Performance boost for primary magnesium cells using iron complexing agents as electrolyte additives
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Aqueous Mg battery technology holds significant appeal, owing to the availability of raw materials, high power densities and the possibility of fast mechanical recharge. However, Mg
batteries have so far been prone to decreased capacity due to self-corrosion of the anodes from the electrochemical redeposition of impurities, such as Fe, which results in parasitic
cathodically active sites on the discharging anode. This work demonstrates that by adding Fe3+-complexing agents like Tiron or salicylate to the aqueous electrolyte of an Mg battery, it was
possible to prevent the redeposition of Fe impurities and subsequent self-corrosion of the anode surface, thereby boosting battery performance. To prevent detrimental fouling of anode
surface by Mg(OH)2, employed Fe3+-complexing agents must also form soluble complexes with Mg2+ of moderate stability. The interplay of these requirements predetermines the improvement of
operating voltage and utilization efficiency. SIMILAR CONTENT BEING VIEWED BY OTHERS IN-SITU ELECTROCHEMICAL ACTIVATION ACCELERATES THE MAGNESIUM-ION STORAGE Article Open access 03 February
2025 HIGH-CAPACITY, FAST-CHARGING AND LONG-LIFE MAGNESIUM/BLACK PHOSPHOROUS COMPOSITE NEGATIVE ELECTRODE FOR NON-AQUEOUS MAGNESIUM BATTERY Article Open access 07 October 2024 HIGH-POWER MG
BATTERIES ENABLED BY HETEROGENEOUS ENOLIZATION REDOX CHEMISTRY AND WEAKLY COORDINATING ELECTROLYTES Article 30 November 2020 INTRODUCTION Aqueous Mg-air batteries possess numerous appealing
qualities for energy storage, including high volumetric capacities of metallic Mg anodes (3832 mA h cm–3, vs. 2061 mA h cm–3 for Li)1. Moreover, they use raw materials that are low in cost
and relatively environmentally benign2,3,4,5,6 - indeed, such batteries for the first time can efficiently work even with ubiquitous electrolytes such as seawater7. Although aqueous Mg
batteries are not electrochemically rechargeable, the option for fast mechanical recharging8 allows this technology to have numerous applications. For example, pilot projects for powering
cars, have been accomplished at the Korea Institute of Technology in Seoul9. But why they are not available in large-scale on the market today? It is interesting that already in 1943
water-activated silver chloride/Mg-battery was commercially accessible10 however, it felt out of favour due to its low efficiency compared with nickel-metal hybrid and lithium batteries. And
even 75 years later, a breakthrough in working efficiency for Mg primary systems has yet to be achieved under real-life conditions, regardless of whether the cathode is air or silver
chloride. The novel concept introduced here might be the key. In addition to obtaining suitable anode11 and cathode materials12,13, the electrolyte itself is a challenging component of any
type of Mg battery13,14. So far economically attractive aqueous electrolytes cause problems related to the self-corrosion of Mg anodes8. First, the electrochemical potential of Mg is highly
negative, and lies lower than the electrochemical stability window of water, thus causing its reduction and self-corrosion of the Mg anode. In contrast, the kinetics of water reduction on a
pure Mg surface covered with an oxide film are rather slow, thus resulting in a lower extent of self-corrosion. Second, Mg is also prone to corrosion when accompanied by noble impurities
such as Fe, Cu or Ni15. Fe-rich particles, present in commercial magnesium, are particularly critical, because they allow for high exchange current densities in the hydrogen evolution
reaction (HER) and cause highly localized microgalvanically induced corrosion of Mg15,16, thereby triggering the growth of corrosion products on the surface of anodes that block the
electrodes17. They consist of a very thin layer of MgO directly at the metallic interface, gradated porous hydroxide on top and partially carbonates. Latter can be a mixture of MgCO3 xH2O,
or mixtures with - (OH)2 depending on pH, solubility product constant and concentration of carbonyl groups. The self-corrosion of Mg anodes through these two phenomena leads to three main
disadvantages: a decrease in utilization efficiency18,19, alternating /unstable dissolution of the anode and a low voltage caused by an IR drop across the layer of corrosion products, which
is far away from theoretical Mg-air cell voltage of 3.1 V (1) $$Mg+0.5{O}_{2}+{H}_{2}O\,\to Mg{(OH)}_{2}\,3.1V$$ (1) Occurring theoretical limits to the anode potential in realistic
scenarios due to the mentioned effects have been already discussed by Chen _et al_.17. However, a strategy to reach these limits in-service conditions has hitherto been lacking. Several
comparative studies aimed at finding effective corrosion inhibitors for Mg alloys have been performed20,21,22. However, little progress has been made in identifying optimal systems for
Mg-air batteries. One of the reported approaches is based on the use of nitrate-based electrolytes instead of chloride-containing counterparts23,24. The non-ionic surfactant decyl glucoside
has been recently shown to improve Mg-air battery performance by inhibiting anode self-corrosion25. Recent work by Höche _et al_.26 has proposed an Fe-redeposition mechanism of Mg
self-corrosion, which triggers a self-propagating process leading to strong microgalvanic corrosion and alkalinisation of the electrolyte and causing precipitation of Mg hydroxides on the
metal surface. On the basis of this hypothesis, a new concept of Mg corrosion inhibition based on iron chelators has been reported27. Using the chelator concept, we propose a non-trivial
solution for controlling self-corrosion during the discharge of Mg anodes. We found that organic additives with dual functionality as strong Fe(III)- and mild Mg(II)-complexing agents
significantly improved the performance of aqueous Mg-air batteries in terms of operating voltage, utilization efficiency and voltage stability. The working mechanism of the chelator
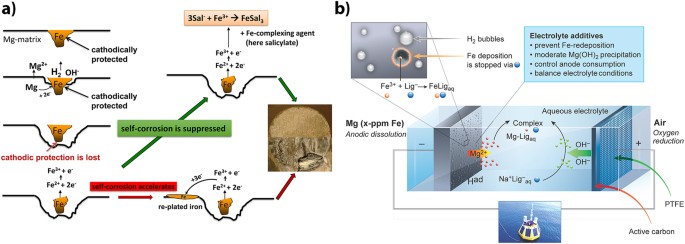
additives (ligands), and recently introduced for being effective for Mg corrosion inhibition27, is illustrated in Fig. 1a. The strong cathodic reaction of water reduction on noble Fe at the
corrosion potential of Mg induces anodic dissolution of Mg around the Fe-rich particles. After trenching of Fe is completed, cathodic protection is lost, and Fe undergoes rapid oxidation to
form Fe2+/Fe3+ ions. These cations can be reduced back and plated onto the Mg surface, forming nanosized patches of pure iron. However, if the concentration of free Fe ions is greatly
decreased by complexation with an organic ligand, self-corrosion of the anode can be inhibited, because the area of cathodic sites is either not growing or decreasing. Moreover, for
effective chelating, the ligands and respective complexes with Fe must be electrochemically stable at the open-circuit potential of the Mg anode and at the potential of the discharging
anode. The importance of this criteria was recently discussed by Hawthorne _et al_.28. For battery applications (Fig. 1b), recent concept of corrosion inhibition27 must be further elaborated
to account for the possible detrimental formation of Mg-containing precipitates. According to preliminary discharge tests in Half-cells29 and given the corrosion inhibition properties
respectively the stability constants for complexes with Mg2+ and Fe3+, the following seven compounds were selected for testing: catechol-3,5-disulfonic acid disodium salt (Tiron),
ethylenediaminetetraacetic acid dipotassium salt (K2-EDTA), and soldium salts of salicylate, glycolate, oxalate, nitrilotriacetate (NTA) and dodecylbenzenesulfonate (DBS). RESULTS AND
DISCUSSION PRE-TESTING OF ADDITIVES – HYDROGEN EVOLUTION AS PERFORMANCE INDICATOR The first parameter to be considered when evaluating the efficiency of electrolyte additives is the effect
of the additives on the self-corrosion kinetics, because this effect directly correlates with the utilization efficiency, and influences the battery discharge voltage. One of the approaches
to measuring the kinetics of Mg anode self-corrosion is based on the measurement of the evolution of cathodic hydrogen during immersion in a relevant electrolyte, because the extent of the
HER directly correlates with the amount of dissolved Mg. Figure 2 shows the kinetics of hydrogen evolution measured for Mg self-corrosion at the open-circuit potential in the presence of
either 0.5% NaCl without additives (reference) or NaCl with selected additives (0.05 M). The reference sample showed substantial HER that increased in rate over time, as is typical for a
self-propagating process controlled by impurity redeposition. Additionally, in line with recent studies26,30,31,32, the sample was heavily corroded after 24 h of operation and was covered by
Mg(OH)2 precipitates. This observation indicates that the Mg anode without adequate additive within the aqueous electrolyte is subject to strong self-corrosion even when the battery is in
open-circuit conditions. Initially (0–6 h), in the pure aqueous NaCl electrolyte, a low HER rate was observed followed by a steep increase of kinetics, because of the intensified cathodic
reaction resulting from the increasing area of active Fe. In electrolytes containing Tiron, NTA or K2-EDTA, initial HER were even higher, in the order Tiron >NTA ≥ K2-EDTA. However, the
anode surfaces did not suffer from Mg(OH)2 precipitation like shown in the top-right of Fig. 2. Presumably, the measured enhancement of hydrogen evolution was related to the formation of
highly soluble Mg complexes that shift the equilibrium toward Mg dissolution. The additives forced the removal of Mg2+ from the anode-electrolyte interface, thus leading to the formation of
a fresh reactive surface, the exposure of new impurity inclusions, and the suppression of passive layer formation. Notably, although the size of all the anode samples was virtually the same
at the beginning, the sample immersed in Tiron-containing electrolyte was consumed to a much greater extent than the one exposed to the NaCl electrolyte without additive (Fig. 2). The
changing slope of the curves (Tiron, NTA, K2-EDTA) relates to the additive concentration decrease and pH increase27. For solutions containing oxalate, glycolate, DBS or salicylate, the
dissolution rate and HER remained low throughout the 24-hour period of data collection. Although the HER measurements in Fig. 2 were similar for these additives; the causes of such behaviour
may be different. In the case of salicylate, this result can be explained by the stable [FeIII(salicylate)3] complex forming at high pH33, after a certain immersion time. Likewise, an
alkaline pH is responsible for the change in dissolution kinetics in the case of DBS, but it causes the formation of Mg-DBS precipitates20 that are stable at high pH. Similar precipitation
was observed after immersion in oxalate. In both cases, the precipitates blocked the anode surface (not displayed) and, as described below, decreased the battery performance in terms of
discharge voltage due to an IR-drop across the precipitated layer of products. Mg dissolution rate in the presence of glycolate became stable at medium level of HER (constant slope after
approx. three hours), probably because of a weaker Fe-chelating ability of the ligand. All additives (except for DBS) formed complexes with both Fe3+ and Mg2+ ions, with varying stability
constants _K_i, (Table 1). Salicylate complexes of Fe and Mg (log _K_1MgII = 4.7)34 were stable even at alkaline pH35,36. Glycolate formed weak complexes with Mg (log _K_1MgII = 0.92)34,37
and Fe-ions. EDTA formed highly stable Fe38 and Mg chelates (log _K_1MgII = 8.64)39. Oxalate40, similarly to DBS20, formed low-solubility Mg complexes that might have blocked the surface
causing observed ohmic drop in Fig. 2, whereas NTA41 and Tiron42 complexes with Mg (both formed highly stable Fe-complexes) were soluble in aqueous electrolytes, thus representing a possible
benefit. It should be noted that there is no general law directly linking dissolubility of Mg chelates with pH value. However, at certain pH Mg-ligand bonds can be broken an lead to
precipitation of Mg(OH)2 from the complex. APPLICATION OF ADDITIVES IN TEST CELLS - DISCHARGE PERFORMANCE: HALF-CELL In the next step, the proposed concept was tested in a Half-cell
configuration using Pt counter electrode ensuring reliable and comparable test conditions for the anode discharge. Constant-current discharge curves for the selected additives are shown in
Fig. 3a. The reference sample discharged in 0.5% NaCl at approximately −1.47 VAg/AgCl and showed a heavily attacked surface with localized corrosion and hydroxide precipitation (bottom-right
Fig. 3b. Addition of oxalate and glycolate showed more negative discharge potentials vs. NaCl 0.5% reference at the beginning of the experiment, a result relating to the Fe complexing
properties and the shift of the equilibrium at the anode surface towards Mg. However, in both cases the potential became more positive over time, and was accompanied by the formation of
insoluble products on the anode surface (Fig. 3b), probably because of the substantially lower stability constants of the Fe3+ and Mg2+ complexes (Table 1). After nine hours, DBS showed a
more positive potential shift than the reference potential, owing to strong adsorption on the Mg surface followed by the precipitation of Mg-DBS and the related IR-drop through this formed
layer (Fig. 3b). After the discharge tests in electrolytes containing salicylate and K2-EDTA, a moderate shift of the electrode potential was observed to persist at more than 110 mV more
negative than that of the anode discharged in the pure aqueous NaCl electrolyte for 24 h. Figure 3b (bottom left) shows that no corrosion products were visible on the sample surfaces
discharged in the presence of Tiron, NTA, K2-EDTA and salicylate. These chelating agents formed the strongest complexes with Mg2+ (Table 1) and correspondingly showed extended but relatively
uniform anode consumption. The samples discharged in the presence of pure aqueous NaCl or NaCl with oxalate, glycolate or DBS salts (Fig. 3b), bottom-right), however, demonstrated minor
anode consumption (depth profiles are masked by precipitated corrosion products). Despite the anode consumption the surfaces of the Mg anodes were brightly shining after the discharge tests
and were found to have granular microstructures in the cases of adding NTA Fig. 3b and Tiron (shown in Figs 2 and 3b, which also demonstrated the most negative discharge potentials. However,
these more negative potentials (high operating voltages) were accompanied by high anode consumption and low utilization efficiency (Table 1), as assessed by weight loss measurements after
the corrosion products were removed. The rate of anode consumption and related weight loss was proportional to the stability of the Mg complex with the additive ligands (Table 1), whereas
the cell voltage correlated with the Fe complex stability. The measured values of utilization efficiency (η = theoretical weight loss/measured weight loss) are lower than previously reported
values18,19. This difference is related to the significantly lower chloride content43 used in the tests and also to the high amount of impurities, thus making the used anodes much more
prone to self-corrosion than alloys used elsewhere18,19. Remarkable, that utilization efficiency was more than twice higher in salicylate containing electrolyte compared to the reference. A
comparison of the weight loss data and the discharge potentials revealed two additive selection strategies based on the specific application: a longer discharge time (durability) could be
obtained by using either K2-EDTA or salicylate if mild operating voltages are sufficient (110–140 mV above reference) or higher voltage (additionally 210–280 mV) could be achieved at the
expense of faster anode consumption by using either NTA or Tiron. These tunable properties enable the possibility of tailored discharge. The utilization efficiency for the most balanced
additive salicylate (compromise voltage/durability), was in the range of 30%, a value twice as high as that of the NaCl electrolyte with no additives. Along with a 140 mV higher operating
voltage than using the pure aqueous NaCl electrolyte, this result represents a considerable improvement. For both strategies, represented here by salicylate and Tiron respectively, the
performance boost was complementary monitored via specific energy measurements as shown in Fig. 3c. The results show a current dependency which originates from the contribution of
self-corrosion related current. At low discharge currents it is stronger and the working mechanism of salicylate takes control. On the contrary when the current is increased, Tiron, which
stimulates dissolution of Mg, triggers the performance. It is assumed that the strength of the enhancement thereby strongly relates to the surface constitution, its polarization properties
and the occurring IR drop (film and/or electrolyte). APPLICATION OF ADDITIVES IN TEST CELLS - DISCHARGE PERFORMANCE: FULL-CELL (MG-AIR) Half-cell tests have demonstrated that addition of
complexants can significantly affect kinetics of discharge and self-corrosion of Mg-based anodes. However, such tests exclude the effect of cathode material (here carbon fabrics) and its
kinetics (here oxygen reduction) on the cell and anode discharge. Thus, only Full-cell tests can validate the additive effect in end-use batteries. Respective Full-cell discharge curves of
the Mg-air battery in the electrolytes with different complexing agent additives are shown in Fig. 4. Despite different impurity grade of anode material the curves of most additives follow
the trend obtained by Half-cell tests. Good performance in terms of battery voltage for cells containing NTA and Tiron was demonstrated; the slightly weaker performance of Tiron probably
relates to the lower Fe impurity content of 50 ppm in the Full-cell test. The better (than in the Half-cell) performance of the cell containing glycolate was probably observed because the
limited stability of the Mg-glycolate complexes, owing to the high pH in the Half-cell, was sufficient during Full-cell testing. K2-EDTA and salicylate performed stable at higher cell
voltage. The expected detrimental effect due to insoluble complex formation and adsorption was explicitly shown for DBS and oxalate. CORRELATION AND SUMMARY OF FINDINGS The outcome of all
tests is summarized in Table 2. For Mg anodes, the self-corrosion and fouling of the electrode should be controlled to increase the discharge voltage and enhance the utilization efficiency
using e.g. NTA, Tiron, K2-EDTA or salicylate additive, whereby anode consumption must be moderated to retain the advantage of volumetric capacity. Thus, for battery applications, the
additives must meet the following requirements: * 1. The additive must be able to form a highly stable iron complex (Fe–ligand) like shown for Tiron or salicylate via discharge tests. * 2.
The ligands and respective complexes with Fe must be stable at the open-circuit potential of the Mg anode and at the potential of the discharging anode (e.g., to prevent
electro-(back)-deposition (analog28); it was not observed for all additives). * 3. The additive must be able to form soluble complexes with Mg2+ (Mg–ligand) to prevent detrimental formation
of precipitates on the anode surface like shown in Fig. 3b. The stability of such complexes must be moderate (e.g. like salicylate) to avoid enhanced dissolution of Mg by shifting the
chemical equilibrium towards the accelerated formation of soluble complexes (e.g. like NTA and Tiron). Formation of Mg complexes is expected to have a two-fold effect that must be balanced
(voltage vs. durability). Its positive influence arises from a) preventing the formation of Mg(OH)2 that otherwise blocks the Mg surface, and b) providing local stable steady-state
conditions to maintain Mg dissolution. The negative effects of the formation of Mg complexes are a) the sparingly soluble Mg complexes, which (despite being effective for corrosion
inhibition) are detrimental for the discharge voltage, owing to anode blockage, and b) strong Mg complexes accelerate the dissolution of Mg and parasitic anode consumption. The desired
balance between the stability of the complexes with Fe and Mg can be achieved by the proper selection of complexing agents that form targeted complexes with both cations, or by combining
different complexing agents in the same electrolyte. In addition, the optimization of the additive concentration is likely to result in considerable improvement of the voltage and
utilization efficiency. For tailoring the battery discharge, adjustment of the interaction processes as shown in Table 2 is required. The additives offer the respective toolbox (three
examples in). Convection (e.g. in flow cells) is named to complete the list since it directly affects cell pH and the adsorption kinetics. Independent from Table 2 the additive concentration
within the electrolyte determines the dissolution of the anode as well. Considering the mentioned aspects, adapting the electrolyte reservoir dimension and the ageing of the electrolyte
itself are mandatory. Consequently, synergistic additive mixtures in combination with a systematic electrolyte design, including the complementary adjustment of battery components, must be
applied to unleash the full potential of this technology sleeping couple of decades. Salicylate (Fig. 2) and K2-EDTA efficiently block anode self-corrosion and consume anode at moderate
rate. Both could be applied to extend the life-time of e.g. rescue-batteries based on Mg. In contrast, NTA and Tiron (Fig. 2), which form soluble complexes, offer adequate Mg dissolution
conditions and a low anodic polarization. Unfortunately, named benefits come at the price of anode consumption and decreased utilization efficiency. They are a good option if a high and
stable voltage is required for short periods. The scientific value of this work becomes evident by comparing the chelator strategy to other approaches based on alloying11,44, microstructural
optimization45 or battery (component) design8. The proposed idea has been shown to be very promising since it allows effective control of anode activity very close to the theoretical limit
thereby avoiding use of hazardous alloying elements. Additionally, the admixture of respective chemicals is economical attractive, its technical handling is simple and most of them are
environmentally benign. CONCLUSION New strategy on improvement of Mg-electrode discharge characteristics via addition of iron complexing agents is reported for the first time. The
electrolyte additives targeting tailored discharge of primary magnesium-air batteries must fulfil three important requirements. * 1. The first is an adequate stability of the resulting Fe
complex at neutral and alkaline conditions. * 2. Second, the complexing agents must be stable under the occurring local (polarized) conditions, to retain Fe-ions during the discharge
process. * 3. Third, the Mg-additive complexes should be soluble to prevent anode fouling. Further technical optimization of the additives is required to identify the compounds with the most
favourable log _K_Mg and log _K_Fe. The best system tested in this work was salicylate, which yielded a utilization efficiency twice that of the reference NaCl at 140–180 mV of higher
discharge potential. An even better potential (210–350 mV vs. reference) could be achieved with Tiron or NTA but at the expense of faster anode consumption. Likewise, additive mixtures and
optimal concentrations should be assessed to achieve the highest possible discharge potential and utilization efficiency. Further progress requires research not only on the interaction of
the additives with novel air cathodes (e.g. activated carbon), but also towards a cell design that optimizes the additive interaction environment (pH etc.). To the end of adapting this
technology towards tailored discharging and enhanced performance, clear requirements have been established for the electrolyte additives and its application towards a renaissance of primary
Mg batteries. METHODS CHEMICALS The following chemicals were used as additives: sodium salicylate (cat. no. 71945, Sigma-Aldrich); glycolic acid (cat. no. 124737, Sigma-Aldrich),
dodecylbenzenesulfonic acid sodium salt (DBS, cat. no. 289957, Sigma-Aldrich), 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate (Tiron, cat. no. 89460, Sigma-Aldrich);
nitrilotriacetic acid disodium salt (NTA, cat. no. N0128, Sigma-Aldrich), ethylenediaminetetraacetic acid dipotassium salt dehydrate (K2-EDTA, cat. no. 819040, Merck) and oxalic acid
dihydrate (cat. no. 006053, Chempure). The pH of 0.05 M solutions of the complexing agents was adjusted by adding NaOH to reach a final value in the range of 6.7 to 7.2. ANODE MATERIALS The
anode Mg materials (CP grade) were produced in the HZG castshop. The Mg impurity content was measured by spark discharge-optical emission spectroscopy (SD-OES) with spark analyser vision
software (SPECTROLAB). The bare material was cut into pieces (16 mm × 16 mm × 4 mm resp. 28 mm × 43 mm × 2.5 mm), ground, polished and rinsed with ethanol. HER-TESTING Hydrogen evolution
tests were performed using eudiometers (cat. no. 2591–10–500 from Neubert-Glas, Germany). The immersion solution was 0.5% (0.085 M) aqueous NaCl with or without a complexing agent/battery
additive. MICROSCOPY Optical microscopy was performed using Leica DMI500 system. 3D maps were produced using a Keyence VK-9700 confocal laser microscope. CELL TESTING The discharge tests
were performed using commercial purity Mg containing 220 ppm Fe in a Half-cell with a Pt cathode connected via a salt bridge (Figure S1). In this setup, the influence of rapid pH change due
to the cathodic reaction was moderated. Discharging (in Half-cell mode) was performed using an Interface 1000 system (Gamry) with a three-electrode setup in a 330 mL cell with Pt as the
counter electrode and Ag/AgClsat as the reference electrode. Weight loss was determined by measuring the weight difference of the samples before and after the discharging tests after removal
of deposits by etching with chromic acid, cleaning and drying. The size of the samples was 16 mm × 16 mm × 4 mm. The additives were also tested in primary Mg-air battery (flow-cell setup
(Figure S2)) by using high-purity Mg containing 50 ppm Fe (already commercial available). The cells were discharged with a constant current density of 0.5 mA cm−2 in 0.1 M NaCl. Plates of
the high-purity material with dimensions of 28 mm × 43 mm × 2.5 mm were used as the anode material, and an activated carbon fabric (Kynol Europa GmbH) with a gas-diffusion layer of PTFE was
used as the cathode material. The electrodes were separated at a distance of 1 cm, and the cell (1 L) was filled with the electrolyte. SPECIFIC ENERGY Half-cell discharge tests with respect
to the quantification of the specific energy were carried out at different currents (ranging from 0.025 mA to 10 mA) with the electrolyte containing Tiron, sodium salicylate and electrolyte
without any additives (NaCl 0.5%). Specific energy obtained from 24 hours of anode discharge was calculated by the following formula: $$specific\,energy=\,\frac{V.I.t}{w}[\frac{Wh}{kg}]$$
(2) where V is the average voltage obtained from discharge curve; I is the applied current; t is the time of discharge; and w is mass loss of anode after discharge. Note that for the cases
of reference electrolyte (NaCl 0.5%) at currents 10 mA and 7.5 mA, and for the electrolyte containing sodium salicylate at current of 10 mA, the battery fails (here, battery failure is when
voltage becomes positive) before reaching the 24 hours of discharge. Discharging was stopped at failure point and all variables were calculated and measured corresponding to that point.
Hydrogen evolution, discharge and corresponding weight loss measurements were performed at least twice for all the chelating additives. The results correlated within 5%. Each specific energy
value was obtained from at least three discharge tests at corresponding applied current and electrolyte. DATA AVAILABILITY The datasets generated during and/or analyzed during the current
study are available from the corresponding author on reasonable request. REFERENCES * Shao, Y. _et al_. Coordination chemistry in magnesium battery electrolytes: how ligands affect their
performance. _Scientific Reports_ 3, 3130 (2013). Article PubMed PubMed Central Google Scholar * Orikasa, Y. _et al_. High energy density rechargeable magnesium battery using
earth-abundant and non-toxic elements. _Scientific Reports_ 4, 5622 (2014). Article CAS PubMed PubMed Central Google Scholar * Yoo, H. D. _et al_. Mg rechargeable batteries: an on-going
challenge. _Energy & Environmental Science_ 6, 2265–2279 (2013). Article CAS Google Scholar * Armand, M. & Tarascon, J. M. Building better batteries. _Nature_ 451, 652–657
(2008). Article ADS CAS PubMed Google Scholar * Blomgren, G. E. Electrochemistry: Making a potential difference. _Nature_ 407, 681–682 (2000). Article ADS CAS PubMed Google Scholar
* Aurbach, D. _et al_. Prototype systems for rechargeable magnesium batteries. _Nature_ 407, 724–727 (2000). Article ADS CAS PubMed Google Scholar * Kim, J.-K. _et al_.
Rechargeable-hybrid-seawater fuel cell. _NPG Asia Mater_ 6, e144 (2014). Article CAS Google Scholar * Zhang, T., Tao, Z. & Chen, J. Magnesium-air batteries: from principle to
application. _Materials Horizons_ 1, 196–206 (2014). Article Google Scholar * KIST develop magnesium-air battery with 800 km range (2013). * Blake, I. C. Fiftieth Anniversary: The
Anniversary Issue on Primary Cell: Silver Chloride‐Magnesium Reserve Battery. _Journal of The Electrochemical Society_ 99, 202C–203C (1952). Article CAS Google Scholar * Yuasa, M., Huang,
X., Suzuki, K., Mabuchi, M. & Chino, Y. Discharge properties of Mg–Al–Mn–Ca and Mg–Al–Mn alloys as anode materials for primary magnesium–air batteries. _Journal of Power Sources_ 297,
449–456 (2015). Article ADS CAS Google Scholar * Cheng, F. & Chen, J. Metal–air batteries: from oxygen reduction electrochemistry to cathode catalysts. _Chemical Society Reviews_ 41,
2172–2192 (2012). Article CAS PubMed Google Scholar * Muldoon, J. _et al_. Electrolyte roadblocks to a magnesium rechargeable battery. _Energy & Environmental Science_ 5, 5941–5950
(2012). Article CAS Google Scholar * Jiang, Z., Sirotina, R. & Iltchev, N. K. Magnesium cell with improved electrolyte. US patent US20100310933 (2010). * Makar, G. & Kruger, J.
Corrosion of magnesium. _International Materials Reviews_ 38, 138–153 (1993). Article CAS Google Scholar * Eaves, D., Williams, G. & McMurray, H. N. Inhibition of self-corrosion in
magnesium by poisoning hydrogen recombination on iron impurities. _Electrochimica Acta_ 79, 1–7 (2012). Article CAS Google Scholar * Chen, L. D., Nørskov, J. K. & Luntz, A. C.
Theoretical limits to the anode potential in aqueous Mg–air batteries. _The Journal of Physical Chemistry C_ 119, 19660–19667 (2015). Article CAS Google Scholar * Cao, D., Wu, L., Sun,
Y., Wang, G. & Lv, Y. Electrochemical behavior of Mg–Li, Mg–Li–Al and Mg–Li–Al–Ce in sodium chloride solution. _Journal of Power Sources_ 177, 624–630 (2008). Article ADS CAS Google
Scholar * Wang, N. _et al_. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery. _Electrochimica Acta_ 149, 193–205 (2014). Article CAS Google Scholar *
Frignani, A., Grassi, V., Zanotto, F. & Zucchi, F. Inhibition of AZ31 Mg alloy corrosion by anionic surfactants. _Corrosion Science_ 63, 29–39 (2012). Article CAS Google Scholar *
Williams, G., Grace, R. & Woods, R. M. Inhibition of the localized corrosion of Mg alloy AZ31 in chloride containing electrolyte. _Corrosion_ 71, 184–198 (2014). Article Google Scholar
* Karavai, O. V. _et al_. Localized electrochemical study of corrosion inhibition in microdefects on coated AZ31 magnesium alloy. _Electrochimica Acta_ 55, 5401–5406 (2010). Article CAS
Google Scholar * Sathyanarayana, S. & Munichandraiah, N. A new magnesium—air cell for long-life applications. _J Appl Electrochem_ 11, 33–39 (1981). Article CAS Google Scholar *
Richey, F. W., McCloskey, B. D. & Luntz, A. C. Mg anode corrosion in aqueous electrolytes and implications for Mg-Air batteries. _Journal of The Electrochemical Society_ 163, A958–A963
(2016). Article CAS Google Scholar * Deyab, M. A. Decyl glucoside as a corrosion inhibitor for magnesium–air battery. _Journal of Power Sources_ 325, 98–103 (2016). Article ADS CAS
Google Scholar * Höche, D. _et al_. Effect of iron re-deposition on corrosion of impurity containing magnesium. _Physical Chemistry Chemical Physics_ 18, 1279–1291 (2016). Article PubMed
Google Scholar * Lamaka, S. V., Höche, D., Petrauskas, R. P., Blawert, C. & Zheludkevich, M. L. A new concept for corrosion inhibition of magnesium: Suppression of iron re-deposition.
_Electrochem Commun_ 62, 5–8 (2016). Article CAS Google Scholar * Hawthorne, K. L., Wainright, J. S. & Savinell, R. F. Studies of iron-ligand complexes for an all-iron flow battery
application. _Journal of The Electrochemical Society_ 161, A1662–A1671 (2014). Article CAS Google Scholar * Höche, D., Lamaka, S. V. & Zheludkevich, M. L. Electrolyte additives for
magnesium air batteries. EU patent EP3291361 A1 (2016). * Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen
measurement and optical imaging. _Electrochimica Acta_ 120, 284–292 (2014). Article CAS Google Scholar * Frankel, G. S., Samaniego, A. & Birbilis, N. Evolution of hydrogen at
dissolving magnesium surfaces. _Corrosion Science_ 70, 104–111 (2013). Article CAS Google Scholar * Brady, M. P. _et al_. Film Breakdown and Nano-Porous Mg(OH)2 Formation from Corrosion
of Magnesium Alloys in Salt Solutions. _Journal of The Electrochemical Society_ 162, C140–C149 (2015). Article CAS Google Scholar * Kramarenko, V. F. Toksikologicheskaya khimiya
(ToxicologicalChemistry). _Kiev_, _High School_ (1989). * Furia, T. E. In _CRC handbook of food additives_ Vol. 1 Ch. 6, 271-294 (CRC Press Boca Raton, FL, 1972). * Radecki, A. &
Weselowski, M. The thermal decomposition of alkaline earth metal salicylates. _Journal of Thermal Analysis_ 9, 29–36 (1976). Article CAS Google Scholar * Drake, S. R., Sanderson, K. D.,
Hursthouse, M. B. & Abdul Malik, K. M. Intensive hydrogen bonding in a monomeric magnesium salicylate tetrahydrate. _Inorganic Chemistry_ 32, 1041–1044 (1993). Article CAS Google
Scholar * Pnspanen, J. & Lajunen, L. H. J. Complex formation euilibria of some aliphatic a-hydroxycarboxylic acids. 1. The determination of protonation constants and the study of
calcium (ll) and magnesium (ll) complexes. _Acta Chemka Scandinavica_ 995, 235–240 (1995). Article Google Scholar * Nowack, B. & Sigg, L. Adsorption of EDTA and metal–EDTA complexes
onto Goethite. _Journal of Colloid and Interface Science_ 177, 106–121 (1996). Article ADS CAS PubMed Google Scholar * Dean, J. A. _Lange’s chemistry handbook_. 15 edn, (McGraw-Hill,
New York, 1999). * Nancollas, G. H. & Purdie, N. Crystallization of magnesium oxalate in aqueous solution. _Transactions of the Faraday Society_ 57, 2272–2279 (1961). Article CAS
Google Scholar * Souaya, R. E., Hanna, G. W., Ismail, H. E. & Milad, E. N. Studies On some acid divalent-metal nitrilotriacetate complexes. _Molecules_ 5, 1121–1129 (2000). Article CAS
Google Scholar * Çam, T., Türkel, N. & Özer, U. In _Main Group Metal Chemistry_ Vol. 30, 203 (2007). * Williams, G., Dafydd, H. & Subramanian, R. Chloride ion concentration
effects on passivity breakdown in magnesium. _ECS Transactions_ 58, 23–34 (2014). Article Google Scholar * Zheng, T., Hu, Y., Zhang, Y., Yang, S. & Pan, F. Composition optimization and
electrochemical properties of Mg-Al-Sn-Mn alloy anode for Mg-air batteries. _Materials & Design_ 137, 245–255 (2018). Article CAS Google Scholar * Xiong, H. _et al_. Effects of
microstructure on the electrochemical discharge behavior of Mg-6wt%Al-1wt%Sn alloy as anode for Mg-air primary battery. _J Alloy Compd_ 708, 652–661 (2017). Article CAS Google Scholar *
Martell, A. E. & Smith, R. M. _Critical stability constants_. Vol. 2–6 (Springer, 1976–1989). * Tezak, D., Strajnar, F. & Sarcevic, D. Solid/liquid equilibria in aqueous systems of
dodecyl benzene sulphonate and alkaline earth ions. _Croatica Chemica Acta_ 57, 93–107 (1984). CAS Google Scholar Download references ACKNOWLEDGEMENTS Dr. S.V. Lamaka acknowledges the
financial support of Alexander von Humboldt Foundation via Experienced Researcher Grant. R.P. Petrauskas appreciates the support of Erasmus-Program, Grant No. 2015-1-LT01-KA103-013105.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Helmholtz-Zentrum Geesthacht (HZG), MagIC-Magnesium Innovation Centre, Max-Planck Str. 1, 21502, Geesthacht, Germany Daniel Höche, Sviatlana V.
Lamaka, Bahram Vaghefinazari & Mikhail L. Zheludkevich * Helmut-Schmidt-University University of the Federal Armed Forces, Faculty of Mechanical Engineering, Holstenhofweg 85, 22043,
Hamburg, Germany Daniel Höche * Helmholtz Institute Ulm (HIU), Helmholtzstr. 11, 89081, Ulm, Germany Tobias Braun & Maximilian Fichtner * University of Vilnius, Department of Inorganic
Chemistry, 03225, Vilnius, Lithuania Rokas P. Petrauskas * Karlsruhe Institute of Technology (KIT), Institute of Nanotechnology, Hermann-von-Helmholtz Platz 1, 76344,
Eggenstein-Leopoldshafen, Germany Maximilian Fichtner * University of Kiel, Faculty of Engineering, Kaiserstrasse 2, 24143, Kiel, Germany Mikhail L. Zheludkevich Authors * Daniel Höche View
author publications You can also search for this author inPubMed Google Scholar * Sviatlana V. Lamaka View author publications You can also search for this author inPubMed Google Scholar *
Bahram Vaghefinazari View author publications You can also search for this author inPubMed Google Scholar * Tobias Braun View author publications You can also search for this author inPubMed
Google Scholar * Rokas P. Petrauskas View author publications You can also search for this author inPubMed Google Scholar * Maximilian Fichtner View author publications You can also search
for this author inPubMed Google Scholar * Mikhail L. Zheludkevich View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.H. and M.L.Z. designed
the research idea S.V.L. selected the electrolyte additives D.H., S.V.L., B.V. and R.P.P performed hydrogen evolution and half-cell tests T.B. and M.F. performed Mg-air full cell tests D.
H., S.V.L. B.V. and M.L.Z. analysed data D. H., S.V.L. and M.L.Z. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Daniel Höche. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPORTING INFORMATION BATTERY BOOST WITH SALICYLATE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Höche, D., Lamaka, S.V., Vaghefinazari, B. _et al._ Performance boost for primary
magnesium cells using iron complexing agents as electrolyte additives. _Sci Rep_ 8, 7578 (2018). https://doi.org/10.1038/s41598-018-25789-8 Download citation * Received: 29 January 2018 *
Accepted: 16 April 2018 * Published: 15 May 2018 * DOI: https://doi.org/10.1038/s41598-018-25789-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative