly being displayed by elephant populations across their threatened habitats. Elephants are thus not unusual
in coping with increasing anthropogenic pressures in their changing environments by displaying significant social flexibility, as has been shown in other mammalian species as well (reviewed
in6,7). The behavioural flexibility, primarily shown by young male elephants in the dispersal stage and manifest through the formation of all-male groups and adoption of novel foraging
strategies, leading to improved body condition, may thus constitute an example of how even large mammals such as elephants can develop behavioural strategies to increase their survival and
reproductive fitness7,8. The mortality of elephants, due to conflict with local human communities and the various resultant interventions, however, is an enormous conservation challenge40,
especially for males in already male-depleted populations41. The lack of mature adult males in such populations, which have also endured high poaching rates in the past13,18, may lead to
reduced population growth rates42 and may have behavioural implications for younger individuals who then grow up in an environment without role models to learn from43. Young male elephants
in the study landscape are known to associate with older males in human-use areas23. Under these circumstances, losing even more males through highly intrusive conflict mitigation measures,
such as captures or retaliatory killings, could have a further negative effect on populations, in a manner like to that of poaching. It may therefore be imperative that future attention is
focused on the management and conservation of young dispersing males of this highly endangered species, as the often-flexible decisions made by these individuals appear to directly influence
the utilization of production landscapes by the species, thus bringing them into direct conflict with local agricultural communities. An understanding of such emergent behaviours in
elephants may also provide us with strategies to reduce human-elephant conflict while keeping in mind the sociality of Asian elephants. Although the management and conservation strategies
for the increasingly threatened Asian elephant populations need to be explored in the near future, we have only now begun to understand the more fundamental aspects of this newly emergent
social organization in male Asian elephants in human-modified landscapes. METHODS STUDY AREA Our prior knowledge of elephant ranging patterns over a nine-year period since 2009, acquired
through following individual elephants, allowed us to demarcate an extended study area of c. 10,000 km2, in southern India. This region was then overlaid with a grid, consisting of 1,000
cells of size 10 km2 each, using Quantum Geographical Information System44. Of these, 778 cells had forest patches within them and using a questionnaire survey, administered to experienced
forest guards, we established the presence of elephants at least for six months annually in 118 of these cells (Fig. 4). These cells were then systematically surveyed by a team, comprising
the researchers and experienced forest staff, for elephant presence at select waterholes, either through direct sightings or by indirect signs such as dung, tracks or evidence of foraging,
over a period of one year, from February 2015 to February 2016, taking care that each cell was surveyed at least once every month. This initial survey also allowed us to determine that only
63 of these 118 cells, comprising c. 630 km2 were logistically amenable for long-term monitoring of elephants using the camera-trap method and was designated as the intensive study area
(Fig. 4). The extended study area is a matrix of human habitations, tropical deciduous forests and cropfields, extending over an area of c. 10,000 km2 and with an approximate human density
of 255 to 431 persons/km2 45, within the administrative boundaries of Karnataka and Tamil Nadu states in southern India. The forested area of c. 1000 km2, included the Protected Areas of
