Usp16 modulates Wnt signaling in primary tissues through Cdkn2a regulation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Regulation of the Wnt pathway in stem cells and primary tissues is still poorly understood. Here we report that Usp16, a negative regulator of Bmi1/PRC1 function, modulates the Wnt pathway
in mammary epithelia, primary human fibroblasts and MEFs, affecting their expansion and self-renewal potential. In mammary glands, reduced levels of Usp16 increase tissue responsiveness to
Wnt, resulting in upregulation of the downstream Wnt target Axin2, expansion of the basal compartment and increased in vitro and in vivo epithelial regeneration. Usp16 regulation of the Wnt
pathway in mouse and human tissues is at least in part mediated by activation of Cdkn2a, a regulator of senescence. At the molecular level, Usp16 affects Rspo-mediated phosphorylation of
LRP6. In Down’s Syndrome (DS), triplication of Usp16 dampens the activation of the Wnt pathway. Usp16 copy number normalization restores normal Wnt activation in Ts65Dn mice models. Genetic
upregulation of the Wnt pathway in Ts65Dn mice rescues the proliferation defect observed in mammary epithelial cells. All together, these findings link important stem cell regulators like
Bmi1/Usp16 and Cdkn2a to Wnt signaling, and have implications for designing therapies for conditions, like DS, aging or degenerative diseases, where the Wnt pathway is hampered.
Wnt signaling has a crucial role in the normal function of several stem cell types, including mammary, neural and embryonic stem cells1,2. Wnt is also very tightly regulated during aging,
and, in the majority of tissues, Wnt signaling declines during senescence3,4. Furthermore, the decline of Wnt signaling with age contributes to the pathogenesis of osteoporosis5, Alzheimer’s
disease, and Parkinson’s disease6. However, despite several decades of studies focusing on this pathway, its regulation in primary tissues, especially stem cells, remains only partially
understood.
Interestingly, the Wnt decline during aging parallels an increase in levels of p16Ink4a, a protein coded at the Cdkn2a locus7,8,9. The Cdkn2a locus is tightly regulated by USP16 and by Bmi1,
a member of the Polycomb Repressive Complex 1 (PRC1). USP16 is a deubiquitination enzyme that plays a crucial role in regulating tissue homeostasis and stem cell self-renewal and
expansion10. USP16 acts by detaching a monoubiquitin protein from histone H2A-K119, opposing the epigenetic repressive function of PRC111. Bmi1 is a member of the PRC1 complex and a crucial
regulator of stem cell self-renewal in several adult tissues, including the bone marrow and the brain12,13. Together, Bmi1/PRC1 and USP16 provide a robust and elaborate mechanism regulating
the epigenetic landscape of stem cells, and governing the equilibrium between self-renewal and senescence10.
Here we show an unexpected link between Wnt signaling and Bmi1/USP16, connecting two important signaling pathways acting on stem cells and primary tissues. We find that USP16 acts as a
negative regulator of Wnt signaling, and that its action is mediated at least in part by the Bmi1/USP16 regulated target Cdkn2a.
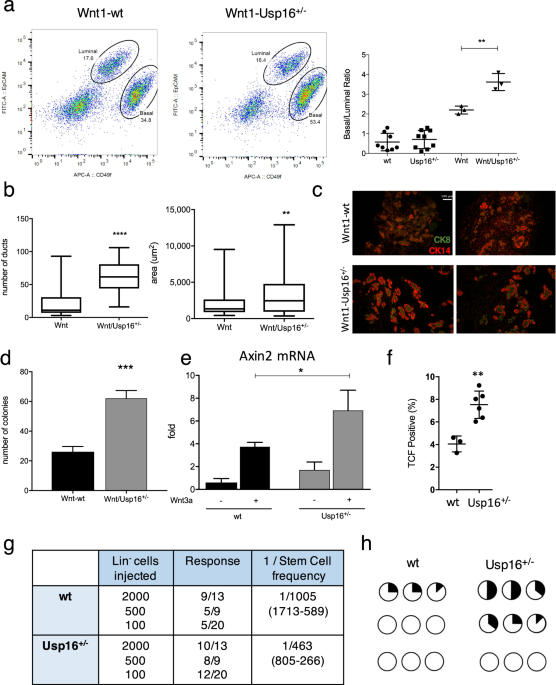
We first investigated mammary tissue, where the Wnt pathway plays a pivotal role in orchestrating proper mammary gland development and stem cell maintenance14. As we previously reported,
increased levels of Usp16 inhibit normal mammary gland stem cell function10. We therefore decided to investigate the effect of Usp16 dosage levels in MMTV-Wnt1 mouse mammary glands.
MMTV-Wnt1 transgenic mice present ectopic activation of Wnt1, causing a powerful mitogenic effect on the mammary epithelium. As a consequence, these animals develop extensive ductal
hyperplasia early in life15. The ratio between basal and luminal cells more than doubles in MMTV-Wnt1 animals compared to wild-type animals (Fig. 1a and Suppl. Fig. S1A), as observed by
Fluorescence Activated Cell Sorting (FACS) analyses. This increase in basal cells is in line with previous studies16, for example evidences from LRP5 mutant mice in which a decrease in the
activation of the Wnt/ß-catenin reduces the percentage of cells within the basal compartment of the mammary gland17. Preneoplastic mammary glands derived from MMTV-Wnt1 mice crossed with
Usp16+/− mice showed that the basal expansion was significantly higher in MMTV-Wnt1-Usp16+/− glands (almost 2-fold over the basal/luminal ratio observed in MMTV-Wnt animals) (P = 0.0073)
(Fig. 1a), suggesting Usp16 might have a role in limiting the activation of the Wnt pathway. Histological analyses also showed an increase in the number (P