Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Transmigration and activation of neutrophils in the lung reflect key steps in the progression of acute lung injury (ALI). It is known that hydrogen sulfide (H2S) can limit
neutrophil activation, but the respective mechanisms remain elusive. Here, we aimed to examine the underlying pathways in pulmonary inflammation. _In vivo_, C57BL/6N mice received the H2S
slow releasing compound GYY4137 prior to lipopolysaccharide (LPS) inhalation. LPS challenge led to pulmonary injury, inflammation, and neutrophil transmigration that were inhibited in
response to H2S pretreatment. Moreover, H2S reduced mRNA expression of macrophage inflammatory protein-2 (MIP-2) and its receptor in lung tissue, as well as the accumulation of MIP-2 and
interleukin-1β in the alveolar space. _In vitro_, GYY4137 did not exert toxic effects on Hoxb8 neutrophils, but prevented their transmigration through an endothelial barrier in the presence
and absence of MIP-2. In addition, the release of MIP-2 and reactive oxygen species from LPS-stimulated Hoxb8 neutrophils were directly inhibited by H2S. Taken together, we provide first
evidence that H2S limits lung neutrophil sequestration upon LPS challenge. As proposed underlying mechanisms, H2S prevents neutrophil transmigration through the inflamed endothelium and
directly inhibits pro-inflammatory as well as oxidative signalling in neutrophils. Subsequently, H2S pretreatment ameliorates LPS-induced ALI. SIMILAR CONTENT BEING VIEWED BY OTHERS RNF128
REGULATES NEUTROPHIL INFILTRATION AND MYELOPEROXIDASE FUNCTIONS TO PREVENT ACUTE LUNG INJURY Article Open access 21 June 2023 GPR84 REGULATES PULMONARY INFLAMMATION BY MODULATING NEUTROPHIL
FUNCTIONS Article Open access 04 April 2023 GSDMD PROMOTES NEUTROPHIL EXTRACELLULAR TRAPS VIA MTDNA-CGAS-STING PATHWAY DURING LUNG ISCHEMIA/REPERFUSION Article Open access 04 October 2023
INTRODUCTION Acute lung injury (ALI) due to pulmonary inflammation still represents a major problem in critical care medicine and is associated with high rates of morbidity and mortality1,2.
In this regard, postoperative pulmonary complications as underlying cause are of great importance3,4. By now, treatment or preventive options are limited, and new therapeutic strategies are
needed. ALI is characterised by alveolar barrier dysfunction, oedema formation, and accumulation of immune competent cells in the lungs. Especially the transmigration of neutrophils through
endothelial cells promote the acute phase of pulmonary inflammation5. Activated neutrophils are attracted by pro-inflammatory cytokines, e.g., macrophage inhibitory protein-2 (MIP-2), to
the side of the injury. Subsequently, neutrophils react with excessive pro-inflammatory cytokine release and oxidative burst, which in turn further aggravate the overall cellular
inflammatory response and lung tissue injury5. Conversely, a reduction of neutrophil transmigration has been described to limit lung injury6. In order to evaluate possible (pre)treatment
options, we and others have previously shown that inhalation of hydrogen sulfide (H2S) prevents neutrophil accumulation in models of ventilator-7,8,9 and lipopolysaccharide (LPS)-induced
lung injury10,11,12,13,14. Although recent data suggest that H2S may limit neutrophil activation and transmigration by downregulating pulmonary expression of chemoattractant molecules15 or
by reducing leukocyte rolling and adhesion15,16,17,18, it remains completely unknown how H2S interacts with the neutrophilic inflammatory response. The current study was designed to
thoroughly investigate the impact of H2S on neutrophil vitality, transmigration, pro-inflammatory response, and oxidative burst _in vivo_ and _in vitro_. We provide first evidence that H2S
prevents neutrophil activation, migration, cytokine release, and oxidative burst upon LPS challenge, subsequently ameliorating lung tissue inflammation and injury. RESULTS EFFECTS OF LPS AND
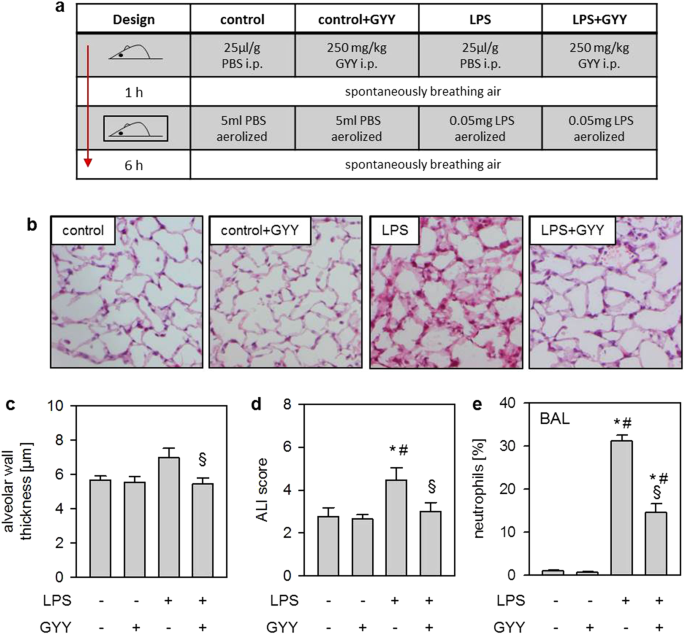
GYY4137 ON ACUTE LUNG INJURY AND NEUTROPHIL TRANSMIGRATION _IN VIVO_ First, we sought to induce acute lung injury in mice by nebulisation of LPS, either in the absence (LPS; study design
Fig. 1a) or in the presence of the H2S releasing compound GYY4137 (LPS + GYY). Compared to controls (control) or controls receiving GYY4137 (control + GYY), treatment with LPS alone resulted
in alveolar wall thickening after 6 h. In contrast, additional GYY4137 significantly decreased alveolar wall thickness (P = 0.0413; Fig. 1b,c). Similar results were obtained by determining
an overall ALI score (Fig. 1d). The percentage of neutrophils in bronchoalveolar lavage (BAL) fluid was negligible in control and control + GYY groups. In contrast, neutrophil counts were
elevated in the LPS group, while additional GYY4137 significantly reduced its number despite LPS treatment (LPS vs LPS + GYY P = 0.0088; Fig. 1e). These results indicate that GYY4137
mediates lung protection _in vivo_ by limiting transmigration of neutrophils. EFFECTS OF LPS AND GYY4137 ON INFLAMMATORY RESPONSE IN THE LUNG _IN VIVO_ Next, we analysed the expression of
the chemotactic cytokine MIP-2 and its receptor C-X-C motif-chemokine receptor 2 (CXCR2) in lung tissue. Both are known mediators of neutrophil transmigration5. In contrast to control and
control + GYY groups, LPS inhalation induced MIP-2 mRNA expression in lung tissue homogenates (LPS, Fig. 2a), an effect that was prevented by supplementary GYY4137 treatment (LPS + GYY, Fig.
2a). Analysis of CXCR2 mRNA expression yielded similar results, but failed statistical significance (Fig. 2b). In BAL fluid, the MIP-2 protein content remained minimal in both control
groups (control, control + GYY, Fig. 2c). While LPS inhalation clearly increased MIP-2 protein (Fig. 2c), the additional application of GYY4137 significantly reduced the amount of MIP-2 as
compared to LPS alone (P = 0.0413, Fig. 2c). Likewise, LPS inhalation increased the amount of interleukin-1β protein (IL-1β) in BAL fluid that was partially prevented in the presence of
GYY4137 (P = 0.0192; Fig. 2d). Interesting to note, GYY4137 application per se tended to decrease IL-1β readings as compared to controls (Fig. 2d). According to these findings, the H2S
releasing compound GYY4137 reduces neutrophil transmigration, most likely by limiting the accumulation of chemoattractant and pro-inflammatory cytokines in the lung. EFFECTS OF GYY4137 ON
HOXB8 NEUTROPHIL VITALITY AND MIGRATION THROUGH AN ENDOTHELIAL MONOLAYER _IN VITRO_ Different pulmonary cell type, e.g., endothelial or neutrophil cells, can produce MIP-2 and IL-1β upon LPS
stimulation5. Because the results from our _in vivo_ experiments would not allow to identify the specific cell type on which GYY4137 exerts its anti-inflammatory effects, we aimed to define
the impacts of GYY4137 on differentiated Hoxb8 neutrophils19 _in vitro_. We first investigated whether the inhibiting effect of GYY4137 on neutrophil accumulation might be a result of
potential toxicity. To address this issue, Hoxb8 neutrophils were subjected to increasing concentrations of GYY4137. After 24 h of incubation, the cell vitality assays showed no differences
between treated and untreated neutrophils (Fig. 3a). After excluding potential toxicity, we further analysed whether GYY4137 directly affects neutrophil transmigration through an endothelial
monolayer. As our results from co-culture experiments demonstrate (experimental design, Fig. 3b), MIP-2 led to an enhanced transmigration of neutrophils from the upper to the lower
compartment (Fig. 3c). In contrast, incubation of neutrophils with GYY4137 in the upper compartment significantly reduced neutrophil transmigration through the endothelial monolayer (Fig.
3c). These effects have been observed in the absence of the stimulating cytokine (control vs control + GYY P = 0.0030; Fig. 3c) and were more pronounced in the presence of MIP-2 (MIP-2 vs
MIP-2 + GYY P = 0.0002, Fig. 3c). EFFECTS OF LPS AND GYY4137 ON HOXB8 NEUTROPHIL CYTOKINE RELEASE AND OXIDATIVE BURST _IN VITRO_ Finally, we tested whether GYY4137 exerts direct
anti-inflammatory and/or anti-oxidative effects on Hoxb8 neutrophils by analysing cytokine accumulation and reactive oxygen species (ROS) formation. LPS incubation profoundly induced MIP-2
accumulation in Hoxb8 neutrophils. In contrast, supplemental GYY4137 significantly reduced MIP-2 liberation despite LPS treatment (P = 0.0010; Fig. 4a). The formation of ROS was clearly
induced by LPS compared to controls. Likewise, GYY4137 treatment in the presence of LPS completely prevented ROS production (P = 0.0130; Fig. 4b). The results from our _in vitro_ experiments
indicate that GYY4137 exerts direct inhibitory effects in neutrophil cells, thus preventing neutrophil migration, cytokine release, and ROS formation. DISCUSSION We and others have
previously shown that hydrogen sulfide prevents lung injury in models of LPS-induced ALI10,11,14. In these studies, protection was clearly associated with the reduction of neutrophil
accumulation in the lungs. Because in a series of injury models7,8,10,13,20, H2S-mediated inhibition of neutrophil influx appeared to be a major factor of its preventive effects, the
rationale of the present study was to examine how H2S interferes with the neutrophilic inflammatory response. In order to specifically address the above mentioned problem of postoperative
pulmonary complications as a source of ALI3,4, and to rule out potential preventive effects of H2S, we chose to apply the H2S releasing compound GYY4137 prior to the inflammatory insult. We
first induced ALI _in vivo_ by nebulisation of LPS21. Lung injury was characterised by enlarged alveolar walls and an elevated ALI score. These findings are in line with the results of other
rodent models, i.e., after intraperitoneal, intranasal, or intratracheal LPS application10,12,13,22,23. By contrast, prophylactic application of GYY4137, a water-soluble compound that
slowly releases H2S24, prevented all signs of lung injury despite LPS treatment. Our data support the findings of two related studies. Here, GYY4137 exerted lung protection in a mouse
endotoxemia model12,13. With regard to LPS-induced ALI, the observed effects of GYY4137 are comparable to those of H2S fast-releasing salts, i.e., sodium hydrosulfide25 or inhaled H2S
gas10,14, underlining the lung protective properties of H2S irrespective of the form of application. Although we chose a ‘prophylactic’ time point of GYY4137 application, it seems likely
that a more ‘therapeutic’ approach, i.e., administration after the injurious insult, can reduce lung inflammation and injury. First, GYY4137 showed anti-inflammatory effects in several
experimental models using a post-injury time points of application12,26. Second, we recently demonstrated the time dependency of application27. After setting the insult, an earlier H2S
inhalation resulted in a more protective effect. However, postponed application still reduced lung inflammation27. Mechanistically, it has been demonstrated in LPS and other models that
H2S-mediated lung protection is associated with the reduction of inflammatory processes. Amongst them, H2S significantly decreases the activity of NF-κB26,28,29,30,31,32,
cystathionine-β-synthetase and cystathionine-γ-lyase14,33, and particularly pro-inflammatory cytokine and neutrophil accumulation in the lungs7,8,9,10,12,13,20,34. Pulmonary neutrophil
activation and transmigration display key events in the development of ALI after bacterial challenge5. Here, we show that upon LPS inhalation a substantial fraction of neutrophils was
recruited into the alveolar space that was inhibited in the presence of GYY4137. These results are in line with previous work on endotoxemia in rats and mice demonstrating a decrease in lung
myeloperoxidase activity and neutrophil influx in response to GYY4137 application11,12,13. While the limitation of neutrophil function appears to be a general mechanism of H2S-mediated
protection in various lung injury models7,8,9,10,12,13, the exact mechanism remains elusive. We therefore investigated the effect of the H2S releasing compound GYY4137 on neutrophil reaction
upon LPS stimulation _in vivo_ and _in vitro_. MIP-2 represents a central chemotactic mediator that is released following LPS challenge in various pulmonary cell types, e.g., endothelial
cells35,36, epithelial cells37,38, or neutrophils5,10, and subsequently activates the neutrophil inflammatory response. In the current study, we found that LPS increased MIP-2 mRNA
expression in lung tissue homogenates and MIP-2 protein in the alveolar space. In contrast, GYY4137 application inhibited both MIP-2 mRNA expression and -protein release even in the presence
of LPS. Furthermore, GYY4137 tended to reduce mRNA expression of the corresponding receptor CXCR2 as compared to LPS alone in the same samples. Similar observations have been made by others
in a model of ventilator-induced ALI15, indicating that the chemotactic response of pulmonary cells may be suppressed in response to H2S administration. These results allow us to
hypothesise that decreased pulmonary chemoattractant signalling due to H2S treatment may lead to reduced neutrophilic transmigration as we observed _in vivo_. Because our results derived
from lung homogenates, we cannot differentiate between pulmonary cell types as effect sites of H2S. We therefore tested the cell specific effects of H2S on differentiated Hoxb8 neutrophils19
as well as in co-culture with endothelial HUVEC cells _in vitro_. In these experiments, we aimed to assess three potential mechanisms: * (1) H2S may induce toxic effects in neutrophils,
thus preventing neutrophil transmigration. After 24 h of incubation with the slow releasing H2S donor GYY4137 in concentrations ranging from 0.1 µM to 1 mM, we were not able to detect any
impact on cellular vitality in Hoxb8 neutrophils. To the best of our knowledge, no other study has yet tested the effect of GYY4137 on neutrophil vitality. The concentrations used in the
current study follow and even exceed GYY4137 concentrations used in comparable vitality studies in other cell lines39,40. It is interesting to note that GYY4137 seems to affect cell
viability after 3 to 5 days at the earliest but not up to 24 h39,40. In this context, incubation with H2S enhanced short-term survival of cultured human neutrophils after 24 h in a recent
study41. Taken together, we suggest that the observed reduction in neutrophil transmigration does not result from toxic side-effects of H2S. * (2) H2S may prevent transmigration of
neutrophils into the lungs. Upon chemoattractant signalling in the lung, neutrophils first pass the endothelial barrier and migrate to the site of inflammation5,21. As it has been
demonstrated in a model of small intestine ischemia-reperfusion injury, H2S can prevent leukocyte adhesion and rolling16,17,18. To address our above mentioned hypothesis, we established an
_in vitro_ neutrophil transmigration model using an endothelial monolayer (HUVEC cells) on a perforated insert (Fig. 3b) that was co-cultured with differentiated Hoxb8 neutrophils42. Adding
the chemoattractant cytokine MIP-2 to the bottom medium led to a substantially increased transmigration of Hoxb8 cells through the endothelial cell layer into the bottom medium as compared
to the control group. Adding GYY4137 to the top medium completely inhibited this process. Our observations can be explained in several ways: First, the permeability of the endothelial
barrier increased upon MIP-2 stimulation and decreased in response to H2S supplementation18,43,44, consequently reducing the capability of neutrophils to cross intercellular gaps. Second,
H2S limits the pro-inflammatory signalling in stimulated HUVEC cells34,45, thus attracting fewer neutrophils. This interpretation is strengthened by our _in vivo_ results in which GYY4137
inhibited MIP-2 mRNA and protein accumulation in lung tissue and BAL. While the latter two points might explain how H2S impacts neutrophil transmigration through the endothelium during
inflammation, the present migration experiment also suggests a direct effect of GYY4137 on neutrophils. As we observed, GYY4137 significantly reduced the number of spontaneously
transmigrating neutrophils even in the absence of MIP-2 stimulation. * (3) H2S may directly inhibit neutrophil pro-inflammatory signalling and oxidative burst. At the site of inflammation,
activated and migrated neutrophils liberate pro-inflammatory cytokines and ROS in order to enhance the inflammatory process5. Upon LPS stimulation, we found a substantial increase of MIP-2
protein in the medium of Hoxb8 cell cultures, a response that is comparable to other neutrophil cell lines46,47. In contrast, we provide first evidence that additional application of GYY4137
prevented MIP-2 release, indicating that H2S can suppress the inflammatory response in neutrophils. In the current study, we show that LPS stimulation directly induced ROS formation in
Hoxb8 neutrophils as previously reported in isolated human neutrophils48,49. In contrast, H2S releasing GYY4137 prevented ROS production even in the presence of LPS, strongly suggesting that
H2S profoundly inhibits the oxidative burst in neutrophils. In conclusion, the slow-releasing H2S compound GYY4137 prevents lung injury and neutrophil transmigration in a mouse model of
LPS-induced ALI. The inhibition of neutrophil transmigration by H2S appears to be a critical step for preventing lung injury. We show _in vivo_ and _in vitro_ that GYY4137 limits neutrophil
migration by reducing chemoattractant signalling in lung tissue and endothelial cells. Moreover, we demonstrate that H2S directly suppresses the pro-inflammatory response and the production
of ROS in neutrophils. These findings allow first insights into the cell specific effects of H2S and underline the beneficial potential of H2S releasing compounds in the prophylaxis of acute
lung injury. METHODS ANIMALS Animal experiments were performed in accordance with the guidelines of the local animal care commission (University of Freiburg, Freiburg, Germany) and in
conformance with the journals’ requirements for human and animal trials (ARRIVE Animals in Research: Reporting _In Vivo_ Experiments). The study was approved by the local government in
consultation with an ethics committee (Regierungspräsidium Freiburg, Referat 35, Fachgebiet Tierschutz und Tierhaltung, [email protected], Freiburg, Germany, permission No. G-12/73).
C57BL/6 N mice (n = 32, weighing 24.6 ± 0.2 g) were obtained from Charles River Laboratories (Sulzfeld, Germany). EXPERIMENTAL SETTING Preliminary experiments were conducted prior to the
study in order to establish the appropriate dose for LPS-induced moderate lung injury and to determine the effective dose of intraperitoneally (i.p.) applied GYY4137 (Supplementary Fig. S1).
Mice were randomly assigned into four experimental groups (n = 8/group; Fig. 1a). Group 1 (control): mice received 25 µl/g body weight phosphate buffered saline (PBS) i.p. and were exposed
to synthetic air for 1 h. Afterwards, mice were treated with 5 ml of aerosolised PBS and subjected to synthetic air for another 6 h. Group 2 (control + GYY): mice received 250 mg/kg body
weight GYY4137 i.p. (freshly dissolved in PBS, 10 mg/ml; Dichloromethane Complex24, Sigma, Taufkirchen, Germany) and were exposed to synthetic air for 1 h. Afterwards, mice were treated with
5 ml of aerosolised PBS and subjected to breathe synthetic air for another 6 h. Group 3 (LPS): mice received 25 µl/g body weight PBS i.p. and were exposed to synthetic air for 1 h.
Afterwards, mice were treated with 0.05 mg of aerosolised LPS (E.coli 055:B5, Sigma14,50; dissolved in 5 ml PBS) and subjected to breathe synthetic air for another 6 h. Group 4 (LPS + GYY):
mice received 250 mg/kg body weight GYY4137 i.p. (freshly dissolved in PBS) and were exposed to breathe synthetic air for 1 h. Afterwards, mice were treated with 0.05 mg of aerosolised LPS
(dissolved 5 ml PBS) and subjected to breathe synthetic air for another 6 h. Experiments were performed in a sealed plexiglas chamber with a constant air flow of 1.5 l/min. Mice had free
access to food and water. Nebulisation was performed in a custom-built cylindrical chamber (20 cm in length, 9 cm in diameter) connected to an air nebuliser (MicroAir; Omron Healthcare,
Vernon Hills, IL, USA), producing particles from 1–5 μm, as previously described21. Nebulisation of the 5 ml solutions was ceased after 14–16 min in all experiments. At the end of the
experiment (6 h after PBS or LPS nebulisation), mice were euthanised by an overdosed injection of ketamine (180 mg/kg, i.p.) and acepromazine (1.8 mg/kg, i.p.). Bronchoalveolar lavage fluid
and lung tissue for histological examination and semi-quantitative polymerase chain reaction (sq RT-PCR) analysis were gained as described previously10,51. HISTOLOGICAL EXAMINATION AND ALI
SCORE Cryosections of the left lung lobes and haematoxylin and eosin staining were performed and analysed in a blinded fashion as described previously10. Alveolar wall thickness, cellular
infiltration and haemorrhage were each rated from 0 (no injury) to 4 (maximal injury) for all individuals. Counts of each score were summed up, and the result was depicted as ALI score as
described previously10. BAL CYTOKINE MEASUREMENTS BAL aliquots were analysed using interleukin-1β and macrophage inflammatory protein-2 ELISA kit (R&D Systems GmbH, Wiesbaden, Germany)
according to the manufacturers’ instructions. RNA PREPARATION AND SEMI-QUANTITATIVE POLYMERASE CHAIN REACTION (SQRT-PCR) RNA from lung tissue samples was extracted and purified as previously
described51. cDNA samples were synthesised from equal amounts of RNA using random hexamer reverse primers and a TaqMan Reverse Transcription kit (Applied Biosystems Inc., Foster City, USA).
TaqMan PCR reactions were performed according to the manufacturers’ instructions. TaqMan Gene Expression Assays for CXCL2 (MIP-2; Mm00436450_m1), CXCR2 (Mm00438258_m1), and GAPDH (TaqMan
Rodent GAPDH Control Reagent) were purchased from Applied Biosystems. The comparative CT (ΔΔCT) method to evaluate the expression profiles of the analysed samples was used. CELL CULTURE
Human umbilical vein endothelial cells (HUVEC, Pelobiotech, Planegg, Germany) were cultured in complete endothelial cell growth medium (Promocell GmbH, Heidelberg, Germany) supplemented with
10% fetal calf serum (FBS, Gibco, Life Technologies GmbH, Darmstadt, Germany) and 1% penicillin-streptomycin (Promocell). Hematopoietic progenitor Hoxb8 neutrophils19 were a generous gift
from Prof. Häcker (Institute of Medical Microbiology and Hygiene, UMC, Freiburg, Germany). Cells were cultured in Opti-modified Eagle medium (Gibco) supplemented with 10% heat-inactivated
FBS (Gibco), 30 µM 2-mercaptoethanol (Thermo Fischer, Paisley, UK), 1 µM estradiol (Sigma) and 1% stem cell factor. Stem cell factor was harvested from chinese hamster ovary cells (a
generous gift from Prof. Häcker) as described earlier19. Prior to the experiments, progenitor Hoxb8 neutrophils were cultured for four days in differentiating medium (estradiol free
medium)19. All cells were grown and experiments were performed at standard growing conditions (37 °C, 5% CO2, sufficient humidity). All neutrophil assays were performed in the presence of
FBS. NEUTROPHIL VITALITY TESTING 106/3 ml differentiated neutrophils were seeded in 6-well plates. Prior to the onset of incubation, 300 µl of the cell suspension were removed, stained for
propidium iodide (PI, Thermo Fisher, Waltham, USA), and analysed by fluorescence-activated cell sorting (FACS, Invitrogen™ Attune™, Thermo Fisher). Remaining cells were incubated in the
absence (control) or presence of GYY4137 (GYY) for 24 h. Different concentrations of GYY4137 were used: 1 mM, 0.1 mM, 10 µM, and 1 µM. At the end of the experiment, cells were harvested,
stained for PI, and analysed by FACS. The relative amount of vital cells was calculated as the ratio between vital cell counts at the beginning and the end of the experiment. For subsequent
short-term experiments, a dose of 1 mM GYY4137 was chosen to achieve appropriate effects without toxic side-effects31,52,53. NEUTROPHIL TRANSMIGRATION ASSAY HUVEC were seeded onto cell
culture inserts (pore size 3.0 µm) and grown to confluence. Prior to experiments, cells were washed and the inserts were transferred to new 6-well companion plates. The lower compartment of
each well was filled with 3 ml of neutrophil cell culture medium. The upper compartments were filled with 1 ml of 5 × 106 differentiated neutrophils (experimental design, Fig. 3b). As
controls, the co-culture setup was performed in the absence (control) or presence of 1 mM GYY4137 (control + GYY). The latter was added to the upper compartment. For stimulation of
transmigration, MIP-2 (1.33 pg/ml) was supplemented to the lower compartment in the absence (MIP-2) or presence of 1 mM GYY4137 (MIP-2 + GYY). Inserts were removed from the companion plates
after 2 h of incubation. Cells from the lower and upper compartment were stained separately with PI (3.75 µM) and subsequently the live/dead cell ratio was analysed by FACS. NEUTROPHIL
CYTOKINE MEASUREMENT Differentiated neutrophils were seeded in 24-well plates. As controls, cells were incubated in the absence (control) or presence of 1 mM GYY4137 (control + GYY) for 4 h.
In addition, cells were incubated with 100 ng/ml LPS in the absence (LPS) or presence of 1 mM GYY4137 (LPS + GYY) for 4 h. Neutrophil cell culture supernatants were analysed using MIP-2
ELISA kit (R&D Systems GmbH) according to the manufacturers’ instructions. NEUTROPHIL DETECTION OF REACTIVE OXYGEN SPECIES Differentiated neutrophils were seeded in 24-well plates. As
controls, cells were incubated in the absence (control) or presence of 1 mM GYY4137 (control + GYY) for 4 h. In addition, cells were incubated with 100 ng/ml LPS in the absence (LPS) or
presence of 1 mM GYY 4137 (LPS + GYY) for 4 h. Subsequently, neutrophils were stained with 2′,7′-dichlorodihydrofluorescein diacetate (DHDHF-DA, Sigma) in order to detect reactive oxygen
species as previously described54. Fluorescence was measured with TECAN infinite 2000 (Thermo Fisher). STATISTICAL ANALYSIS _In vivo_ experiments were performed with n = 8 mice per group.
Power calculations were performed prior to the study in order to define group sizes. Cell culture experiments were performed from at least three subsequent cell passages with n = 3 per
group. Graphs represent means ± standard error of means (SEM) and were created with SigmaPlot 11.0 software (Systat Software Inc., Erkrath, Germany). In Figs 2a,b and 4b, data were depicted
as fold induction compared to untreated controls. Data were further analysed for normal variation prior to one way analysis of variance (ANOVA) followed by the Tukey’s post hoc test. P <
0.05 was considered significant. All calculations were performed with GraphPad Prism 7.01 (GraphPad Software, Inc., La Jolla, CA, USA). DATA AVAILABILITY All data generated or analysed
during this study are included in this published article (and its Supplementary Information file). REFERENCES * Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of
sepsis in the United States from 1979 through 2000. _N. Engl. J. Med._ 348, 1546–1554 (2003). Article Google Scholar * Wheeler, A. P. & Bernard, G. R. Acute lung injury and the acute
respiratory distress syndrome: a clinical review. _Lancet_ 369, 1553–1564 (2007). Article Google Scholar * Choudhuri, A. H., Chandra, S., Aggarwal, G. & Uppal, R. Predictors of
postoperative pulmonary complications after liver resection: Results from a tertiary care intensive care unit. _Indian J. Crit Care Med._ 18, 358–362 (2014). Article Google Scholar *
Kelkar, K. V. Post-operative pulmonary complications after non-cardiothoracic surgery. _Indian J. Anaesth._ 59, 599–605 (2015). Article Google Scholar * Grommes, J. & Soehnlein, O.
Contribution of neutrophils to acute lung injury. _Mol. Med._ 17, 293–307 (2011). Article CAS Google Scholar * Savov, J. D., Gavett, S. H., Brass, D. M., Costa, D. L. & Schwartz, D.
A. Neutrophils play a critical role in development of LPS-induced airway disease. _Am. J. Physiol Lung Cell Mol. Physiol_ 283, L952–L962 (2002). Article CAS Google Scholar * Faller, S.
_et al_. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. _Anesthesiology_ 113, 104–115 (2010). Article CAS Google Scholar * Spassov, S. _et al_. Genetic targets
of hydrogen sulfide in ventilator-induced lung injury–a microarray study. _PLoS. One._ 9, e102401 (2014). Article ADS Google Scholar * Spassov, S. _et al_. Hydrogen Sulfide Prevents
Formation of Reactive Oxygen Species through PI3K/Akt Signaling and Limits Ventilator-Induced Lung. _Injury._ 2017, 3715037 (2017). Google Scholar * Faller, S. _et al_. Inhaled hydrogen
sulfide protects against lipopolysaccharide-induced acute lung injury in mice. _Med. Gas Res._ 2, 26 (2012). Article CAS Google Scholar * Kaya-Yasar, Y., Karaman, Y., Bozkurt, T. E.,
Onder, S. C. & Sahin-Erdemli, I. Effects of intranasal treatment with slow (GYY4137) and rapid (NaHS) donors of hydrogen sulfide in lipopolysaccharide-induced airway inflammation in
mice. _Pulm. Pharmacol. Ther._ 45, 170–180 (2017). Article CAS Google Scholar * Li, L., Salto-Tellez, M., Tan, C. H., Whiteman, M. & Moore, P. K. GYY4137, a novel hydrogen
sulfide-releasing molecule, protects against endotoxic shock in the rat. _Free Radic. Biol. Med._ 47, 103–113 (2009). Article CAS Google Scholar * Zhang, H. X. _et al_. H2S Attenuates
LPS-Induced Acute Lung Injury by Reducing Oxidative/Nitrative Stress and Inflammation. _Cell Physiol Biochem._ 40, 1603–1612 (2016). Article CAS Google Scholar * Zimmermann, K. K. _et
al_. Hydrogen Sulfide Exerts Anti-oxidative and Anti-inflammatory Effects in Acute Lung Injury. _Inflammation_ 41, 249–259 (2018). Article CAS Google Scholar * Francis, R. C., Vaporidi,
K., Bloch, K. D., Ichinose, F. & Zapol, W. M. Protective and Detrimental Effects of Sodium Sulfide and Hydrogen Sulfide in Murine Ventilator-induced Lung Injury. _Anesthesiology_ 115,
1012–1021 (2011). Article CAS Google Scholar * Zuidema, M. Y. _et al_. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of
BK channels. _Am. J. Physiol Heart Circ. Physiol_ 299, H1554–H1567 (2010). Article CAS Google Scholar * Zuidema, M. Y., Peyton, K. J., Fay, W. P., Durante, W. & Korthuis, R. J.
Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of heme oxygenase-1. _Am. J. Physiol Heart Circ. Physiol_ 301, H888–H894
(2011). Article CAS Google Scholar * Zuidema, M. Y. & Korthuis, R. J. Intravital microscopic methods to evaluate anti-inflammatory effects and signaling mechanisms evoked by hydrogen
sulfide. _Methods Enzymol._ 555, 93–125 (2015). Article CAS Google Scholar * Wang, G. G. _et al_. Quantitative production of macrophages or neutrophils _ex vivo_ using conditional Hoxb8.
_Nat. Methods_ 3, 287–293 (2006). Article CAS Google Scholar * Tokuda, K. _et al_. Inhaled hydrogen sulfide prevents endotoxin-induced systemic inflammation and improves survival by
altering sulfide metabolism in mice. _Antioxid. Redox. Signal._ 17, 11–21 (2012). Article CAS Google Scholar * Reutershan, J., Basit, A., Galkina, E. V. & Ley, K. Sequential
recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. _Am. J. Physiol Lung Cell Mol. Physiol_ 289, L807–L815 (2005). Article CAS Google
Scholar * Hirano, S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. _Am. J. Physiol_ 270, L836–L845 (1996). CAS PubMed Google
Scholar * Yoshida, T. _et al_. Relationship between neutrophil influx and oxidative stress in alveolar space in lipopolysaccharide-induced lung injury. _Respir. Physiol Neurobiol._ 191,
75–83 (2014). Article CAS Google Scholar * Li, L. _et al_. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of
hydrogen sulfide. _Circulation_ 117, 2351–2360 (2008). Article CAS Google Scholar * Du, Q. _et al_. _In vivo_ study of the effects of exogenous hydrogen sulfide on lung mitochondria in
acute lung injury in rats. _BMC. Anesthesiol._ 14, 117 (2014). Article Google Scholar * Li, L. _et al_. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model
of acute joint inflammation and in human cartilage cells. _J. Cell Mol. Med._ 17, 365–376 (2013). Article ADS CAS Google Scholar * Faller, S. _et al_. Pre- and posttreatment with
hydrogen sulfide prevents ventilator-induced lung injury by limiting inflammation and oxidation. _PLoS. One._ 12, e0176649 (2017). Article Google Scholar * Benedetti, F. _et al_.
Anti-inflammatory effects of H2S during acute bacterial infection: a review. _J. Transl. Med._ 15, 100 (2017). Article Google Scholar * Bourque, C. _et al_. H2S protects
lipopolysaccharide-induced inflammation by blocking NFkappaB transactivation in endothelial cells. _Toxicol. Appl. Pharmacol._ 338, 20–29 (2018). Article CAS Google Scholar * Li, H. D.
_et al_. Treatment with exogenous hydrogen sulfide attenuates hyperoxia-induced acute lung injury in mice. _Eur. J. Appl. Physiol_ 113, 1555–1563 (2013). Article ADS CAS Google Scholar *
Lohninger, L. _et al_. Hydrogen sulphide induces HIF-1alpha and Nrf2 in THP-1 macrophages. _Biochimie_ 112, 187–195 (2015). Article CAS Google Scholar * Luo, Z. L. _et al_. The Role of
Exogenous Hydrogen Sulfide in Free Fatty Acids Induced Inflammation in Macrophages. _Cell Physiol Biochem._ 42, 1635–1644 (2017). Article CAS Google Scholar * Wagner, F. _et al_.
Cardiopulmonary, Histologic, and Inflammatory Effects of Intravenous Na2S After Blunt Chest Trauma-Induced Lung Contusion in Mice. _J. Trauma_ 71, 1659–1667 (2011). CAS PubMed Google
Scholar * Faller, S. _et al_. Hydrogen sulfide prevents hyperoxia-induced lung injury by downregulating reactive oxygen species formation and angiopoietin-2 release. _Curr. Pharm. Des_ 19,
2715–2721 (2013). Article CAS Google Scholar * Driscoll, K. E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. _Exp. Lung Res._ 20, 473–490 (1994). Article
CAS Google Scholar * Laudes, I. J. _et al_. Expression and function of C5a receptor in mouse microvascular endothelial cells. _J. Immunol._ 169, 5962–5970 (2002). Article CAS Google
Scholar * Chuang, K. H. _et al_. Attenuation of LPS-induced lung inflammation by glucosamine in rats. _Am. J. Respir. Cell Mol. Biol._ 49, 1110–1119 (2013). Article CAS Google Scholar *
Hecker, M. _et al_. PPAR-alpha activation reduced LPS-induced inflammation in alveolar epithelial cells. _Exp. Lung Res._ 41, 393–403 (2015). Article Google Scholar * Lee, Z. W. _et al_.
The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects _in vitro_ and _in vivo_. _PLoS. One._ 6, e21077 (2011). Article ADS CAS Google Scholar * Wei, W.
B., Hu, X., Zhuang, X. D., Liao, L. Z. & Li, W. D. GYY4137, a novel hydrogen sulfide-releasing molecule, likely protects against high glucose-induced cytotoxicity by activation of the
AMPK/mTOR signal pathway in H9c2 cells. _Mol. Cell Biochem._ 389, 249–256 (2014). Article CAS Google Scholar * Rinaldi, L. _et al_. Hydrogen sulfide prevents apoptosis of human PMN via
inhibition of p38 and caspase 3. _Lab Invest_ 86, 391–397 (2006). Article CAS Google Scholar * Schnyder-Candrian, S. _et al_. Neutrophil Inhibitory Factor Selectively Inhibits the
Endothelium-Driven Transmigration of Eosinophils _In Vitro_ and Airway Eosinophilia in OVA-Induced Allergic Lung Inflammation. _J. Allergy (Cairo.)_ 2012, 245909 (2012). Google Scholar *
Yuan, S. _et al_. Hydrogen sulfide metabolism regulates endothelial solute barrier function. _Redox. Biol._ 9, 157–166 (2016). Article CAS Google Scholar * Zhang, L. J., Tao, B. B., Wang,
M. J., Jin, H. M. & Zhu, Y. C. PI3K p110alpha isoform-dependent Rho GTPase Rac1 activation mediates H2S-promoted endothelial cell migration via actin cytoskeleton reorganization. _PLoS.
One._ 7, e44590 (2012). Article ADS CAS Google Scholar * Monti, M., Terzuoli, E., Ziche, M. & Morbidelli, L. H2S dependent and independent anti-inflammatory activity of zofenoprilat
in cells of the vascular wall. _Pharmacol. Res._ 113, 426–437 (2016). Article CAS Google Scholar * Kukulski, F. _et al_. The P2 receptor antagonist PPADS abrogates LPS-induced neutrophil
migration in the murine air pouch via inhibition of MIP-2 and KC production. _Mol. Immunol._ 47, 833–839 (2010). Article CAS Google Scholar * Kukulski, F. _et al_. NTPDase1 controls IL-8
production by human neutrophils. _J. Immunol._ 187, 644–653 (2011). Article CAS Google Scholar * Martire-Greco, D. _et al_. Interleukin-10 controls human peripheral PMN activation
triggered by lipopolysaccharide. _Cytokine_ 62, 426–432 (2013). Article CAS Google Scholar * Ren, X. _et al_. Anesthetic agent propofol inhibits myeloid differentiation factor
88-dependent and independent signaling and mitigates lipopolysaccharide-mediated reactive oxygen species production in human neutrophils _in vitro_. _Eur. J. Pharmacol._ 744, 164–172 (2014).
Article CAS Google Scholar * Weifeng, Y. _et al_. Inhibition of Acute Lung Injury by TNFR-Fc through Regulation of an Inflammation-Oxidative Stress Pathway. _PLoS. One._ 11, e0151672
(2016). Article Google Scholar * Strosing, K. M. _et al_. Inhaled Anesthetics Exert Different Protective Properties in a Mouse Model of Ventilator-Induced Lung Injury. _Anesth. Analg_
(2016). * Fitzgerald, R. _et al_. H2S relaxes isolated human airway smooth muscle cells via the sarcolemmal K(ATP) channel. _Biochem. Biophys. Res. Commun._ 446, 393–398 (2014). Article CAS
Google Scholar * Whiteman, M. _et al_. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. _Antioxid. Redox. Signal._
12, 1147–1154 (2010). Article CAS Google Scholar * Rosenkranz, A. R. _et al_. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. _J.
Immunol. Methods_ 156, 39–45 (1992). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Prof. Häcker (Institute of Medical Microbiology and Hygiene, UMC,
Freiburg, Germany) for sharing hematopoietic progenitor Hoxb8 neutrophils, chinese hamster ovary cells, and expertise on these cells. The article processing charge was funded by the German
Research Foundation (DFG) and the University of Freiburg in the funding programme Open Access Publishing. AUTHOR INFORMATION Author notes * Simone Faller and Florian Hausler contributed
equally. AUTHORS AND AFFILIATIONS * Department of Anesthesiology and Critical Care Medicine, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg,
Germany Simone Faller, Florian Hausler, Andreas Goeft, Marc-Nicolas André von Itter, Veronica Gyllenram, Alexander Hoetzel & Sashko G. Spassov Authors * Simone Faller View author
publications You can also search for this author inPubMed Google Scholar * Florian Hausler View author publications You can also search for this author inPubMed Google Scholar * Andreas
Goeft View author publications You can also search for this author inPubMed Google Scholar * Marc-Nicolas André von Itter View author publications You can also search for this author
inPubMed Google Scholar * Veronica Gyllenram View author publications You can also search for this author inPubMed Google Scholar * Alexander Hoetzel View author publications You can also
search for this author inPubMed Google Scholar * Sashko G. Spassov View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.F., A.H. and S.G.S.
developed the concept and designed the study; F.H., A.G., M.-N.A.I., V.G. and S.G.S. performed the experiments; S.F., F.H. and S.G.S. analysed the data; S.F., F.H., A.G., M.-N.A.I., V.G.,
A.H. and S.G.S. interpreted the results of the experiments. S.F. and S.G.S. prepared the figures; S.F., A.H. and S.G.S. drafted, edited, and revised the manuscript. All authors approved the
final version of the manuscript. CORRESPONDING AUTHOR Correspondence to Simone Faller. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL
SUPPLEMENTARY FIGURE S1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Faller, S., Hausler, F., Goeft, A. _et al._ Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung
injury. _Sci Rep_ 8, 14676 (2018). https://doi.org/10.1038/s41598-018-33101-x Download citation * Received: 01 February 2018 * Accepted: 20 September 2018 * Published: 02 October 2018 * DOI:
https://doi.org/10.1038/s41598-018-33101-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Neutrophil Transmigration * C-X-C Motif Chemokine
Receptor 4 (CXCR2) * Pulmonary Cell Types * Neutrophilic Inflammatory Response * Receptor CXCR2