Bannerghatta National Park and parts of Cauvery and Cauvery North Wildlife Sanctuaries, with a combined elephant density of around 1/km2 46,47, as also the Reserved Forests of Ramanagara,
Bengaluru and Hosur Forest Divisions (Fig. 4). The vegetation is mostly tropical dry deciduous and scrub woodland forests, with numerous riparian patches. The elevation ranges from 250 to
1,000 m above msl, sloping from north to south. The region records a mean monthly maximum and minimum temperatures of 35 °C (April) and 18 °C (January) respectively, and receives an annual
rainfall of c.800 mm, with a mean monthly maximum and minimum of 170 mm (October) and 5 mm (January) respectively45. Agriculture is the major occupation in the region, with June to December
comprising the main cultivation season. Agriculture is mostly rain-fed, with the region receiving both the southwest (summer) and northeast (winter) monsoons. The main crops grown were
finger millet _Eleusine coracana_ and maize _Zea mays_45. However, in well irrigated areas, paddy _Oryza sativa_ cultivation was prevalent. In addition, plantation crops such as mango
_Mangifera indica_, coconut _Cocus nucifera_, sugarcane _Saccharum officinale_ and banana _Musa_ spp. are cultivated alongside agro-forestry crops such as acacia _Acacia auriculiformi_s and
eucalyptus _Eucalyptus_ spp. The landscape is dotted with numerous artificial waterbodies and quarries and highly fragmented by road and rail networks. Human-elephant conflict was prevalent
across the region with almost daily crop-foraging by elephants and ten human- and elephant lives each being lost during the study period of 15-month duration. A record high level of
human-elephant conflict, was reported from this landscape, in the years 2005 and 200646. COLLECTION AND ANALYSIS OF PHOTOGRAPHIC DATA A single camera trap was placed within each of these 63
cells, at waterholes with elephant usage established through the initial survey (Fig. 4). Information on elephant age, sex, group size, group composition and musth state was collected and
individual identification carried out, simultaneously across these locations using photographs obtained48,49,50,51. These cameras covered a gradient of forest fragmentation and human use
within the study area (Srinivasaiah N.M., Sinha, A., Vaidyanathan, S. & Sukumar, R. unpublished data) and were operational both during the day and at night, ensuring spatio-temporal
coverage. Systematic camera trapping for elephants was conducted in the intensive study area between February 2016 and December 2017 for 10,705 days of 24-h period each (mean ± SE of 169.92
± 18.94 days/camera), resulting in a total of 20,124 records. A comparable effort was invested in camera-trapping across a gradient of fragmentation within the intensive study area with 142
trap-days/nights in the highly fragmented regions (CONTIG 0.63 to 0.95), 185 trap-days/nights (CONTIG 0.95 to 0.97) and 153 trap-days/nights (CONTIG 0.97 to 0.98) on an average. We selected
any one sighting of an individual elephant or a group, as defined below, from all operational cameras within a 12-h period for analysis; this resulted in a sample size of 1445 independent
records (mean ± SE of 5.80 ± 0.57 sightings/individual, range from 1 to 53). Accurate recordings of group size were not possible on five of these occasions and of musth status on ten
occasions. Hence, the total sample size to assess the impact of biological and environmental influences on the social organization of the study male elephants was reduced from 1445 to 1430
records. A group, comprising both male and female elephants, was considered as a mixed-sex group, one with only males an all-male group and single male elephants as solitary individuals. It
may be noted here that, in general, the study elephant groups were periodically observed over space and time across our study area. More specifically, the all-male groups could be stably
identified through the regular association of individuals, as identified by systematic camera-trapping over the study area, as described above. A group was defined using a temporal measure.
An elephant photographed within a three-hour interval of another individual at the same camera location was considered to be part of the same group. This was based on our observation that
typically, all individuals of a known group arrived at specific waterholes between two and three hours of any of its members visiting the same. Moreover, our earlier observations, during
this long-term study23, indicated that if behavioural observations were conducted within a single sampling session of three hours, there was no change in group membership within this period
of time. The number of elephants within a group was counted based on the identification of individuals by a number of morphological features14,52, as recognised in their camera-trap images
obtained both diurnally and nocturnally (Supplementary Fig. 10). From the inception of the study, every individual elephant was identified either through direct observations or camera-trap
photographs, all its visible morphological features recorded and a database of all the identified individuals in the study area created. Every subsequently recorded individual was compared
with this database and an algorithm used to estimate the probability of match of the newly sighted elephant with the known elephants in the database. The top 10 matches were then manually
compared independently by two observers to arrive at the identity of the individual. If there was no final match, the individual was entered into the database as a new elephant. The age of
an elephant was estimated based on relative shoulder height measures18,52, which is the standard surrogate measure for age estimation in wild elephants. This method was used to classify our
study elephants using camera trap images, into five age categories namely, Calf (birth to 1 years of age), Juvenile (1 to 5 years), Subadult Stage One (5 to 10 years), Subadult Stage Two (10
to 15 years) and Adult (>15 years). It should be noted that the shoulder height of a 15 to 20 year-old male is comparable to that of a fully grown adult female52. Beyond 20–25 years of
age, the shoulder height of elephants is known to asymptote and hence, other morphological features such as degree of folding and depigmentation of the ears, temporal and buccal cavity
depression and prominence of domes53. Our observations of male elephants in the study area along with other such studies on Asian elephant socioecology suggests that male Asian elephants do
not express periodic and long _musth_ periods at least until about the age above 20 to 25 years13,17, similar to African elephants19. The forays of SIM males away from their natal herds too
started from around the age of 10 years and increased in frequency after the 10th year13. Hence, it was essential to categorise elephants using the maturity classes that we have, as it
provides adequate importance to males in the adolescent age class (10 to 20 years of age), which have seldom been studied. Our repeated observations of wild male elephants and of those in
captivity allowed us to establish a strong correlation between the age categories of male elephants and their maturity classes, namely, SIM, SM and SSM. Thus, individual males from 1 to 10
years constituted SIM males, those from 10 to 20 years SM males and those beyond 20 years SSM males. The scoring of Body Condition of the study male elephants was carried out based on the
prominence in visibility of an elephant’s backbone, ribs, shoulder- and pelvic bones (Supplementary Fig. 8). The elephants were scored on a scale of I to V54 (Supplementary Table 3). Musth
in male elephants was noted based on the different stages of secretion of musth fluid from the temporal glands and urine dribbling18,55,56,57. To assess the level of human- and associated
activities such as livestock grazing at each camera-trap location, we developed a Human Use Index or HUI (modified from58,59,60,61), defined as the number of photographs of humans/livestock
within one hour of one another, considered as a single event (Table 1). LANDUSE AND OTHER HABITAT CHARACTERISTICS The study landscape was classified into different landuse types, based on
geospatial data obtained from the National Remote Sensing Agency of the Government of India (downloaded from http://bhuvan3.nrsc.gov.in/cgi-bin/LULC250K.exe). The original 19 Landuse and
Landcover (LULC) categories62 were merged to derive eight LULC categories: Deciduous Forest, Degraded Forest, Plantation (orchards), Crop (seasonal and multicrop), Current Fallow, Wasteland,
Waterbody and Built-Up Area (Table 2). For the 63 cells in the intensive study area, selected as described above, we estimated the percentage of different LULC categories (Table 2). To
estimate the contiguity of forests within the study area, we developed a Contiguity Index (CONTIG), using FRAGSTATS63, by a 3 × 3 moving window on a layer containing forest-only patches
(adapted from64,65). The Contiguity Index layer assigned larger values to large contiguous forest patches across cells and smaller values to small isolated patches (Table 1), which resulted
in a measure of spatial connectedness of forested patches. The intensive study area, used extensively by elephants, primarily comprised Deciduous Forest, although it also included
significant proportions of human-modified landuse, such as Crop, Plantation and Wasteland (Table 2).There was also a significant gradient of the Human Use Index across the study area, a
reflection of the simultaneous use of these habitats by the local human communities (Table 1). Fragmented deciduous forests and cropfields were thus the predominant landuse types in the
study area. STATISTICAL ANALYSIS To examine the influence of biological and environmental factors on the decision by males to associate in particular social group types, we constructed
recursive partitioning classification trees in R, version 3.4.02366. We used classification trees as it allows for intuitive visualization of the results obtained from a dataset having both
categorical and continuous variables67,68,69. They also aid in the stepwise, hierarchical expression of the relative importance of the different variables investigated. The ten input
variables included two biological parameters, Maturity and Musth, and eight environmental parameters, namely Deciduous Forest, Degraded Forest, Wasteland, Crop, Plantation, Waterbody, Human
Use Index (HUI) and Contiguity Index (CONTIG). The response variables measured were Group Size, Social Group Type, and Body Condition. Two of the landuse types, Built-Up Area and Current
Fallow, were not used in the final analysis, as they did not offer any resource to the study elephants. We assessed the statistical significance of the differences in the propensity of
occurrence of male Asian elephants in the three social group types, referred to above, as response to varying levels of the above biological and environmental parameters using
multiplicity-adjusted Monte-Carlo simulated (n = 9999) p-values66. The G-test of independence was used to assess differences in the occurrence of different classes of males in the
population, the demographic composition of associations and also as a post-hoc procedure to test for the statistical significance of the recursive partitioning classification trees obtained
above70. The Research Ethics Committee of our host institution, the National Institute of Advanced Studies (NIAS) in Bangalore, approved all the natural observation and camera trapping
protocols under the NIAS Research Ethics Policy. Permission to conduct the natural observations on the study elephants was obtained from the Principal Chief Conservators of Forests
(Wildlife) and Chief Wildlife Warden of the Karnataka and Tamil Nadu Forest Departments (permit number: PCCF(WL)/E2/CR-103/2013-14 and WL5(A)/21591/67/2015). DATA AVAILABILITY The datasets
generated during and/or analysed during the current study are available in the Dryad digital repository, [https://doi.org/10.5061/dryad.957f2tv]. REFERENCES * Crook, J. H. Social
organization and the environment: Aspects of contemporary social ethology. _Anim Behav_ 18, 197–209 (1970). Article Google Scholar * Caro, T. M. & Bateson, P. Organization and ontogeny
of alternative tactics. _Anim Behav_ 34, 1483–1499 (1986). Article Google Scholar * Sinha, A., Mukhopadhyay, K., Datta-Roy, A. & Ram, S. Ecology proposes, behaviour disposes:
ecological variability in social organization and male behavioural strategies among wild bonnet macaques. _Current Science_ 89, 1166–1179 (2005). Google Scholar * Sih, A., Ferrari, M. C. O.
& Harris, D. J. Evolution and behavioural responses to human-induced rapid environmental change. _Evol Appl_ 4, 367–387 (2011). Article Google Scholar * Kappeler, P. M., Barrett, L.,
Blumstein, D. T. & Clutton-Brock, T. H. Constraints and flexibility in mammalian social behaviour: introduction and synthesis. _Philos Trans R Soc B_ 368, 1618 (2013). Article Google
Scholar * Schradin, C. Intraspecific variation in social organization by genetic variation, developmental plasticity, social flexibility or entirely extrinsic factors. _Philos Trans R Soc
B_ 368, 1618 (2013). Article Google Scholar * Wong, B. B. M. & Candolin, U. Behavioral responses to changing environments. _Behav Ecol_ 26, 665–673 (2015). Article Google Scholar *
Silk, J. B. The adaptive value of sociality in mammalian groups. _Philos Trans R Soc B_ 362, 539–559 (2007). Article Google Scholar * Sinha, A. Not in their genes: Phenotypic flexibility,
behavioural traditions and cultural evolution in wild bonnet macaques. _J. Biosci_ 30, 51–64 (2005). Article Google Scholar * Sol, D., Lapiedra, O. & González-Lagos, C. Behavioural
adjustments for a life in the city. _Anim Behav_ 85, 1101–1112 (2013). Article Google Scholar * Sukumar, R., Joshi, N. V. & Krishnamurthy, V. Growth in the Asian elephant. _J. Biosci_
97, 561–571 (1988b). Google Scholar * Evans, K. E. & Harris, S. Adolescence in male African elephants, Loxodonta africana, and the importance of sociality. _Anim Behav_ 76, 779–787
(2008). Article Google Scholar * Sukumar, R. _The Living Elephants: Evolutionary Ecology, Behavior and Conservation_. (New York, USA: Oxford University Press 2003). * de Silva, S.,
Rangeewa, A. & Kryazhimskiy, S. The dynamics of social networks among female Asian elephants. _BMC Ecology_ 11, 1–17 (2011). Article Google Scholar * Fishlock, V. & Lee, P. C.
Forest elephants: fission-fusion and social arenas. _Animal Behaviour_ 85, 357–363 (2013). Article Google Scholar * Nandini, S., Keerthipriya, P. & Vidya, T. N. C. Group size
differences may mask underlying similarities in social structure: a comparison of female elephant societies. _Behavioral Ecology_ 29, 145–159 (2018). Article Google Scholar * Jainudeen, M.
R., McKay, G. M. & Eisenberg, J. F. Observations on musth in the domesticated Asiatic elephant (_Elephas maximus_). _Mammalia_ 36, 247–261 (1972). Article Google Scholar * Sukumar, R.
_The Asian Elephant: Ecology and Management_. Cambridge, UK: (Cambridge University Press 1992). * Poole, J. H. Rutting behaviour in African elephants: the phenomenon of musth. _Behaviour_
102, 283–316 (1987). Article Google Scholar * Poole, J. H. Mate guarding, reproductive success and female choice in African elephants. _Anim Behav_ 37, 842–849 (1989). Article Google
Scholar * Chiyo, P. I. _et al_. The influence of forage, protected areas, and mating prospects on grouping patterns of male elephants. _Behav Ecol_ 25, 1494–1504 (2014). Article Google
Scholar * Wittemyer, G., Douglas-Hamilton, I. & Getz, W. M. The socioecology of elephants: analysis of the processes creating multitiered social structures. _Anim Behav_ 69, 1357–1371
(2005). Article Google Scholar * Srinivasaiah, N. M., Anand, V. D., Vaidyanathan, S. & Sinha, A. Usual populations, unusual individuals: Insights into the behavior and management of
Asian elephants in fragmented landscapes. _PLoS ONE_ 7, e42571 (2012). Article ADS CAS Google Scholar * Chiyo, P. I. _et al_. Association patterns of African elephants in all-male
groups: The role of age and genetic relatedness. _Anim. Behav_ 81, 1093–1099 (2011). Article Google Scholar * Sukumar, R. & Gadgil, M. Male-female differences in foraging on crops by
Asian elephants. _Anim Behav_ 36, 1233–1235 (1988a). Article Google Scholar * Chiyo, P. I. _et al_. No risk, no gain: effects of crop raiding and genetic diversity on body size in male
elephants. _Behav Ecol_ 22, 552–558 (2011). Article Google Scholar * Chelliah, K. & Sukumar, R. The role of tusks, musth and body size in male-male competition among Asian elephants,
_Elephas maximus_. _Anim Behav_ 86, 1207–1214 (2013). Article Google Scholar * Pokharel, S. S., Singh, B., Seshagiri, P. B. & Sukumar, R. Lower levels of glucocorticoids in
crop-raiders: diet quality as a potential ‘pacifier’ against stress in free-ranging Asian elephants in a human-production habitat. _Anim Conserv_. https://doi.org/10.1111/acv.12450 (2018).
Article Google Scholar * Shannon, G. _et al_. Effects of social disruption in elephants persist decades after culling. _Front. Zool_ 10, 62 (2013). Article Google Scholar * Burke, T.,
Page, B., Van Dyk, G., Millspaugh, J. & Slotow, R. Risk and Ethical Concerns of Hunting Male Elephant: Behavioural and Physiological Assays of the Remaining Elephants. _PLoS ONE_ 3,
e2417 (2008). Article ADS Google Scholar * Rangarajan, M. _et al_. Gajah: Securing the Future for Elephants in India. Ministry of Environment and Forests, New Delhi, India (2010). *
Vijayakrishnan, S., Kumar, M. A., Umapathy, G., Kumar, V. & Sinha, A. Physiological stress responses in wild Asian elephants _Elephas maximus_ in a human-dominated landscape in the
Western Ghats, southern India. _Gen. Com. Endocrinol_, https://doi.org/10.1016/j.ygcen.2018.05.009 (2018). Article CAS Google Scholar * Stamps, J. A. Habitat selection by dispersers:
Integrating proximate and ultimate approaches. In: Clobert, J., Danchin, E., Dhondt, A. A. & Nichols, J. D. (Eds), Dispersal (pp 230–242). Oxford, UK: Oxford University Press (2001). *
McNamara, J. M. & Houston, A. I. Optimal foraging and learning. _J. Theor. Biol_ 117, 231–249 (1985). Article MathSciNet Google Scholar * Kumar, S. R. _Ecology of Asian Elephants,
their Habitats and Interactions with People in Hosur and Dharmapuri Forest Division, Tamil Nadu, South Indian_. Ph.D. thesis. Bharathidasan University, Trichirapally, Tamil Nadu, India
(1994). * Adhikari, S., Southworth, J. & Nagendra, H. Understanding forest loss and recovery: a spatiotemporal analysis of land change in and around Bannerghatta National Park, India.
_J. Land Use Sci_ 10, 402–424 (2015). Article Google Scholar * Ims, R. A. & Hjermann, D. O. Condition-dependent dispersal. In: Clobert, J., Danchin, E., Dhondt, A. A. & Nichols, J.
D. (Eds), Dispersal (pp 203–216). Oxford, UK: Oxford University Press (2001). * Doligez, B., Danchin, E. & Clobert, J. Public information and breeding habitat selection in a wild bird
population. _Science_ 297, 1168–1170 (2002). Article ADS CAS Google Scholar * Srinivasaiah, N. M., Vaidyanathan, S. & Sinha, A. Elephas flexibilis: Behavioural plasticity in Asian
elephants in a human-dominated landscape in southern India. In: Kumar, A. & Vasudev, D. (Eds), _Ecological and Anthropological Studies in India: The Science of Conserving Wildlife_. New
Delhi, India: Springer India. In press. * Goswami, V. R., Vasudev, D. & Oli, M. K. The importance of conflict-induced mortality for conservation planning in areas of human-elephant
co-occurrence. _Biol Conserv_ 176, 191–198 (2014). Article Google Scholar * Sukumar, R. The management of large mammals in relation to male strategies and conflict with people. _Biol
Conserv_ 55, 93–102 (1991). Article Google Scholar * Arivazhagan, C. & Sukumar, R. Comparative demography of Asian elephant populations (_Elephas maximus_) in southern India. Technical
Report No. 106. Indian Institute of Science, Bangalore, India: Centre for Ecological Sciences (2005). * Slotow, R., van Dyk, G., Poole, J., Page, B. & Klocke, A. Older bull elephants
control young males. _Nature_ 408, 425–426 (2000). Article ADS CAS Google Scholar * QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Found Project,
http://www.qgis.org/ (2015). * Census. District Census Handbook Karnataka and Tamilnadu. Ministry of Home Affairs. Government of India, New Delhi: Office of the Registrar General and Census
Commissioner, http://www.censusindia.gov.in/2011census/dchb/DCHB.html (2011). * Baskaran, N. & Venkatesh, P. Human Elephant Conflict in Hosur Forest Division, Tamilnadu, India. An
Interim Report to Hosur Forest Division, Tamil Nadu Forest Department. Bengaluru, India: Asian Nature Conservation Foundation. p. 1–30 (2009). * Project Elephant. Synchronized elephant
population estimation India 2017. New Delhi, India: Ministry of Environment, Forest and Climate Change, Government of India (2017). * Bridges, A. S. & Noss, A. J. Behaviour and Activity
Patterns. In O’Connell, A. F., Nichols, J. D. & Karanth, K. U. (Eds), _Camera Traps in Animal Ecology: Methods and Analyses_ (pp 57–69). New York, NY: Springer New York (2011). *
Ranjeewa, A. D. G., Tharanga, Y. J. S., Sandanayake, G. H. N. A., Perera, B. V. & Fernando, P. Camera traps unveil enigmatic crop raiders in Udawalawe, Sri Lanka. _Gajah_ 42, 7–14
(2015). Google Scholar * Caravaggi, A. _et al_. A review of camera trapping for conservation behaviour research. _Remote Sens Ecol Conserv_ 3, 109–122 (2017). Article Google Scholar *
Smit, J., Pozo, R. A., Cusack, J. J., Nowak, K. & Jones, T. Using camera traps to study the age–sex structure and behaviour of crop-using elephants Loxodonta africana in Udzungwa
Mountains National Park, Tanzania. _Oryx_. https://doi.org/10.1017/S0030605317000345 (2017). Article Google Scholar * Arivazhagan, C. & Sukumar, R. Constructing age structures of Asian
elephant populations: A comparison of two field methods of age estimation. _Gajah_ 29, 11–16 (2008). Google Scholar * Sukumar, R. Ecology of the Asian Elephant (_Elephas maximus_) and its
Interaction with Man in South India. Doctoral thesis, Indian Institute of Science, Bangalore (1985). * Pokharel, S. S., Seshagiri, P. B. & Sukumar, R. Assessment of season-dependent body
condition scores in relation to faecal glucocorticoid metabolites in free-ranging Asian Elephants. _Conserv Physiol_ 5, 191–206 (2017). Article Google Scholar * Gale, U. T. Burmese Timber
Elephant. Rangoon, Burma: Trade Corporation (1974). * Ghosal, R., Ganswindt, A., Seshagiri, P. B. & Sukumar, R. Endocrine correlates of musth in free-ranging Asian elephants (_Elephas
maximus_) determined by non-invasive faecal steroid hormone metabolite measurements. _PLoS ONE_ 8, 4–8 (2013). Article Google Scholar * Chelliah, K. & Sukumar, R. Interplay of male
traits, male mating strategies and female mate choice in the Asian elephant, _Elephas maximus_. _Behaviour_ 152, 1113–1144 (2015). Article Google Scholar * Foster, R. J., Harmsen, B. J.
& Doncaster, C. P. Habitat use by sympatric jaguars and pumas across a gradient of human disturbance in Belize. _Biotropica_ 42, 724–731 (2010). Article Google Scholar * Rowcliffe, J.
M., Kays, R., Kranstauber, B., Carbone, C. & Jansen, P. A. Quantifying levels of animal activity using camera trap data. _Methods Ecol Evol_ 5, 1170–1179 (2014). Article Google Scholar
* Sollmann, R. A gentle introduction to camera‐trap data analysis. _Afr J Ecol_ 56, 740–749 (2018). Article Google Scholar * Lashley, M. A. _et al_. _Sci Rep_ 8, 4173 (2018). Article
ADS Google Scholar * National Remote Sensing Agency Manual of national land use land cover mapping using multi-temporal satellite data. Hyderabad, India: Department of Space (2006). *
McGarigal, K., Cushman, S. A. & Ene, E. FRAGSTATS v4. 2. FRAGSTATS Help (2015). * LaGro, J. Assessing patch shape in landscape mosaics. _Photogramm Eng Remote Sensing_ 57, 285–293
(1991). Google Scholar * Wang, X., Blanchet, F. G. & Koper, N. Measuring habitat fragmentation: An evaluation of landscape pattern metrics. _Methods Ecol Evol_ 5, 634–646 (2014).
Article Google Scholar * Hothorn, T., Hornik, K. & Zeileis, A. Unbiased recursive partitioning: A conditional inference framework. _J. Comput Graph Stat_ 15, 651–674 (2006). Article
MathSciNet Google Scholar * Jerome, F. H. & Meulman, J. J. Multiple additive regression trees with application in epidemiology. _Statistics in Medicine_ 22.9, 1365–1381 (2003). Google
Scholar * Rick, L. L. & Wright, A. Rule-based classification systems using classification and regression tree (CART) analysis. _Photogramm Eng Remote Sensing_ 67, 1137–1142 (2001).
Google Scholar * Katharina, D. C. S. & Pfeiffer, D. U. The application of non-parametric techniques to solve classification problems in complex data sets in veterinary epidemiology - an
example. _Intelligent Data Analysis_ 3, 23–35 (1999). Article Google Scholar * McDonald, J. H. _Handbook of Biological Statistics_ (3rd ed.). Baltimore, Maryland, USA: Sparky House
Publishing (2014). Download references ACKNOWLEDGEMENTS We would like to thank the two anonymous reviewers for their extremely insightful comments, which helped us improve the quality of our
manuscript. We would also like to thank the Rufford Foundation, Business Transactions Group Legal, Prince Bernhard Nature Fund and the Ashirvadam Trust for their support towards the
long-term study of Asian elephant behavior and ecology in the human-dominated landscapes of the Eastern Ghats. We would like to express our gratitude to the officials of the Forest
Departments of Karnataka and Tamil Nadu states for their constant support and involvement in the study and for necessary research permissions. NS and VK would like to thank the field staff
of the respective Forest Departments, farmers living alongside the elephants in the study area and the elephants themselves for their patience during the many hours of interactions. RS was a
JC Bose National Fellow during the tenure of this study. We would also like to thank the administrative staff, colleagues and friends at the National Institute of Advanced Studies, Indian
Institute of Science, Asian Nature Conservation Foundation and the Foundation for Ecological Research, Advocacy and Learning (FERAL) for their constant support and encouragement during this
study. NS is thankful to Manipal Academy of Higher Education for permitting this research as part of the PhD programme. Finally, we are grateful to Rajat Nayak for his untiring help with the
GIS analysis. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * National Institute of Advanced Studies, Animal Behaviour and Cognition Programme, Bengaluru, 560012, India Nishant Srinivasaiah
& Anindya Sinha * Foundation for Ecological Research, Advocacy and Learning, Pondicherry, 605101, India Vinod Kumar & Srinivas Vaidyanathan * Indian Institute of Science, Centre for
Ecological Sciences, Bengaluru, 560012, India Raman Sukumar * Indian Institute of Science Education and Research Kolkata, Mohanpur, 741246, India Anindya Sinha Authors * Nishant Srinivasaiah
View author publications You can also search for this author inPubMed Google Scholar * Vinod Kumar View author publications You can also search for this author inPubMed Google Scholar *
Srinivas Vaidyanathan View author publications You can also search for this author inPubMed Google Scholar * Raman Sukumar View author publications You can also search for this author
inPubMed Google Scholar * Anindya Sinha View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.S., S.V., R.S. and A.S. conceived the study, N.S.
and V.K. conducted the field work, N.S., V.K., S.V. and A.S. analysed the results, All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Nishant Srinivasaiah. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION SUPPLEMENTARY VIDEO 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Srinivasaiah, N., Kumar, V., Vaidyanathan, S. _et al._ All-Male
Groups in Asian Elephants: A Novel, Adaptive Social Strategy in Increasingly Anthropogenic Landscapes of Southern India. _Sci Rep_ 9, 8678 (2019). https://doi.org/10.1038/s41598-019-45130-1
Download citation * Received: 07 February 2019 * Accepted: 29 May 2019 * Published: 04 July 2019 * DOI: https://doi.org/10.1038/s41598-019-45130-1 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative