Aloperine Protects Mice against Bleomycin-induced Pulmonary Fibrosis by Attenuating Fibroblast Proliferation and Differentiation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Aloperine is a quinolizidine alkaloid extracted from Sophora alopecuroides. It has been proven to alleviate oxidative stress and effectively promote tumor cell apoptosis in mice. Herein, we
investigated whether aloperine could also mediate its protective effects on bleomycin (BLM)-induced pulmonary fibrosis. Pathological staining, western blot, RT-PCR and flow cytometry were
used to evaluate the impact of aloperine on the development of pulmonary fibrosis. The effect of aloperine on fibroblast proliferation, differentiation and related signaling pathways were
next investigated to demonstrate the underlying mechanisms. In the present report, we showed that aloperine provided protection for mice against BLM-induced pulmonary fibrosis as manifested
by the attenuated lung injury and reduced fibrosis along with alleviated fibroblast proliferation and differentiation. Additionally, we provided in vitro evidence revealing that aloperine
inhibited cellular proliferation in PDGF-BB-stimulated mouse lung fibroblasts by repressed PI3K/AKT/mTOR signaling and fibroblast to myofibroblast differentiation by repressed TGF-β/Smad
signaling. Overall, our data showed that aloperine could protect the mice against BLM-induced pulmonary fibrosis by attenuated fibroblast proliferation and differentiation, which indicated
that aloperine may be therapeutically beneficial for IPF patients.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and devastating lung disease with unknown etiology and manifested the poorest prognosis1. Despite past extensive studies, the
underlying mechanisms of IPF pathogenesis are not fully understood2. As a result, therapeutic strategies for IPF have been largely unsuccessful, and the average survival time for this
category of patients is only 3 to 5 years after diagnosis2.
The pathology of IPF is characterized by migration, proliferation and differentiation of fibroblast and remodeling of the extracellular matrix (ECM)3. Fibroblasts have been noted to play a
central role in the fibrotic processes regulated by transforming growth factor-β (TGF-β)4,5 or other profibrotic mediators, such as platelet-derived growth factor (PDGF)6,7 and connective
tissue growth factor (CTGF)8. These fibroblasts characterized by abnormal α-SMA and fibrillar collagens expression are called myofibroblasts9. Fibroblast to myofibroblast differentiation is
a key step during the course of fibrotic process10. It has been shown that myofibroblasts in IPF lung tissues exhibited a profibrotic secretory phenotype, with aberrantly proliferative rates
and lower spontaneous apoptosis11. To reduce the fibrogenesis in IPF, the production of these profibrotic mediators and myofibroblast differentiation must be attenuated12,13,14,15.
Aloperine is a kind of alkaloid extracted from sophora alopecuroides and has been reported to execute therapeutic effects against pulmonary hypertension16, renal injury17 and neuropathic
pain18 through attenuating oxidative stress, and multiple myeloma19, and colon cancer20 though increasing cell apoptosis. These observations prompted us to hypothesize that aloperine may be
a good candidate drug for the prevention and treatment of bleomycin (BLM) -induced pulmonary fibrosis, since oxidative stress and apoptosis are involved in its pathogenesis21,22. To address
this feasibility, we conducted studies in a pulmonary fibrosis mouse model, and then assessed the impact of aloperine on the disease development. We found that administration of aloperine
provided protection for mice against pulmonary fibrosis as manifested by the attenuated lung injury and reduced fibrosis along with a marked alleviation of fibroblast proliferation and
differentiation in the lung. Mechanistic studies revealed that aloperine could regulate the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and
TGF-β/Smad signaling pathways, and by which it reduced fibroblast proliferation and differentiation, respectively. Our data suggested that treatment with aloperine could be a viable strategy
for the prevention and treatment of pulmonary fibrosis in clinical settings.
Aloperine is known to treat various diseases. However, whether it could be used as a viable approach for BLM-induced pulmonary fibrosis has not been extensively researched. To study the
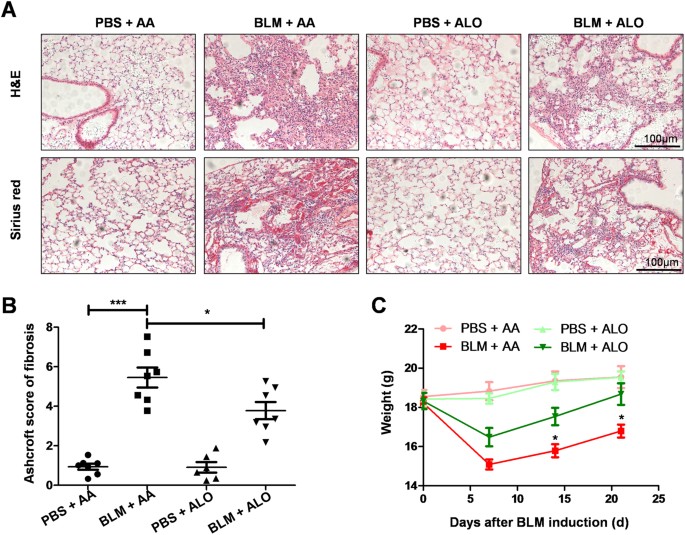
effect of aloperine on BLM-induced pulmonary fibrosis, the mice were treated with aloperine for 21 days after exposure to BLM. Mice treated with phosphate-buffered saline (PBS) + Alo or PBS
+ AA served as controls. We first sought to address the impact of aloperine on pulmonary fibrosis. A significantly attenuated lung injury and pulmonary fibrosis were noted in
aloperine-treated mice as evidenced by hematoxylin and eosin (H&E) and Sirius red staining (Fig. 1A). Particularly, the severity of pulmonary fibrosis was much lower as manifested by the
lower Ashcroft scores (5.45 ± 0.51 versus 3.78 ± 0.43, p