Global variation of nutritional status in children undergoing chronic peritoneal dialysis: a longitudinal study of the international pediatric peritoneal dialysis network
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT While children approaching end-stage kidney disease (ESKD) are considered at risk of uremic anorexia and underweight they are also exposed to the global obesity epidemic. We sought
to investigate the variation of nutritional status in children undergoing chronic peritoneal dialysis (CPD) around the globe. The distribution and course of body mass index (BMI) standard
deviation score over time was examined prospectively in 1001 children and adolescents from 35 countries starting CPD who were followed in the International Pediatric PD Network (IPPN)
Registry. The overall prevalence of underweight, and overweight/obesity at start of CPD was 8.9% and 19.7%, respectively. Underweight was most prevalent in South and Southeast Asia (20%),
Central Europe (16.7%) and Turkey (15.2%), whereas overweight and obesity were most common in the Middle East (40%) and the US (33%). BMI SDS at PD initiation was associated positively with
current eGFR and gastrostomy feeding prior to PD start. Over the course of PD BMI SDS tended to increase on CPD in underweight and normal weight children, whereas it decreased in initially
overweight patients. In infancy, mortality risk was amplified by obesity, whereas in older children mortality was markedly increased in association with underweight. Both underweight and
overweight are prevalent in pediatric ESKD, with the prevalence varying across the globe. Late dialysis start is associated with underweight, while enteral feeding can lead to obesity.
Nutritional abnormalities tend to attenuate with time on dialysis. Mortality risk appears increased with obesity in infants and with underweight in older children. SIMILAR CONTENT BEING
VIEWED BY OTHERS THE CHANGE IN GERIATRIC NUTRITIONAL RISK INDEX IS ASSOCIATED WITH MORTALITY IN PATIENTS WHO START HEMODIALYSIS: KOREAN RENAL DATA REGISTRY, 2016–2018 Article Open access 27
November 2022 COMPARATIVE PERFORMANCE OF BODY COMPOSITION PARAMETERS IN PREDICTION OF DEATH IN HOSPITALIZED PATIENTS ON MAINTENANCE HEMODIALYSIS: A COHORT STUDY Article Open access 23 June
2020 ASSOCIATION OF BLOOD UREA NITROGEN TO GLUCOSE RATIO WITH 365-DAY MORTALITY IN CRITICALLY ILL PATIENTS WITH CHRONIC KIDNEY DISEASE: A RETROSPECTIVE STUDY Article Open access 25 February
2025 INTRODUCTION The nutritional status is a principal concern when caring for children undergoing chronic peritoneal dialysis (CPD). While early studies revealed providing sufficient
nutrition was essential for adequate growth in this population, advances in enteral feeding practices have enabled the elimination of underweight but have not improved linear growth as much
as expected1,2,3. Recent concerns have emerged on the potential for adverse effects of excessive caloric intake in patients who receive supplemental feeding1,2,3. The majority of published
studies assessing the nutritional status of dialyzed children were performed at highly specialized pediatric dialysis units in North America and Western Europe. In contrast, on a global
scale the risk of nutritional abnormalities in individual regions and countries is likely to be affected by a range of medical and non-medical factors including the patient case-mix
regarding age, underlying disease and co-morbidities, national economic strength and healthcare expenditure, cultural acceptability of dietary and feeding prescriptions, availability of
special formula diets and enteral feeding equipment, and differences in local, national or regional nutritional recommendations4. The International Pediatric Peritoneal Dialysis Network
(IPPN) has been collecting comprehensive clinical and laboratory data in a standardized manner from children undergoing CPD worldwide since 2007. Since these data include detailed
anthropometric measures, feeding prescriptions and outcome measures, it provides an opportunity to address the global demographics of nutritional abnormalities in children receiving CPD. The
objective of this study was to examine and follow prospectively the nutritional status of 1,001 children commencing CPD around the globe, analyze factors associated with the nutritional
status at the start and during the course of dialysis, and to analyze the impact of nutritional abnormalities on patient survival. METHODS DATA COLLECTION The IPPN Registry was established
in 2007 and currently collects comprehensive clinical and laboratory information from children undergoing CPD at 95 pediatric dialysis centers in 37 countries around the globe. Patient
status is updated every 6 months via an Internet-based web platform (www.pedpd.org). The complete list of data items collected has been published previously2,4. Data is automatically checked
for plausibility and completeness. Data protection is ensured by pseudonymized data input. The study is performed in accordance with the relevant medical association’s professional codes of
conduct with the Declaration of Helsinki from 2008. Approval for the registry project was obtained from The Children’s Mercy Hospital Pediatric Institutional Review Board, Kansas City, USA
and local Institutional Review Boards or ethical committees. Informed consent was obtained from the patients and/or their legal guardians as required by local review boards. CALCULATION OF
BMI SDS AND EGFR Body Mass Index (BMI), i.e. weight/height2 (kg/m2), was normalized to standard deviation scores (SDS) according to height age, utilizing the WHO (2006) and CDC (2000)
standards for children aged younger and older than 5 years, respectively (see www.who.int/childgrowth/en/)5,6. Normalization to height age, i.e. the chronological age of a child with the
same height growing at the 50th height percentile, was made to adjust for the high prevalence of growth failure in the cohort7. BMI SDS values were used to categorize patients into three BMI
groups: underweight (<2.5th percentile, i.e. <−2 SDS), normal (2.5th to 85th percentile, i.e. −2 to 1.036 SDS), overweight (>85th −95th percentile, i.e. >1.036 to 1.645 SDS),
and obesity (>95th percentile, i.e. >1.645 SDS). The Schwartz bedside formula was used to estimate GFR at initiation of CPD8. STATISTICS Data collection was complete for all
observations except residual urine output (7.6% missing data), daily ultrafiltration rate (5.2%), eGFR (1.2%), total PD fluid turnover and dialytic glucose exposure (0.8%), PD modality
(0.5%), serum bicarbonate (0.3%), serum albumin (0.2%), and estimated dry weight (0.2%). Multiple imputation by chained equations was conducted to replace these missing values9. All analyses
were performed using the imputed dataset. Additionally, sensitivity analyses were performed using only cases with complete data sets. ANOVA or Kruskal-Wallis tests were conducted to compare
differences between BMI groups. Differences in proportions were assessed using Chi2 tests. Linear mixed modeling was used to identify factors affecting BMI SDS at baseline and during
follow-up. The initial cross sectional model included age, sex, eGFR, gross national income (GNI), renal diagnosis, presence of comorbidities, ethnicity, urine output, nutritional support
(oral caloric supplements, nasogastric tube (NGT) and gastrostomy feeding), and growth hormone use as independent variables. The region of residence was accounted for as random intercept.
For the longitudinal analysis, the change in BMI SDS between two observations, projected to 12 months, was used as the dependent variable and region and patients were used as nested random
effects. Potential covariates included in the initial model were age at baseline, sex, presence of comorbidities, renal diagnosis, GNI and the time-varying variables BMI SDS, height SDS,
eGFR, % deviation from estimated dry weight, PD modality, duration of PD, serum albumin, serum bicarbonate, total PD fluid volume, urine output, ultrafiltration, growth hormone use,
nutritional support, glucose exposure, biocompatible PD fluid use, and amino acid PD fluid use. A stepwise variable selection procedure was applied to identify the relevant covariates for
the cross sectional model as well as for the longitudinal model, using p = 0.2 as a cutoff criterion for model entry. Kaplan–Meier analysis with log-rank testing was used to assess
differences in patient survival. Cox proportional hazard modeling with time dependent covariates and interaction term was applied to identify risk factors of death on dialysis. Data were
analyzed using SAS, version 9.3 (SAS Institute, Inc., Cary, NC), and R, version 3.1.110. RESULTS STUDY POPULATION All children and adolescents enrolled in the IPPN registry with initiation
of CPD between March 2007 and December 2014 were analyzed for this study. Five patients with syndromic and metabolic disorders associated with intrinsic abnormalities of growth and body
composition were excluded from the analysis. The final dataset comprised a total of 1,001 incident patients from 85 nephrology centers in 35 countries. Children originated from Western
Europe (n = 300), Central Europe (n = 120), Turkey (n = 105), the Middle East (n = 15), China and Hong Kong (n = 77), Korea (n = 24), India and South East Asia (n = 30), New Zealand (n =
18), USA (n = 97), Canada (n = 13) and Latin America (n = 202). One or more comorbidities were reported in 369 patients (35.6%); these included mainly defined syndromic disorders (n = 107),
impaired cognitive development (n = 119), cardiac (n = 130) and pulmonary abnormalities (n = 52). Of the 1,001 patients, 702 (70%) patients had at least two BMI records available. Median
follow-up time was 14.5 (IQR 17.8) months. Altogether, the data set contained 2,931 follow-up entries. NUTRITIONAL STATUS AT DIALYSIS INITIATION The overall prevalence of underweight, normal
weight and overweight/obesity at the start of CPD was 8.9%, 71.4%, and 19.7%, respectively. The detailed patient characteristics according to nutritional status at dialysis entry are shown
in Table 1. Overweight/obese children originated from countries with higher GNI per capita, had higher eGFR at CPD initiation and were more growth retarded. Gastrostomy feeding was performed
in almost 17% of the overweight children as compared to 8% and 6% in the normal and low BMI groups (p < 0.001 for comparison of gastrostomy feeding between overweight and normal, as well
as between overweight and low BMI). Children starting PD with underweight more often received amino acid PD fluid than children without underweight (p = 0.013). The distribution of
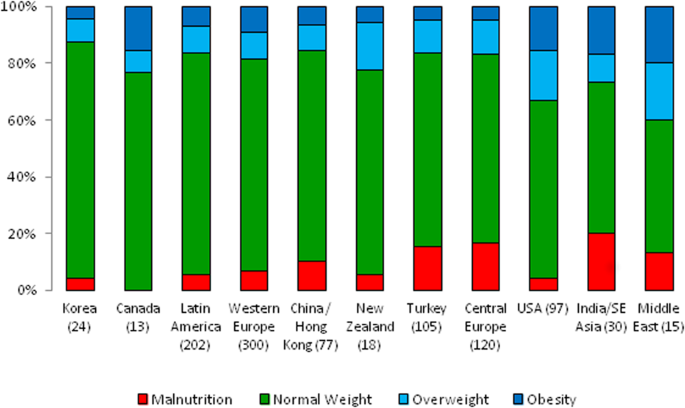
underweight, normal weight, and overweight/obese patients according to geographical region is depicted in Fig. 1. The prevalence of nutritional abnormalities varied significantly across
world regions. Underweight was most prevalent in South and Southeast Asia (20%), followed by Central Europe (16.7%) and Turkey (15.2%), whereas overweight and obesity were most common in the
Middle East (40%) and the US (33%). The prevalence of underweight was highest in the first year of life (14.2%), decreasing to 6.5%, 9.4% and 7.7% in children aged 1–<6, 6–12 and older
than 12 years, respectively (Fig. S1 in supplement). The prevalence of underweight was correlated with the eGFR at initiation of PD, increasing from 5.3% at eGFR 9–12 ml/min/1.73 m2 to 11%
at eGFR < 6 ml/min/1.73 m2 (p = 0.03) (see also Fig. S2). The prevalence of obesity was higher in the younger children than in adolescents, whereas the prevalence of being overweight was
largely independent of age (see Fig. S1). By multivariate analysis, BMI SDS at dialysis initiation was positively predicted by eGFR and the use of gastrostomy feeding and negatively
predicted by the presence of comorbidities, whereas age, sex, ethnicity, GNI, renal diagnosis, and growth hormone use were not predictive (Table S1). ENTERAL FEEDING PRACTICES To further
explore and compare regional characteristics in nutrition management, enteral feeding patterns were investigated. Enteral (NGT or gastrostomy) feeding was used at baseline in 57.4%, 32.9%,
5.6%, and 2.3% of children <1, 1–<6, 6–12 and >12 years, respectively (p < 0.001). The variation of feeding practices varied markedly by region (Fig. 2). Enteral feeding was
rarely applied in Central Europe, Turkey, India, South East Asia, and China. Gastrostomy usage was confined to North America, Western Europe, Korea and New Zealand. Among the 386 children
<6 years, 166 were followed for at least 12 months on dialysis. The fraction of children with enteral tube feeding was 38% at baseline and 41% at follow up (ns). CHANGES IN NUTRITIONAL
STATUS WITH TIME ON CPD Changes in BMI SDS were analyzed using 702 patients with at least two available BMI records. During a median follow-up time of 15 (interquartile range 18) months, BMI
SDS tended to increase on CPD in both underweight and normal weight children, whereas it decreased in the overweight patients (Fig. 3). Out of 74 underweight children at the start of CPD,
51.4% were non-underweight at last observation; among 125 overweight/obese children at CPD initiation, 36.0% achieved a normal BMI at follow-up. Gastrostomy feeding was associated with an
increase in BMI SDS during follow-up (Table 2). Additional factors independently associated with a positive change in BMI SDS included a lower BMI SDS and higher height SDS, higher serum
albumin and the diagnosis of CAKUT. In contrast, greater fluid overload (expressed as % deviation from estimated dry weight) was predictive of a negative change in BMI SDS during follow-up.
NUTRITION AND MORTALITY A total of 54 children died during the observation period. The most common causes of death were non-PD related infections (39%), followed by congestive heart failure
(17%), PD-related infections (7%) and malignances (7%). The 1-, 2- and 4-year survival rates were 91%, 84%, and 74% in patients who were underweight at last observation, as compared to 95%,
93% and 89% in those with final higher BMI SDS values (p = 0.03) (Fig. 4). Cox proportional hazard analysis identified the presence of comorbidities and younger age as risk factors for death
on dialysis (Table 3). Whereas BMI SDS per se was not predictive, the interaction term of BMI SDS and age affected the risk of death at borderline significance (p = 0.06). To further
illustrate the interaction of age and BMI SDS with respect to mortality risk on dialysis, we modeled the hazard ratio of death by age for patients with a BMI SDS of −2, 0, and 2 (Fig. 5).
While the globally increased risk of death of children younger than 5 years of age is more marked in obese children, at older age underweight children appear at higher risk of death than
obese ones. DISCUSSION At variance with historical epidemiological studies on nutrition in children with CKD, which focused mainly on uremic underweight, this study highlights a changing
trend of the nutritional status in pediatric end stage kidney disease (ESKD). Both underweight and overweight/obesity were common. Using the 2.5th and the 85th BMI percentiles as cutoffs for
underweight and overweight/obesity respectively, 28.6% of patients receiving CPD, as compared to an expected 17.5%, exhibited an abnormal body composition. A relatively low rate of
underweight, but an increased prevalence of overweight and obesity was recently also observed in North American CKD cohorts11,12 and in European children undergoing dialysis13. While it is
tempting to merely attribute this trend to the global childhood obesity epidemic, the comprehensive and worldwide data collection in the IPPN Registry allowed us to dissect in detail and on
a global scale, the impact of macroeconomic factors, patient characteristics, and pediatric CKD and dialysis management practices on nutritional outcome. The regional breakdown and
comparison with the general childhood population prevalence data revealed that overweight and obesity in the CPD patients was indeed most prevalent in children and adolescents from the US
and the Middle East, and comparable to the national prevalence rates in these countries which show the highest prevalence of childhood obesity worldwide14. On the other hand, when relating
the observed obesity rates to the current prevalences in the general pediatric populations of the respective regions, obesity was slightly less common in patients from Western Europe, but
was substantially more frequent in children from South and South East Asia14. The latter finding might be explained in part by overrepresentation of families from upper socioeconomic strata
to whom chronic dialysis is affordable. Noteworthy also was the finding that whereas gross national income was positively correlated with overweight/obesity prevalence by univariate
analysis, it was not included in the multivariate models predicting BMI at initiation of dialysis and during follow-up. This suggests that the observed regional differences can be explained
in large part by the medical factors included in the multivariate analysis. Two factors were consistently associated with BMI SDS at the time of PD initiation: the most current eGFR and the
use of gastrostomy feeding prior to PD start. The positive association with eGFR is compatible with the notion that the risk of uremic underweight increases as residual kidney function
declines in the late pre-dialytic phase. Centers in countries where late referral to pediatric nephrology care is common may be more likely to start PD in underweight patients with little
residual kidney function. In the case of children for whom PD is to be started electively, our findings add a nutritional perspective to the list of factors to be considered when determining
the optimal timing of PD initiation. The most important single factor associated with higher BMI SDS both at PD start and during follow-up was the presence of a gastrostomy. In the
multivariate longitudinal analysis, gastrostomy feeding accounted for an increase in BMI by almost two standard deviations per three years of treatment. Accordingly, gastrostomy feeding was
almost three times more common among overweight and obese as compared to underweight patients. These findings confirm and extend a previous IPPN study in young infants, which identified PEG
feeding as a risk factor for obesity2. Since the use of gastrostomy feeding was found to be largely restricted to high-income countries, enteral feeding via gastrostomy appears to be the
most important factor underlying the observed link between Gross National Income and higher BMI. An unexpected observation was the fact that obese - but not underweight - patients were
significantly shorter than patients with normal nutritional status at the initiation of renal replacement therapy. This finding, which is in keeping with recent findings in the ESPN/ERA-EDTA
registry13 and previous reports15, may reflect previous frustraneous interventions to correct growth failure by hypercaloric feeding. An important and reassuring finding from the
longitudinal analysis is the observation that nutritional abnormalities, both underweight and overweight/obesity, tend to level off over the course of dialysis. The prevailing BMI SDS was a
highly significant inverse predictor of the subsequent change in BMI SDS in the longitudinal model. This ‘funneling’ of the nutritional status is likely to be a consequence of regular
nutritional monitoring and dietary advice. Direct effects of dialysis such as control of uremia, correction of acidosis and dialytic glucose resorption probably play a role in the correction
of underweight, but these factors were not included as significant predictors of prospective BMI SDS change in the overall model since they are less likely to increase BMI in patients with
normal nutritional status. Remarkably, patients with congenital kidney malformation disorders (CAKUT) were more likely to gain BMI SDS over the course of dialysis than patients with other
underlying renal diseases. Since CAKUT is the most common renal diagnosis in infants with ESKD and was slightly more common among initially underweight patients, we speculate that catch-up
weight gain due to enteral feeding was more common in this patient group. In addition, as CAKUT patients are often polyuric even at commencement of dialysis, part of the observed BMI gain
may be explained by relative fluid gain to the gradual loss of diuresis with time on PD. The estimated fractional deviation from dry weight was associated with a subsequent negative change
in BMI SDS, possibly reflecting the impact of early fluid imbalances (e.g. hypervolemia) and their correction with time on CPD. When patients with substantial fluid overload have their body
weight decreased because of improved fluid management, this will be recorded as a negative change in BMI, illustrating the limitation of BMI SDS as a measure of nutritional status.
Nutritional status is a well recognized, major global determinant of mortality in the general adult and pediatric population, as well as in adults and children with ESKD16,17,18. The overall
patient survival observed in this global sample of children and adolescents starting CPD (91% at 2 years) was not dissimilar from survival rates noted in North American and European
registry cohorts19,20,21. Wong _et al_., in the only other large study examining BMI and mortality in pediatric ESRD, demonstrated a U-shaped association between the risk of death and BMI
SDS, with both extremes of BMI associated with increased mortality19. An increased death risk was also observed in children with a hematological malignancy who were either underweight or
obese22. In the present study, underweight patients were twice as likely to die on dialysis as non-underweight children by univariate Kaplan Meier survival analysis, whereas obesity did not
affect the risk of death. On the other hand, when accounting for age and the presence of comorbidities in a Cox regression model, although BMI SDS did not predict the risk of death, age and
BMI SDS showed an interactive effect; specifically, an increased infant mortality was amplified by obesity, whereas mortality in older children and adolescents was markedly increased by
underweight. Hence, our findings are compatible with an age-dependent impact of the extremes of body composition on patient survival. We hypothesize that the morbid obesity generated in a
subset of young infants by enteral feeding may, in fact, put these patients at increased risk of death. Possible underlying causes of this risk association might include enhanced
cardiovascular risk due to the difficulty of assessing fluid status in obese infants and the susceptibility of obese subjects to severe outcomes following viral respiratory infections23.
While the strength of our study relates to the robust set of data available from a large group of pediatric CPD patients, several limitations of our study should be mentioned. Although BMI
is a generally accepted method to assess nutritional status in healthy children24,25,26, it is an imperfect measure of body composition. This is particularly true in pediatric dialysis
patients as it does not account for abnormalities of fluid status and is impacted by abnormal body height. While the latter issue was accounted for by calculation of BMI SDS for height
age27,28, a more refined auxological assessment was not possible in this global registry. Another important limitation of our study was the lack of detailed data on dietary prescriptions and
caloric intake, which would be difficult to obtain due to complexity of data collection in such a large and diverse cohort of patients. In summary, this longitudinal assessment of 1001
children from 35 countries commencing CPD demonstrates that both underweight and obesity are observed at increased frequency in pediatric ESRD. The prevalence of both abnormalities varies
substantially across the globe. Delayed start of dialysis is a risk factor for underweight whereas enteral tube feeding, while protecting from underweight, increases the risk of developing
obesity. Nutritional abnormalities tend to attenuate with time on dialysis. REFERENCES * Sienna, J. L. _et al_. Body size in children with chronic kidney disease after gastrostomy tube
feeding. _Pediatr Nephrol._ 25, 2115–21 (2010). Article Google Scholar * Rees, L. _et al_. International Pediatric Peritoneal Dialysis Network (IPPN) registry. Growth in very young
children undergoing chronic peritoneal dialysis. _J Am Soc Nephrol._ 22, 2303–2312 (2011). Article Google Scholar * Rees, L. & Jones, H. Nutritional management and growth in children
with chronic kidney disease. _Pediatr Nephrol._ 28, 527–36 (2013). Article Google Scholar * Schaefer, F. _et al_. IPPN investigators. Impact of global economic disparities on practices and
outcomes of chronic peritoneal dialysis in children: insights from the International Pediatric Peritoneal Dialysis Network Registry. _Perit Dial Int._ 32, 399–409 (2012). Article Google
Scholar * WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. _Acta Paediatr Suppl_ 450, 76–85 (2006). Google Scholar *
Kuczmarski, R. J. _et al_. 2000 CDC Growth Charts for the United States: methods and development. _Vital Health Stat_ 246, 1–190 (2002). Google Scholar * Bonthuis, M. _et al_. Application
of body mass index according to height-age in short and tall children. _PLoS One_. 8(8) (2013). * Schwartz, G. J. & Work, D. F. Measurement and estimation of GFR in children and
adolescents. _Clin J Am Soc Nephrol._ 4(11), 1832–43 (2009). Article Google Scholar * Van Buuren, S. & Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R.
_J Stat Softw._ 45(3), 1–67 (2011). Article Google Scholar * R Core Team R: A language and environment for statistical computing. R Foundation for Statistical. Computing, Vienna, Austria.
http://www.R-project.org/ (2014). * Wilson, A. C. _et al_. Prevalence and correlates of multiple cardiovascular disease risk factors in children with chronic kidney disease. _Clin J Am Soc
Nephrol._ 6, 2759–65 (2011). Article CAS Google Scholar * Yasin, A., Benidir, A. & Filler, G. Are Canadian pediatric nephrology patients really overweight? _Clin Nephrol._ 78(5),
359–64 (2012). Article Google Scholar * Bonthuis, M. _et al_. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. _Nephrol Dial Transplant._ 28(Suppl
4), iv195–iv204 (2013). Article Google Scholar * Ng, M. _et al_. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013:a systematic
analysis for the Global Burden of Disease Study 2013. _Lancet_ 384, 766–81 (2014). * Rashid, R. _et al_. Body composition and nutritional intake in children with chronic kidney disease.
_Pediatr Nephrol._ 11, 1730–8 (2006). Article Google Scholar * De Mutsert, R. _et al_. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular
disease in chronic dialysis patients. _Nephrol Dial Transplant_ 23, 2957–2964 (2008). Article Google Scholar * Kang, S. S., Chang, J. W., Park, Y. Nutritional Status Predicts 10-Year
Mortality in Patients with End-Stage Renal Disease on Hemodialysis. _Nutrients_ 9, pii: E399 (2017). * Ku, E. _et al_. Association of Body Mass Index with Patient-Centered Outcomes in
Children with ESRD. _J Am Soc Nephrol_ 27, 551–558 (2016). Article CAS Google Scholar * Chesnaye, N. C. _et al_. Mortality risk in European children with end-stage renal disease on
dialysis. _Kidney Int_ 89, 1355–1362 (2016). Article Google Scholar * NAPRTCS Report: https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf (2011) * Wong, C. S. _et al_.
Anthropometric measures and risk of death in children with end-stage renal disease. _Am J Kidney Dis_ 36, 811–819 (2000). Article CAS Google Scholar * Lange, B. J. _et al_. Mortality in
overweight and underweight children with acute myeloid leukemia. _JAMA_ 293, 203–211 (2005). Article CAS Google Scholar * Almond, M. H., Edwards, M. R., Barclay, W. S. & Johnston, S.
L. Obesity and susceptibility to severe outcomes following respiratory viral infection. _Thorax_ 68, 684–686 (2013). Article Google Scholar * Cole, T. J. _et al_. Establishing a standard
definition for child overweight and obesity worldwide: international survey. _BMJ_ 320, 1240–1243 (2000). Article CAS Google Scholar * Cole, T. J. _et al_. Body mass index cut offs to
define thinness in children and adolescents: international survey. _BMJ_ 335, 194 (2007). Article Google Scholar * Cole, T. J. _et al_. What is the best measure of adiposity change in
growing children: BMI, BMI%, BMI z-score or BMI centile? _Eur J Clin Nutr_ 59, 419–425 (2005). Article CAS Google Scholar * Gao, T. _et al_. Interpretation of body mass index in children
with CKD. _Clin J Am Soc Nephrol_ 7, 558–564 (2012). Article Google Scholar * Foster, B. J. & Leonard, M. B. Measuring nutritional status in children with chronic kidney disease. _Am J
Clin Nutr_ 80, 801–814 (2004). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors gratefully acknowledge the support by the International Society for Peritoneal
Dialysis, Baxter Health Care, and Fresenius Medical Care. We also appreciate the continued dedicated support of the IPPN by the medical and nursing staff in all collaborating centers. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Center for Pediatrics and Adolescent Medicine, Heidelberg, Germany Franz Schaefer * Institute of Medical Biometry and Informatics, University of
Heidelberg, Heidelberg, Germany Laura Benner & Anja Sander * Medical University of Gdansk, Department of Pediatrics, Nephrology and Hypertension, Gdańsk, Poland Dagmara Borzych-Dużałka *
Nemours/A.I. duPont Hospital for Children, Wilmington, DE, USA Joshua Zaritsky * Children’s Hospital of Fundan University, Shanghai, China Hong Xu * Great Ormond Street Hospital, London,
United Kingdom Lesley Rees * Department of Pediatric Nephrology, National Kidney and Transplant Institute, Quezon City, Philippines Zenaida L. Antonio * Dr. Behcet Uz Children Research and
Educational Hospital, Izmir, Turkey Erkin Serdaroglu * Iran University of Medical Sciences, Tehran, Iran Nakysa Hooman * Nationwide Children’s Hospital, Columbus, OH, USA Hiren Patel *
Carrahpasa School of Medicine, Istanbul, Turkey Lale Sever * University Hospital Motol, Prague, Czech Republic Karel Vondrak * Seattle Children’s Hospital, Seattle, WA, USA Joseph Flynn *
S.E.N.N.I.A.D, Montevideo, Uruguay Anabella Rébori * Department of Nephrology, Starship Children’s Hospital, Auckland, New Zealand William Wong * HUCH Hospital for Children and Adolescents,
Helsinki, Finland Tuula Hölttä * Istanbul University, Istanbul Faculty of Medicine, Istanbul, Turkey Zeynep Yuruk Yildirim * Service de Néphrologie Pédiatrique, Hôpital Femme Mère Enfant,
Hospices Civils de Lyon, Lyon, France Bruno Ranchin * Children’s Memorial Health Institute, Warsaw, Poland Ryszard Grenda * Pediatric nephrology, Dialysis and Transplantation Unit Fondazione
IRCCS Ca’ Granda Osp. Maggiore Policlinico, Milan, Italy Sara Testa * Jagellonian University Medical College, Kraków, Poland Dorota Drożdz * Semmelweis University, Budapest, Hungary Attila
J. Szabo * Dubai Hospital, Dubai, United Arab Emirates Loai Eid * NRS Medical College & Hospital, Kolkata, India Biswanath Basu * Children Hospital, affiliate of Vilnius University
Hospital Santaros Klinikos, Vilnius, Lithuania Renata Vitkevic * Lucile Packard Children’s Hospital at Stanford, Palo Alto, USA Cynthia Wong * Children’s Medical Center Dallas, Dallas, Tx,
USA Stephen J. Pottoore * Department of Pediatric Gastroenterology, Nephrology and Metabolism, Charité, Berlin, Germany Dominik Müller * Erciyes University, Kayseri, Turkey Ruhan Dusunsel *
Hospital Sotero del Rio, Santiago, Chile Claudia Gonzalez Celedon * CHU Arnaud de Villeneuve, Université de Montpellier, Montpellier, France Marc Fila * Barnkliniken, Lund, Sweden Lisa Sartz
* Children’s Mercy Hospital, Kansas City, MO, USA Bradley A. Warady * Hospital de Pediatria Garrahan, Buenos Aires, Argentina M. Adragna * Hospital Italiano de Buenos Aires, Buenos Aires,
Argentina P. A. Coccia * Hospital de Ninos Sor. Maria Ludovica La Plata, La Plata, Argentina A. Suarez * Hospital Pediatrico Humberto Notti, Mendoza, Argentina P. G. Valles * R.S.A., Salta,
Argentina R. Salim * Hospital Interzonal General, Bahia Blanca, Argentina L. Alconcher * Medical University Vienna, Vienna, Austria K. Arbeiter * University Hospital Antwerp, Edegem, Belgium
K. van Hoeck * Instituto da CrianÇa - Hospital das Clinicas FMUSP, Sao Paulo, Brazil V. Koch * Children’s Hospital of Eastern Ontario, Ottawa, Canada J. Feber * Hospital for Sick Children,
Toronto, Canada E. Harvey * BC Children’s Hospital, Vancouver, Chile C. White * Hospital Guillermo Grant Benavente, Concepcion, Chile M. Valenzuela * Hospital Base, Osorno, Chile J. Villagra
* Hospital Luis Calvo Mackenna, Santiago, Chile F. Cano * Roberto del Rio Hospital, Santiago, Chile M. A. Contreras * Pontivicia Universidad Catolica de Chile, Santiago, Chile A. Vogel *
Hospital Dr. Gonzales Cortes, Santiago, Chile P. Zambrano * Hospital San Juan de Dios, Santiago, Chile P. Hevia * Department of Pediatric & Adolescent Medicine, Hong Kong, China M. C.
Chiu * Peking First Hospital, Beijing, China Jie Ding * Instituto del Rinon, Medellin, Colombia J. J. Vanegas * Baxter Servicio al Cliente Colombia, Medellin, Colombia L. M. Higuita * CHU
Nantes, Nantes, France G. Roussey * Armand Trousseau Hospital, Paris, France T. Ulinski * Hopital Necker_Enfants Malades, Paris, France S. Krid * Children’s Dialysis Center, Strasbourg,
France M. Fischbach * Hopital de Enfants, Bordeaux, France J. Harambat * Hopital Jeanne de Flandre, Lille, France Ch. Samaille * Children’s Hospital Essen, Essen, Germany R. Büscher *
University Medical Center, Hamburg, Germany J. Oh * Medical School, Hannover, Germany L. Pape * Kidney Center for Children and Adolescents, Jena, Germany U. John * KfH Kidney Center,
Marburg, Germany G. Klaus * Children’s University Hospital, TÜbingen, Germany H. Billing * A&P Kyriakou Children’s Hospital, Athens, Greece C. Stafanidis * Aristoteles University,
Thessaloniki, Greece F. Papachristou * All India Institute of Medical Sciences, New Delhi, India A. Bagga * Armed Forces Medical College, Pune, India M. Kanitkar * Institute of Child Health,
Kolkata, India R. Sinha * The Medicity, Gurgaon, India S. Sethi * G. Gaslini Institute, Genova, Italy E. Verrina * Pediatric Nephrology, Dialysis and Transplant Unit, Padova, Italy E. Vidal
* Department of Nefrologia-Urologia, Rome, Italy G. Leozappa * Soroka Medical Center, Beer-Sheva, Israel D. Landau * Seoul National University Children’s Hospital, Seoul, Korea I. S. Ha *
Samsung Medical Center, Seoul, South Korea K. H. Paik * Rafik Hari University Hospital, Beirut, Lebanon A. Bilal * Pediatric Clinic, Skopje, Macedonia E. Sahpazova * Kuala Lumpur Hospital,
Kuala Lumpur, Malaysia Y. N. Lim * Pediatric Hospital Medical Center SXXI, Cuahutemoc, Mexico L. Sanchez Barbosa * Academic Medical Center, Amsterdam, The Netherlands J. W. Groothoff *
Wilhelmina Children’s Hospital, Utrecht, The Netherlands Y. Konijenberg * Hospital Infantil de Nicaragua, Managua, Nicaragua Y. Silva * Royal Hospital, Muscat, Oman M. Al Ryami * Cayetano
Heredia Hospital, Lima, Peru R. Loza Munarriz * Public Pediatric Teaching Hospital, Warsaw, Poland B. Leszczynska * Dialysis Division for Children, Zabrze, Poland M. Szczepanska * St. Maria
Children’s Hospital, Iasi, Romania O. Brumariu * King Abdul Aziz University Hospital, Jeddah, Saudi Arabia J. Kari * University Children’s Hospital, Belgrade, Serbia D. Kruscic *
Shaw-NKF-NUH Children’s Kidney Center, Singapore, Singapore H. K. Yap * University Hospital Materno-Infantil Vall d’Hebron, Barcelona, Spain G. Ariceta * Hospital de Cruces, Baracaldo, Spain
M. Aguirre * Hospital Universitario Central de Asturias, Oviedo, Spain F. Santos * Karolinska University Hospital, Stokholm, Sweden B. Niwinska-Faryna * Cukrova University, Adana, Turkey A.
Bayazit * Hacettepe University, Ankara, Turkey C. A. S. Bakkaloglu * Gazi University, Ankara, Turkey S. Bakkaloglu * Department of Pediatric Nephrology, Capa-Istambul, Istanbul, Turkey I.
Bilge * Tepecik Children and Research Hospital, Izmir, Turkey O. Yavascan * Ege University Faculty of Medicine, Izmir-Bornova, Turkey S. Mir * Al Jalila Children’s Specialty Hospital, Dubai,
United Arab Emirates Eva Simkova * Children & Young People’s Kidney Unit, Notthingham, United Kingdom M. Christian * Children’s Healthcare Pediatric Dialysis Unit, Atlanta, United
States L. Greenbaum * Johns Hopkins Hospital, Baltimore, UK A. Neu * Children’s Hospital of Alabama, Birmingham, UK D. Askenazi * Driscoll Children’s Hospital, Corpus Christi, USA A.
Al-Akash * Texas Children’s Hospital, Houston, USA S. Swartz * University of Iowa Children’s Hospital, Iowa, USA P. Brophy * University of Minnesota Amplatz Children’s Hospital, Minneapolis,
USA M. Rheault * The Children’s Hospital of Philadelphia, Philadelphia, USA M. Pradhan AUTHOR NOTES * A COMPREHENSIVE LIST OF CONSORTIUM MEMBERS APPEARS AT THE END OF THE PAPER Authors *
Franz Schaefer View author publications You can also search for this author inPubMed Google Scholar * Laura Benner View author publications You can also search for this author inPubMed
Google Scholar * Dagmara Borzych-Dużałka View author publications You can also search for this author inPubMed Google Scholar * Joshua Zaritsky View author publications You can also search
for this author inPubMed Google Scholar * Hong Xu View author publications You can also search for this author inPubMed Google Scholar * Lesley Rees View author publications You can also
search for this author inPubMed Google Scholar * Zenaida L. Antonio View author publications You can also search for this author inPubMed Google Scholar * Erkin Serdaroglu View author
publications You can also search for this author inPubMed Google Scholar * Nakysa Hooman View author publications You can also search for this author inPubMed Google Scholar * Hiren Patel
View author publications You can also search for this author inPubMed Google Scholar * Lale Sever View author publications You can also search for this author inPubMed Google Scholar * Karel
Vondrak View author publications You can also search for this author inPubMed Google Scholar * Joseph Flynn View author publications You can also search for this author inPubMed Google
Scholar * Anabella Rébori View author publications You can also search for this author inPubMed Google Scholar * William Wong View author publications You can also search for this author
inPubMed Google Scholar * Tuula Hölttä View author publications You can also search for this author inPubMed Google Scholar * Zeynep Yuruk Yildirim View author publications You can also
search for this author inPubMed Google Scholar * Bruno Ranchin View author publications You can also search for this author inPubMed Google Scholar * Ryszard Grenda View author publications
You can also search for this author inPubMed Google Scholar * Sara Testa View author publications You can also search for this author inPubMed Google Scholar * Dorota Drożdz View author
publications You can also search for this author inPubMed Google Scholar * Attila J. Szabo View author publications You can also search for this author inPubMed Google Scholar * Loai Eid
View author publications You can also search for this author inPubMed Google Scholar * Biswanath Basu View author publications You can also search for this author inPubMed Google Scholar *
Renata Vitkevic View author publications You can also search for this author inPubMed Google Scholar * Cynthia Wong View author publications You can also search for this author inPubMed
Google Scholar * Stephen J. Pottoore View author publications You can also search for this author inPubMed Google Scholar * Dominik Müller View author publications You can also search for
this author inPubMed Google Scholar * Ruhan Dusunsel View author publications You can also search for this author inPubMed Google Scholar * Claudia Gonzalez Celedon View author publications
You can also search for this author inPubMed Google Scholar * Marc Fila View author publications You can also search for this author inPubMed Google Scholar * Lisa Sartz View author
publications You can also search for this author inPubMed Google Scholar * Anja Sander View author publications You can also search for this author inPubMed Google Scholar * Bradley A.
Warady View author publications You can also search for this author inPubMed Google Scholar CONSORTIA INTERNATIONAL PEDIATRIC PERITONEAL DIALYSIS NETWORK (IPPN) REGISTRY * M. Adragna * , P.
A. Coccia * , A. Suarez * , P. G. Valles * , R. Salim * , L. Alconcher * , K. Arbeiter * , K. van Hoeck * , V. Koch * , J. Feber * , E. Harvey * , C. White * , M. Valenzuela * , J. Villagra
* , F. Cano * , M. A. Contreras * , A. Vogel * , P. Zambrano * , P. Hevia * , M. C. Chiu * , Jie Ding * , J. J. Vanegas * , L. M. Higuita * , G. Roussey * , T. Ulinski * , S. Krid * , M.
Fischbach * , J. Harambat * , Ch. Samaille * , R. Büscher * , J. Oh * , L. Pape * , U. John * , G. Klaus * , H. Billing * , C. Stafanidis * , F. Papachristou * , A. Bagga * , M. Kanitkar * ,
R. Sinha * , S. Sethi * , E. Verrina * , E. Vidal * , G. Leozappa * , D. Landau * , I. S. Ha * , K. H. Paik * , A. Bilal * , E. Sahpazova * , Y. N. Lim * , L. Sanchez Barbosa * , J. W.
Groothoff * , Y. Konijenberg * , Y. Silva * , M. Al Ryami * , R. Loza Munarriz * , B. Leszczynska * , M. Szczepanska * , O. Brumariu * , J. Kari * , D. Kruscic * , H. K. Yap * , G. Ariceta *
, M. Aguirre * , F. Santos * , B. Niwinska-Faryna * , A. Bayazit * , C. A. S. Bakkaloglu * , S. Bakkaloglu * , I. Bilge * , O. Yavascan * , S. Mir * , Eva Simkova * , M. Christian * , L.
Greenbaum * , A. Neu * , D. Askenazi * , A. Al-Akash * , S. Swartz * , P. Brophy * , M. Rheault * & M. Pradhan CONTRIBUTIONS All co-authors have participated sufficiently in the work by
submitting patient related data, providing intellectual concept of data analysis and revising manuscript draft. Franz Schaefer, Dagmara Borzych-Dużałka, Joshua Zaritsky and Bradley A.
Warady directed the project and wrote the first draft of the manuscript. Bruno Ranchin, Hiren Patel, Stephen J. Pootore, Joseph Fynn, Karel Vondrack, Willian Wong, Ryszrd Grenda, Sara Testa,
Dorota Drożdz, Attila J. Szabo, Hong Xu, Zenaida Antonio, Erkin Sedaroglu, Zaynep Yuruk Yildrim, Loai Eid, Biswanath Basu, Renate VItkevic, Naksysa Hooman, Anabella Rebori, Lale Sever,
Ruhan Dusunsel, Lesley Rees, Tuula Holtta, Cynthia Wong, Dominik Muller, Marc Fila, Ruhan Dusunsel, Claudia Gonzalez Celedon, Lisa Sartz, and Eva Simkova were involved in the design of the
study, provided substantial original data, and reviewed and revised the manuscript in several iterations. Laura Benner and Anja Sander performed the statistical analysis of the project.
CORRESPONDING AUTHOR Correspondence to Franz Schaefer. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION DATASET1 RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Schaefer, F., Benner, L., Borzych-Dużałka, D.
_et al._ Global Variation of Nutritional Status in Children Undergoing Chronic Peritoneal Dialysis: A Longitudinal Study of the International Pediatric Peritoneal Dialysis Network. _Sci
Rep_ 9, 4886 (2019). https://doi.org/10.1038/s41598-018-36975-z Download citation * Received: 27 June 2018 * Accepted: 26 November 2018 * Published: 20 March 2019 * DOI:
https://doi.org/10.1038/s41598-018-36975-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative