Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Indoleamine 2,3-dioxygenase-1 (IDO1) mediates the degradation of L-tryptophan (L-Trp) and is constitutively expressed in the chorionic vascular endothelium of the human placenta
with highest levels in the microvasculature. Given that endothelial expression of IDO1 has been shown to regulate vascular tone and blood pressure in mice under the condition of systemic
inflammation, we asked whether IDO1 is also involved in the regulation of placental blood flow and if yes, whether this function is potentially impaired in intrauterine growth restriction
(IUGR) and pre-eclampsia (PE). In the large arteries of the chorionic plate L-Trp induced relaxation only after upregulation of IDO1 using interferon gamma and tumor necrosis factor alpha.
However, _ex vivo_ placental perfusion of pre-constricted cotyledonic vasculature with L-Trp decreases the vessel back pressure without prior IDO1 induction. Further to this finding, IDO1
protein expression and activity is reduced in IUGR and PE when compared to gestational age–matched control tissue. These data suggest that L-Trp catabolism plays a role in the regulation of
placental vascular tone, a finding which is potentially linked to placental and fetal growth. In this context our data suggest that IDO1 deficiency is related to the pathogenesis of IUGR and
PE. SIMILAR CONTENT BEING VIEWED BY OTHERS _NR4A2_ EXPRESSION IS NOT ALTERED IN PLACENTAS FROM CASES OF GROWTH RESTRICTION OR PREECLAMPSIA, BUT IS REDUCED IN HYPOXIC CYTOTROPHOBLAST Article
Open access 19 October 2021 PLACENTAL HYPOXIA-INDUCED ALTERATIONS IN VASCULAR FUNCTION, MORPHOLOGY, AND ENDOTHELIAL BARRIER INTEGRITY Article 30 July 2020 DOCK1 DEFICIENCY DRIVES PLACENTAL
TROPHOBLAST CELL DYSFUNCTION BY INFLUENCING INFLAMMATION AND OXIDATIVE STRESS, HALLMARKS OF PREECLAMPSIA Article 08 October 2024 INTRODUCTION L-Tryptophan (L-Trp) is the least abundant
essential amino acid1. It is required for protein synthesis and also as precursor of key biomolecules such as serotonin, melatonin and NAD+ 1,2,3,4. L-Trp catabolism mainly follows the
kynurenine (kyn) pathway (KP), with the first and rate-limiting step being the oxidation of the indole ring of L-Trp. This step is catalyzed by one of three enzymes capable of converting of
L-Trp into N-formylkynurenine5,6, namely tryptophan 2,3-dioxygenase (TDO), indoleamine 2,3-dioxygenase-1 (IDO1) and −2 (IDO2). These cytosolic enzymes display varying expression
patterns2,7,8, a factor which likely dictates their relative ability to contribute to the degradation of L-Trp along the KP. It is well established that IDO1 is induced during infection
where it displays antimicrobial activity9 and immune regulation10,11. Indeed, recent studies have probed the ability of the enzyme to mediate the immune system in tumour survival12. In the
term placenta high levels of IDO1 expression and activity have been reported, where it is implicated in establishing and maintaining feto-maternal tolerance13,14,15,16, a role mediated via
the induction of regulatory T cells17. Other recent works have shown that during inflammation, IDO1 becomes expressed in vascular endothelial cells and contributes to arterial relaxation18.
The enzyme is constitutively expressed on both the maternal and the chorionic vascular endothelium with a positive gradient towards the feto-maternal interface13. This led us to question
whether expression of IDO1 in the fetal vessels of the chorion is involved in the regulation of placental perfusion and consequently placental and fetal growth. Furthermore, we wondered
whether pathologies which display reduced fetal growth (such as intrauterine growth retardation (IUGR) and preeclampsia (PE))19,20, are associated with a defect in IDO1-mediated regulation
of placental vascular tone. In fact, previous reports present some evidence in favour of this hypothesis. For instance, L-Trp degradation is reduced in placental tissue in IUGR21, and IDO1
expression is decreased in PE22,23. In addition, disruption of the IDO1 gene induces IUGR and preeclampsia phenotypes in pregnant mice24. Changes in the chorionic vasculature are associated
with IUGR, as increased vascular resistance leads to abnormal Doppler waveforms of the umbilical artery25. Remodeling of the arteries of placental stem villi contributes to IUGR26. However,
the role of IDO1 in the regulation of the placental vascular tone and its potential link with these pregnancy complications has not been investigated so far. RESULTS EXPRESSION OF IDO1 IN
ISOLATED ENDOTHELIAL CELLS FROM CHORIONIC PLATE ARTERIES (PLAECS) As a basis for subsequent functional studies involving chorionic plate arteries, we studied IDO1 expression at the level of
mRNA and protein in freshly isolated primary PLAECs and in PLAECs stimulated with IFNγ (80 ng/mL) and TNFα (80 ng/mL) for 48 h27 (Supplementary Fig. S1). As revealed by qPCR, IDO1 mRNA
expression was markedly augmented following cytokine stimulation (Supplementary Fig. S1a). IDO1 protein contents varied between the different isolations, and markedly increased upon cytokine
stimulation (Supplementary Fig. S1b). The calculated MW of IDO1 is 45 kDa but the observed band was found at a somewhat lower level as previously described9. Immunocytochemical staining of
cellular pellets failed to detect IDO1 protein in non-stimulated cells (Supplementary data, Fig. S1c1). However, strong IDO1-staining was found in PLAECs stimulated with IFNγ and TNFα
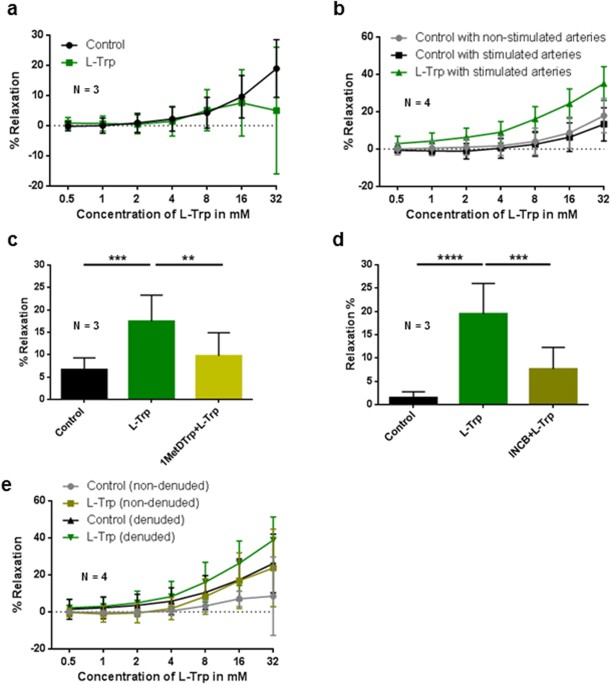
(Supplementary Fig. S1c2). EFFECT OF L-TRP ON THE RELAXATION OF PRECONTRACTED ARTERIAL RINGS OF THE CHORIONIC PLATE Compared to vehicle control, L-Trp had no discernible effects on U46619
pre-constricted arterial rings (P = 0.528) (Fig. 1a). In contrast, L-Trp- elicited relaxation of the arterial rings preincubated overnight with IFNγ and TNFα was significantly higher
compared to vehicle-treated control rings (P < 0.001). Non-stimulated vehicle control arteries responded similar to stimulated control (P = 0.931) (Fig. 1b). THE RELAXING EFFECT OF L-TRP
ON STIMULATED TERM CHORIONIC PLATE ARTERIES IS PARTLY IDO1-DEPENDENT To determine whether the relaxing effect of L-Trp depends on IDO1, we examined L-Trp-elicited relaxation of the rings
preincubated with IFNγ and TNFα in the absence or presence of two different competitive inhibitors of IDO1, 1-methyl-D-tryptophan (Fig. 1c; 1MetDTrp) and INCB024360 (Fig. 1d; INCB024360). In
comparison to vehicle treated control rings, L-Trp-elicited relaxation was significantly higher (Fig. 1c,d) (P ≤ 0.001 and P < 0.0001, resp.). Pre-treatment with either 1MetDTrp (P ≤
0.01) (Fig. 1c) or INCB024369 (P ≤ 0.001) (Fig. 1d) resulted in reduced L-Trp-induced relaxation in stimulated rings. THE EFFECT OF L-TRP ON THE VASCULAR TONE OF STIMULATED CHORIONIC PLATE
ARTERIES IS NOT EXCLUSIVELY ENDOTHELIUM-DEPENDENT To test the role of the endothelium in the L-Trp-elicited relaxation, myography was performed in rings with intact or denuded endothelium.
The success of the denudation was assessed by immunohistochemistry (Supplementary Fig. S2). In denuded rings L-Trp elicited relaxation that was higher compared to (also denuded) control
rings (P < 0.001) (Fig. 1e). We then performed myography measurements in the absence or presence of 1MetDTrp. Treatment with L-Trp relaxed the denuded arterial rings (P = 0.006,
Supplementary Fig. S3). This effect was reduced by pre-incubation with 1MetDTrp (P = 0.0259), albeit to a small extent only (Supplementary Fig. S4). Therefore, our data indicate on one hand
the presence of an IDO1-dependent pathway that catabolizes L-Trp when the endothelium is removed, on the other hand they suggest that there may be an additional element of IDO1-independent
relaxation in denuded rings. Immunohistochemistry of a cytokine-stimulated artery ruled out an expression of IDO1 in smooth muscle cells, however, a few isolated cells in the vessel wall
(possibly macrophages) stained positive for IDO1 (Supplementary Fig. S5). IMPACT OF L-TRP ON THE VESSEL BACK PRESSURE OF A PLACENTAL COTYLEDON IDO1 expression is higher in the placental
microcirculation than the big chorionic plate arteries used for the above myography experiments. To examine the vascular relaxant effect of L-Trp in the placental microcirculation, we
measured fetal vessel back pressure differences in response to L-Trp as a measure for the relaxation of the microvascular tone in single cotyledons from normal term placentas by _ex-vivo_
placental perfusion. Perfusion of non-preconstricted cotyledons obtained from 5 different placentas with 8 mM L-Trp yielded a 13.5% median decrease (range between −16.3 and +46.5%) of the
pressure measured in the artery after 40 min (a representative experiment is shown in Fig. 2a). The coefficient for the linear relationship between pressure and time was different from zero
(−0.175, SE 0.020, P < 0.001), and the vessel back pressure was overall significantly decreased by 0.175 mmHg per min after attaining the stable phase (Fig. 2c). In a second set of
experiments we pre-constricted the vascular bed of normal term placental cotyledons with 10 nM of U46619. After achieving stable contraction perfusion of cotyledons with 8 mM L-Trp in the
presence of U46619 resulted in a 18.5% median decrease (range between −4.9 and +47.7%) of the pressure measured in the artery after 40 min (a representative experiment is shown in Fig. 2b);
the coefficient for the linear relationship between pressure and time was different from zero (−0.651, SE 0.059, P < 0.001), and the vessel back pressure was overall significantly
decreased by 0.651 mmHg per min after pre-constriction with U46619 (Fig. 2c). IDO1 EXPRESSION AND ACTIVITY IN HEALTHY AND PATHOLOGIC PLACENTA IDO1 mRNA, protein, activity and tissue
distribution were examined in banked chorionic tissues from pre-term controls (placenta praevia with a mean gestational age similar to IUGR and PE), normal term placenta, IUGR and PE (Table
1). When normalized to the endothelial marker CD34 (Fig. 3a) IDO1 mRNA was increased in term (n = 10) pregnancies compared to pre-term controls (n = 5) (P < 0.05). However, when
normalized to the housekeeping gene RPL30 (Supplementary Fig. S6) we found no such difference. No differences were found in IUGR (n = 6) and PE (n = 13) tissue samples compared to pre-term
controls (Fig. 3a). In contrast, IDO1 protein content was lower in the IUGR (n = 6; P ≤ 0.05) and PE (n = 13; P ≤ 0.01) samples compared to the pre-term controls (n = 5) (Fig. 3b,c). In line
with protein content, IDO activity was lower in IUGR (n = 7; P < 0.01) and PE (n = 13; P < 0.05) compared to the pre-term controls (n = 11), whereas it was higher in term placenta
tissues (n = 9; P < 0.05) (Fig. 3c). To determine whether the tissue distribution pattern of IDO1 protein was different in the chorion of IUGR and PE compared to normal term and pre-term
controls, we performed immunohistochemistry on paraffin sections of banked placental tissues (Fig. 4). The expression profile of vascular endothelial IDO1 was reduced in some cases of IUGR
(3 cases out of 5) and PE (4 cases out of 11) in comparison with pre-term controls (1 case out of 5). Non-endothelial localization of IDO1 was found in maternal macrophages aggregated within
the intervillous spaces in areas affected by chronic villitis and intervillositis. These were found mostly in the PE group and in one pre-term control sample. L-TRP-ELICITED RELAXATION IN
RINGS FROM PATHOLOGIC PREGNANCIES L-Trp did not induce relaxation of unstimulated rings obtained from PE placentas (Fig. 5a). In contrast, in cytokine-stimulated rings L-Trp-induced
relaxation compared to vehicle responses in both cytokine-stimulated and –unstimulated rings (P < 0.001). Furthermore, in stimulated PE arteries the sensitivity to L-Trp appeared slightly
increased in compared to arteries from stimulated normal term pregnancies (Fig. 1b vs. Fig. 5b). Interestingly, in arterial rings from IUGR placenta L-Trp induced contraction of
unstimulated rings (P < 0.001, compared to control rings) (Fig. 5c). Preliminary data from one placenta only suggest that L-Trp might induce relaxation in cytokine-stimulated arterial
rings from IUGR compared to vehicle control rings (Supplementary Fig. S7). DISCUSSION The placental circulation is characterized by low vascular resistance28,29 and a lack of neuronal
innervation30, suggesting that regulation of vascular tone is determined by physical and/or local factors. Our study focussed on the biological significance of the endothelial expression of
IDO1 in the chorionic vasculature described earlier13. Apart from the placenta, constitutive endothelial expression of IDO1 has been found in some tumors31 (and Astrid Blaschitz, unpublished
observation), whereas in other vascular beds, endothelial IDO1 is not normally expressed unless during inflammation. In this state the enzyme contributes to the regulation of the vascular
tone18 and probably also to hypotension in human sepsis32. Based on studies with commercial preparations, the Trp metabolite kynurenine was originally reported to mediate relaxation of
vascular tone. More recently, however, HPLC-purified kynurenine was reported to be inactive, and arterial relaxation attributed to a yet to be identified Trp metabolite33. The distribution
of endothelial IDO1 expression in the placenta shows a positive gradient on both the maternal and the fetal side towards the feto-maternal interface13. This finding implies that only a
minority of the bigger arteries of the chorionic plate show IDO1 protein in their endothelium. This could explain why L-Trp did not induce arterial relaxation in this vascular bed, and why
arterial relaxation was only seen after cytokine-mediated upregulation of endothelial IDO1. The fact that 1MetDTrp as well as INCB024360 blocked the effect of L-Trp implies IDO1 in the
production of a vasoactive L-Trp metabolite in endothelial intact arteries34,35,36, as both of these inhibitors do not sufficiently inhibit IDO2 or TDO34,35,36,37,38. Cytokine-stimulated
intact and endothelium-denuded vessels relax similarly in response to L-Trp. Our data suggest that following cytokine stimulation, the relaxation of denuded arteries does not depend
exclusively on IDO1, which is expressed in the tunica media in a few cells that remain to be characterized. There might be an increased availability of L-Trp to these cells once the
endothelium is removed. It seems unlikely that this arterial relaxation is mediated by IDO2 given that the L-Trp degrading effect of this enzyme is very low compared to IDO137,39, and that
50% of Caucasians lack functional IDO2 alleles40. A possible role of serotonin seems also unlikely as this tryptophan metabolite acts as a vasoconstrictor in placental arteries41. As a means
to test the possibility that L-Trp-induced arterial relaxation occurs in the microvasculature and that this in turn influences organ perfusion pressure we _ex vivo_ perfused single
cotyledons with L-Trp. In these experiments L-Trp caused decreased vessel back pressure in pre-constricted and non-preconstricted circulatory beds in the absence of previous cytokine
stimulation. Despite the fact that the myography experiments showed that L-Trp -induced relaxation is slight in the larger chorionic plate arteries, the effect of L-Trp on decreasing vessel
back pressure is more substantial and potentially suggests a greater physiological relevance, even though also in these experiments the variability of the response to L-Trp was substantial.
Whilst the role of IDO1 was not directly proven in this experiment by specific blocking, we speculate that this enzyme plays a role in this response given its constitutive expression in this
vasculature13. Also, we are reasoning that in otherwise healthy arteries with intact endothelium L-Trp does not relax. Indeed these arteries require cytokine stimulation before relaxation
to L-Trp is observed, an effect which is inhibited by IDO1 inhibition. As we localized IDO1 in the endothelium of placental capillaries very early on in pregnancy13, it will be interesting
to study to which extent smooth muscle cells and other cells, such as pericytes, are involved in L-Trp–mediated regulation of the vascular tone. Cellular uptake of L-Trp is the pre-requisite
for the effect of IDO1 a cytosolic enzyme. IDO1 has been shown to induce the expression of a L-Trp transporter in human tumor cells42. Recent studies indicate that L-Trp transporters can be
induced by IFNγ43 and that 1MetDTrp does not only competitively inhibit IDO1 but also blocks L-Trp transport into the trophoblast44. However, our data gained by _ex vivo_ placental
perfusion (in the absence of IFNγ) and the results of myography experiments using INCB024360, a competitive IDO1 inhibitor that does not affect L-Trp transport37, renders the possibility
unlikely that the vasorelaxing effect of L-Trp on cytokine-stimulated arteries is based solely on upregulation of an endothelial L-Trp transporter. Previously we had reported increasing
expression of IDO1 in the chorionic vascular endothelium during the course of pregnancy13,14 that correlated with increased IDO activity assessed by the kynurenine-to-Trp ratio13. Similar
results were found in term placentas of rhesus monkeys and common marmosets45. Conflicting data regarding the localization of IDO1 in the placenta46,47,48 are discussed extensively
elsewhere13. Regarding placental pathology, earlier reports21,49 indicate a decrease in L-Trp catabolism by placental IDO1 in IUGR and PE. In accordance with this, we found decreased IDO
activity in IUGR and PE compared with pre-term controls, as determined by LC/MS. Based on semi-quantitative assessment by immunohistochemistry, a recent publication reported decreased IDO1
in villous stromal endothelial cells in placentas with PE50, supporting our data that show decreased IDO protein expression in PE and IUGR in comparison to gestational age-matched normal
placentas. Thus, the decrease in IDO1 expression reported earlier for PE placentas22,23 cannot be explained solely by the fact that term placentas are an inappropriate control for PE
placentas, as PE and IUGR pregnancies are associated with pre-term delivery. Indeed, we show that PE and IUGR placentas have a decreased IDO activity and express less IDO1 protein than
pre-term placentas delivered. Although the IUGR and PE tissue samples showed some diversity in the extent of IDO1 protein expression, the difference to gestational age-matched controls is
striking. This is particularly so when considering that in some PE placental samples part of the IDO1 protein was localized to macrophages in the foci of chronic villitis and intervillositis
rather than to endothelial cells. It should be noted, however, that we cannot exclude an influence of the mode of delivery on placental expression of IDO1. In our sample this might
potentially affect the difference between term and preterm placentae (see Table 1). At the level of analysis of vascular tone, our data suggest that in PE cytokine-stimulated chorionic
arteries are sensitive to low concentrations of L-Trp, possibly due to a constitutive expression of L-Trp transporters51. In contrast, we observed a contracting effect of L-Trp in
non-stimulated arteries in IUGR. These data should be interpreted with caution, however, as gestational age–matched chorionic plate arteries were unavailable to our study. While IDO1 mRNA
did not differ between normal and pathological placentas, there were clear differences in IDO1 protein content and in functional assays. We speculate that posttranscriptional or
posttranslational modifications might differentially affect IDO1 protein stability in normal and diseased placenta52,53,54. As IDO1 deficient mice have a PE phenotype24, our data suggest a
possible pathogenic role of a deficiency in IDO1-mediated L-Trp catabolism for placenta pathology. It should be kept in mind that, whereas our study focussed on the chorionic vasculature,
alterations of IDO1 expression in the endothelium of the vasculature of the decidua (where the enzyme is also constitutively expressed13) might as well pertain to human pregnancy pathology.
Further analysis of _ex vivo_ placental perfusion of IUGR and PE placentas and of the uterine and placental blood flow in patients are warranted to shed more light on this issue. Our
findings provide evidence for a novel mechanism of the regulation of the placental vascular tone, based on IDO1-mediated metabolism of L-Trp, and suggest that dysregulation of this mechanism
may be involved in the pathogenesis of the pregnancy complications, such as IUGR and PE. MATERIALS AND METHODS ETHICAL APPROVAL The study, including the isolation of placental arterial
endothelial cells, was performed in accordance with the national guidelines and approved by the Ethics Committee of the Medical University of Graz, Austria (No. 25–366 ex 12/13 and No.
21–079 ex 09/10). All participants involved in the study gave written informed consent. Samples obtained from the Biobank Graz also included informed consent. PATIENTS IUGR was defined as
the presence of signs for placental insufficiency and fetal compromise (without the characteristics of PE). These signs included asymmetric fetal biometry and estimated fetal weight below
the 3rd centile (with confirmed gestational age by first trimester crown-rump-length measurement), oligohydramnios and centralized fetal circulation. From January 2016 on the Barcelona
calculator was used for staging IUGR http://medicinafetalbarcelona.org/calc/55 and included cases with at least IUGR stage 1. PE was defined after week 20 of pregnancy as displaying a blood
pressure higher than 140/90 mmHg (without pre-existing hypertension) and proteinuria. All patients were treated at the Department of Obstetrics and Gynaecology of the Medical University of
Graz. WIRE MYOGRAPHY Placentas were obtained from normal, IUGR and PE pregnancies within 30 min after caesarean section or vaginal delivery. Arterial rings (2 mm length) were cut from
arteries of the chorionic plate using a calibrated eyepiece micrometre. The arterial rings were either used immediately or after overnight incubation at 37 °C in 95% air/5% CO2 in DMEM
culture medium (Life Technologies, Paisley, UK) supplemented with 10% (v/v) FBS (Life Technologies), and stimulated with human TNFα (80 ng/mL, Peprotech, Vienna, Austria) and human IFNγ (80
ng/mL, Peprotech). For some experiments, arterial rings were denuded of their endothelium by perfusion with 0.1% (v/v) Triton X-100 (Sigma, St. Louis, MO, USA), successful denudation was
confirmed by immunohistochemistry. Arterial rings were carefully mounted on two pin-supports on a myograph 620 M (Danish MyoTechnology, Aarhus, Denmark) in a modified Krebs-Henseleit buffer
(Sigma Aldrich, Schnelldorf, Germany) made up in deionized water (6.9 g/L NaCl, 0.35 g/L KCl, 0.16 g/L KH2PO4, 0.141 g/L MgSO4, 2.1 g/L NaHCO3, 2 g/L D-glucose, 0.97 g/L EDTA·2H2O and 0.017
g/L CaCl2, penicillin/streptomycin 1% (w/v), pH 7.4) and continuously aerated with 5% CO2/95% O2 at 37 °C. Isometric tensions were recorded using a PowerLab and LabChart software
(ADInstruments), optimal resting tension was calculated using the length-tension method as previously described56,57. After establishing resting tension arteries were allowed to equilibrate
for 30 min. Arteries were then subject to a “wake up” protocol involving depolarising constriction to modified Krebs-Henseleit buffer containing 60 mM KCl (KPSS; 9.2 g/L KCl, 0,289 g/L
MgSO4·7H2O, 0.161 g/L KH2PO4, 0.277 g/L CaCl2, 2.1 g/L NaHCO3, 0.01 g/L EDTA-Na2·2H2O, 0.991 g/L D-glucose), washout and then receptor mediated constriction using the thromboxane A2 analogue
U46619 (10−9–10−6 M) (Sigma-Aldrich). After the “wake up” protocol contractile arteries were equilibrated in Krebs-Henseleit buffer containing indomethacin (10 μM, Sigma Aldrich), L-NAME
(300 μM, Sigma Aldrich), and where necessary the IDO inhibitors 1-methyl-D-tryptophan (1 mM, 1MetDTrp; Sigma Aldrich, Schnelldorf, Germany) or INCB024360 (30 μM, MedChem Express, New Jersey,
USA) for 30 min. Arteries were then constricted by a single-dose of U46619 to achieve a 50% maximal KPSS contraction. Depending on the experimental aims, arteries were either treated with a
single dose of L-Trp (8 mM) or with increasing doses of L-Trp (0.5–32 mM). The extent of relaxation (% relaxation) was defined as the reversal of U46619-induced tone. _EX-VIVO_ PERFUSION OF
A SINGLE COTYLEDON OF THE HUMAN TERM PLACENTA The _ex-vivo_ perfusion system was adapted from Schneider _et al_.58 to study changes in the vessel back pressure of a placental cotyledon.
Within 20 min after delivery of the placenta, the fetal circulation of one intact cotyledon was established by cannulating its chorionic artery and corresponding vein. Once the circuit was
established, the cotyledon was flushed with pre-warmed (37 °C) perfusion medium (Earl´s buffer (6.8 g/L NaCl, 0.4 g/L KCl, 0.14 g/L NaH2PO4, 0.2 g/L MgSO4·7H2O, 0.2 g/L CaCl2, 2.2 g/L
NaHCO2, all from Merck, Darmstadt, Germany), supplemented with 10 g/L dextran FP40 (Serva, Heidelberg, Germany), and 2 g/L D-glucose (Merck). The cotyledon together with surrounding tissue
was cut and carefully positioned into the pre-warmed perfusion chamber. Perfusion medium kept at 37 °C was connected to the fetal artery cannula where a micro-catheter pressure sensor
(Millar, Houston, Texas, US) was also inserted to monitor in real time the pressure changes. The fetal circulation was gassed with 95% N2, 5% CO2 through a gas exchanger (Living Systems, St.
Albans, VT, US). A continuous fetal artery influx of 4 mL/min was applied by a magnetic pump (Codan, Salzburg, Austria). In order to establish the maternal circulation, three rounded
needles were inserted and fixed into the intervillous space of the perfused cotyledon. A constant volumetric flow rate of 8 mL/min was administered to the maternal circulation; the medium
was gassed with 5% CO2, 20% O2 and 75% N2. After a pre-stabilization period of at least 10 min, (i) either 8 mM L-Trp (Sigma Aldrich, Steinheim, Germany) (ii) or 10 nM U4661959, attaining a
plateau of pre-contraction for at least another 10 min, followed by 8 mM L-Trp (in the continued presence of 10 nM U46619) were applied to the fetal reservoir so ensuing pressure changes
could be studied. Only experiments with a fetal flow recovery of at least 85% within the first 30 min were continued for analysis according to defined quality criteria60. Perfusates from
maternal and fetal circulation were collected every 30 min during the experiment in order to measure the metabolic and oxygenation status (pO2, pCO2, pH, lactate production and glucose
consumption) of the perfused cotyledon by a blood gas analyser (Radiometer, Copenhagen, Denmark). ISOLATION AND CULTURE OF PLACENTAL ARTERIAL ENDOTHELIAL CELLS (PLAECS) Human term placentas
from uncomplicated pregnancies were obtained under informed consent after vaginal delivery. After removal of the amnion, arterial chorionic blood vessels were resected from the fetal surface
of the chorionic plate as previously published61. After removal, arteries were washed with Hank’s balanced salt solution (HBSS) (Life Technologies, Paisley, UK) to remove residual blood.
PLAECs were then isolated by perfusion of vessels with DMEM containing 185 U/mL collagenase type II (Sigma, St. Louis, MO, USA), 2 mg/mL bovine serum albumin (Sigma) 0.5 mM CaCl2 (Roth,
Karlsruhe, Germany), 1 unit amino acids 50x (PAA, Pasching, Austria), 1 unit amino acids 100x (PAA), 0.01 unit vitamin 1x (PAA) and 5% FBS, pre-warmed to 37 °C. The perfusion time was
limited to 8 min to avoid contamination with non-endothelial cells. Detached cells were collected in FBS. Finally, the cell suspension obtained was centrifuged (200 × _g_ for 5 min), and the
pellet re-suspended with EGM-MV medium (Lonza, Walkersville, MD, USA). The cells were plated on culture plates pre-coated with 1% (v/v) gelatin (Sigma-Aldrich, Schnelldorf, Germany). The
purity of the cultures was assessed by staining for the specific endothelial marker von Willebrand factor (1:8000, A0082, Dako, Glostrup, Denmark) and the absence of fibroblast specific
antigen (1:2000, DIA−100, Dianova, Hamburg, Germany), smooth muscle actin (1:600, M0851, Dako) and desmin (1:1000, Dako). Only PLAECs cultures with a purity of > 99% were used for the
present study. Cells were used only between the third and the sixth passage. IMMUNOHISTOCHEMISTRY Tissues were fixed between 24 h and 3 days in 4% (w/v) buffered formalin (Sanova, Vienna,
Austria) at room temperature and embedded in paraffin. 5 μm sections were cut on a rotation microtome, mounted on Superfrost+ glass slides (Sanova) and dried over-night on a 50 °C hot plate.
Slides were then deparaffinised with Tissue Clear (Sanova) and rehydrated followed by heat induced antigen retrieval buffer at pH 9 (Leica Biosystems, Vienna, Austria) under pressure at 120
°C for 7 min, cooled down for 20 min and washed in distilled water. Endogenous peroxidase activity was blocked by 10 min exposure to peroxidase-blocking solution (Dako, Carpinteria, CA),
washed and then application of Ultra-V block (Thermo Scientific, Cheshire, UK) for 5 min. Samples were incubated first with anti-IDO1 antibody (IgG1 1 μg/mL, generously provided by O.
Takikawa9) for 45 min at room temperature and then 10 min with primary antibody enhancer (Thermo Scientific). Bound antibodies were detected using a polyvalent horseradish-peroxidase polymer
system (Thermo Scientific) for 15 min followed by 10 min incubation with 3-amino-9-ethylcarbazole (Thermo Scientific). All samples were counterstained with Mayer’s hematoxylin and aqueously
mounted with Kaiser’s glycerol gelatine (Merk, Damstadt, Germany). Washing steps were performed with TBS/0.05% (v/v) Tween 20 (Merck). Irrelevant mouse anti-Aspergillus niger IgG1 (1 μg/mL,
Dako) was used as a negative control antibody. Anti-CD34 class II (0.04 μg/mL, Dako) was used for staining vascular endothelium, anti-CD68 (0.025 μg/mL; Thermo Scientific) for staining
macrophages. FIXATION OF CELL PELLETS Accutase-detached cells were re-suspended in EGM-MV medium (Lonza, Walkersville, MD, USA) and centrifuged (630 × _g_ for 5 min), the supernate was
carefully removed. The pellet was transferred into a 1.5 mL Eppendorf tubes, re-suspended and fixed in 1 mL 4% paraformaldehyde (PFA) for 30 min at room temperature. After two washes with
PBS (630 × _g_ for 5 min), the pellet was re-suspended in 5% (v/v) gelatine (Merck, Darmstadt, Germany) for 30 min at 37 °C. Following centrifugation at 37 °C, the pellet was placed
overnight at 4 °C and then fixed for an hour at 4 °C by adding 4% PFA. Once the PFA was removed, the pellet was carefully placed in a new vessel with 4% PFA and incubated overnight at 4 °C.
The pellet was then washed with PBS and embedded in paraffin as mentioned above. PROTEIN ISOLATION AND QUANTIFICATION Total cell or tissue protein lysates were prepared in RIPA buffer (Sigma
Aldrich, St. Louis, MO, USA) containing protease inhibitors (Roche, Mannheim, Germany). Protein concentration was determined according to the Lowry method. WESTERN BLOTTING ANALYSIS Cell
and/or tissue lysates were mixed with LDS sample buffer (4×) (Life Technologies, Carlsbad, CA) and sample reducing agent (10×) (Life Technologies). Samples were then denatured at 70 °C for
10 min. Equal amounts of total protein (25 µg/well) were loaded onto 10% Bis-Tris SDS-PAGE gels (Life Technologies) and resolved at 120 V for approximately 75 min. Proteins were transferred
to a nitrocellulose membrane (Life Technologies) and nonspecific binding sites blocked for 30 min with blocking solution (Life Technologies). After blocking, the membranes were incubated by
the anti-IDO1 antibody9 (1 μg/mL) mixed together with an anti-GAPDH antibody (Novus Biologicals; 50 ng/mL) on a shaker overnight at 4 °C. Membranes were washed with wash solution (Life
Technologies) and incubated with the appropriate secondary antibody solution conjugated with horseradish peroxidase (Life Technologies). After washing, membranes were visualized using an
enhanced chemiluminescent imaging system (Life Technologies). SEMI-QUANTITATIVE POLYMERASE CHAIN REACTION Total RNA was isolated according to manufacturer’s instructions using peqGOLDTriFast
(VWR, Erlangen, Germany). cDNA was synthetized from 2 μg of RNA in the presence of random primers and MultiScribeTM reverse transcriptase (Applied Biosystems, Cheshire, UK). IDO1 specific
fragments (297 bp) were amplified (62 °C, 35 cycles) by PCR and detected by 2% (w/v) agarose gel electrophoresis. REAL TIME POLYMERASE CHAIN REACTION (RT-QPCR) Total RNA from placental
tissue was isolated using the peqGOLD TriFast reagent (VWR) according to the manufacturer’s protocol. For RNA extraction, small tissue pieces were cut and homogenized using a tissue
homogenizer and the TriFast reagent. RNA quality was assessed on 1.5% (w/v) denaturing agarose gels (Biozym, Vienna, Austria) and stained with GelRed™ Nucleic Acid Gel Stain (Biotium,
Fremont, CA). RNA quantity and purity were assessed using a NanoDrop1000 Spectrophotometer (Thermo Scientific). After the quality check, 2 µg of total RNA was reverse transcribed with the
High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Reverse transcription was performed with and without reverse transcriptase to check for a possible
contamination with genomic DNA. cDNA was employed for quantitative real-time PCR using TaqMan Gene Expression Assays (Applied Biosystems) for human IDO1 [Hs00158027_m1], human CD34
[Hs00990732_m1] and ribosomal protein L30 (RPL30)[Hs00265497_m1] and the TaqMan Universal PCR Mastermix (Applied Biosystems). cDNA and kit compounds were mixed in a total volume of 20 μL per
well (96 well plates, Thermo Scientific) according to the manufacturer’s instructions and amplified using a Bio-Rad CFX96 Real-Time PCR System (Bio-Rad, Vienna, Austria). Ct values were
automatically generated by the CFX Manager 2.0 Software (Bio-Rad). Relative quantification of gene expression was calculated by standard ∆∆Ct method using the expression of CD34 or RPL30.
Data are presented as mean of 2−∆∆Ct values. IDO ACTIVITY ANALYZED BY LIQUID CHROMATOGRAPHY – TANDEM MASS SPECTROMETRY (LC-MS/MS) IDO activity in placental tissue homogenates was measured as
the amount of kynurenine formed from L-Trp using the ascorbate/methylene blue assay as previously described62 with modifications. Placental tissue homogenates were generated by placing
frozen placental tissue (cut into small pieces on dry ice) in 500 μL of 100 mM phosphate buffer pH 7.4 containing 2x protease inhibitor cocktail (Roche Diagnostics, Sydney, Australia) and
homogenizing the tissue using a Heidolph homogenizer. Homogenates were centrifuged at 17,000 x _g_ for 15 min at 4 °C and the supernate used for IDO activity assay. The standard assay
mixture (in 300 μL final volume) consisted of 100 mM phosphate buffer, pH 7.4, containing 200 μL supernate, 200 μM L-Trp, 10 mM ascorbic acid, and 0.2 mg/mL catalase. The reaction was
initiated by adding 15 μL 500 μM methylene blue (25 μM final concentration) to the above reaction mixture, followed by incubation for 30 min at 37 °C. Aliquots (100 μL) were removed at 0 min
(before incubation at 37 °C) and at 30 min and the reaction stopped by the addition of cold trichloroacetic acid (4% (w/v) final concentration). Mixtures were stored on ice for 15 min and
then incubated at 65 °C for a further 15 min to convert N-formyl-kynurenine to kynurenine before centrifugation at 17,000 x _g_ for 15 min at 4 °C. To 25 μL supernate, equal volume of 1 M
phosphate buffer, pH 7.4 was added, the mixture vigorously mixed for 30 s and then centrifuged at 17,000 × _g_ for 5 min at 4 °C. Five microliters of the supernate were then injected onto an
Agilent 1290 UHPLC system connected to an Agilent 6490 triple-quadrupole mass spectrometer equipped with an electrospray ionization source (ESI) operating in positive ion mode. Analytes
were separated on a 5 μm Luna C18 (2) column (30 × 2.1 mm; Phenomenex, USA) by gradient elution using 0.1% acetic (mobile phase A) and 0.1% acetic acid in acetonitrile (mobile phase B) at
0.15 mL/min. The gradient consisted of 0–5% mobile phase B from 0 to 6 min, 5–100% from 6 to 8 min. Mass spectrometer parameters were as follows: gas temperature = 250 °C; gas flow = 20
L/min; nebulizer pressure = 241.3 kPa); sheath gas heater = 325 °C; sheath gas flow = 12 L/min; capillary voltage = 3,500 V. Detection of kynurenine was by multiple reaction monitoring (MRM)
in positive ion mode using the above general mass spectrometry parameters with fragmentor voltage at 380 V and cell accelerator voltage at 5 V. The largest fragment ion generated by
collision-induced dissociation of the [M + H]+ ion were used for quantification with the following transition (parent ion → fragment ion) m/z 209 → 146 with CE = 10 V. Kynurenine was
quantified against a standard curve of authentic commercial standard obtained from SigmaAldrich (USA). STATISTICS Statistical analyses were performed using GraphPadPrism (version 6.01) and R
(version 3.3.2). Data were expressed as mean ± standard deviation. Normality of distribution was assessed by Kolmogorov-Smirnov and Shapiro-Wilk tests. Unpaired data were analysed with
Dunnett’s test for comparisons against one reference treatment, paired non-parametric data with Wilcoxon signed-rank test and paired parametric data with _t_-test. The relaxation percentages
were related to the concentration (mM) using a linear regression model on original scale including treatments, a linear and a quadratic component for the concentration, an interaction term
for treatments and concentration as fixed effects and additionally intercept and concentration as random effects. We will state that two treatments have a different effect if the
coefficients of the linear component differ. P-values less than 0.05 were considered statistical significant. DATA AVAILABILITY The datasets generated during and/or analysed during the
current study are available from the corresponding author on reasonable request. REFERENCES * Sainio, E. L., Pulkki, K. & Young, S. N. L-Tryptophan: Biochemical, nutritional and
pharmacological aspects. _Amino Acids_ 10, 21–47, https://doi.org/10.1007/bf00806091 (1996). Article CAS PubMed Google Scholar * Ball, H. J., Jusof, F. F., Bakmiwewa, S. M., Hunt, N. H.
& Yuasa, H. J. Tryptophan-catabolizing enzymes - party of three. _Front Immunol_ 5, 485, https://doi.org/10.3389/fimmu.2014.00485 (2014). Article PubMed PubMed Central Google Scholar
* Wurtman, R. J., Hefti, F. & Melamed, E. Precursor control of neurotransmitter synthesis. _Pharmacol Rev_ 32, 315–335 (1980). CAS PubMed Google Scholar * Richard, D. M. _et al_.
L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. _International Journal of Tryptophan Research:_. _IJTR_ 2, 45–60 (2009). Article CAS PubMed
PubMed Central Google Scholar * Efimov, I. _et al_. Structure and reaction mechanism in the heme dioxygenases. _Biochemistry_ 50, 2717–2724, https://doi.org/10.1021/bi101732n (2011).
Article CAS PubMed PubMed Central Google Scholar * Rafice, S. A., Chauhan, N., Efimov, I., Basran, J. & Raven, E. L. Oxidation of L-tryptophan in biology: a comparison between
tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. _Biochem Soc Trans_ 37, 408–412, https://doi.org/10.1042/bst0370408 (2009). Article CAS PubMed Google Scholar * Sedlmayr, P.,
Blaschitz, A. & Stocker, R. The role of placental tryptophan catabolism. _Front Immunol_ 5, 230, https://doi.org/10.3389/fimmu.2014.00230 (2014). Article PubMed PubMed Central Google
Scholar * Pantouris, G., Serys, M., Yuasa, H. J., Ball, H. J. & Mowat, C. G. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from
those of indoleamine 2,3-dioxygenase-1. _Amino Acids_ 46, 2155–2163, https://doi.org/10.1007/s00726-014-1766-3 (2014). Article CAS PubMed Google Scholar * Takikawa, O., Kuroiwa, T.,
Yamazaki, F. & Kido, R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the
enzyme-mediated tryptophan degradation in its anticellular activity. _J Biol Chem_ 263, 2041–2048 (1988). CAS PubMed Google Scholar * Mellor, A. L. & Munn, D. H. IDO expression by
dendritic cells: tolerance and tryptophan catabolism. _Nat Rev Immunol_ 4, 762–774, https://doi.org/10.1038/nri1457 (2004). Article CAS PubMed Google Scholar * Mellor, A. L., Keskin, D.
B., Johnson, T., Chandler, P. & Munn, D. H. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. _J Immunol_ 168, 3771–3776 (2002). Article CAS PubMed Google Scholar
* Munn, D. H. _et al_. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. _J Clin Invest_ 114, 280–290,
https://doi.org/10.1172/jci21583 (2004). Article CAS PubMed PubMed Central Google Scholar * Blaschitz, A. _et al_. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms
a positive gradient towards the feto-maternal interface. _PLoS One_ 6, e21774, https://doi.org/10.1371/journal.pone.0021774 (2011). Article ADS CAS PubMed PubMed Central Google Scholar
* Sedlmayr, P. _et al_. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. _Mol Hum Reprod_ 8, 385–391 (2002). Article CAS PubMed Google
Scholar * Munn, D. H. _et al_. Prevention of allogeneic fetal rejection by tryptophan catabolism. _Science_ 281, 1191–1193 (1998). Article ADS CAS PubMed Google Scholar * Mellor, A. L.
_et al_. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. _Nat Immunol_ 2, 64–68 (2001). Article CAS PubMed Google Scholar *
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. _Nat Immunol_ 5, 266–271, https://doi.org/10.1038/ni1037 (2004). Article
CAS PubMed Google Scholar * Wang, Y. _et al_. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. _Nat Med_ 16, 279–285, https://doi.org/10.1038/nm.2092
(2010). Article CAS PubMed PubMed Central Google Scholar * Scifres, C. M. & Nelson, D. M. Intrauterine growth restriction, human placental development and trophoblast cell death. _J
Physiol_ 587, 3453–3458, https://doi.org/10.1113/jphysiol.2009.173252 (2009). Article CAS PubMed PubMed Central Google Scholar * Eiland, E., Nzerue, C. & Faulkner, M. Preeclampsia.
_J Pregnancy_ 2012, 586578, https://doi.org/10.1155/2012/586578 (2012). Article PubMed PubMed Central Google Scholar * Kamimura, S., Eguchi, K., Yonezawa, M. & Sekiba, K.
Localization and developmental change of indoleamine 2,3-dioxygenase activity in the human placenta. _Acta Med Okayama_ 45, 135–139 (1991). CAS PubMed Google Scholar * Liu, X., Liu, Y.,
Ding, M. & Wang, X. Reduced expression of indoleamine 2,3-dioxygenase participates in pathogenesis of preeclampsia via regulatory T cells. _Mol Med Rep_ 4, 53–58,
https://doi.org/10.3892/mmr.2010.395 (2011). Article CAS PubMed Google Scholar * Santoso, D. I. _et al_. Localization of indoleamine 2,3-dioxygenase and 4-hydroxynonenal in normal and
pre-eclamptic placentae. _Placenta_ 23, 373–379, https://doi.org/10.1053/plac.2002.0818 (2002). Article CAS PubMed Google Scholar * Santillan, M. K. _et al_. Pregnant mice lacking
indoleamine 2,3‐dioxygenase exhibit preeclampsia phenotypes. _Physiological Reports_ 3, e12257, https://doi.org/10.14814/phy2.12257 (2015). Article PubMed PubMed Central Google Scholar *
Arbeille, P. Fetal arterial Doppler-IUGR and hypoxia. _Eur J Obstet Gynecol Reprod Biol_ 75, 51–53 (1997). Article CAS PubMed Google Scholar * Lu, L., Kingdom, J., Burton, G. J. &
Cindrova-Davies, T. Placental Stem Villus Arterial Remodeling Associated with Reduced Hydrogen Sulfide Synthesis Contributes to Human Fetal Growth Restriction. _Am J Pathol_ 187, 908–920,
https://doi.org/10.1016/j.ajpath.2016.12.002 (2017). Article CAS PubMed PubMed Central Google Scholar * Robinson, C. M., Shirey, K. A. & Carlin, J. M. Synergistic transcriptional
activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. _J Interferon Cytokine Res_ 23, 413–421, https://doi.org/10.1089/107999003322277829 (2003). Article CAS
PubMed PubMed Central Google Scholar * Trudinger, B. J., Giles, W. B., Cook, C. M., Bombardieri, J. & Collins, L. Fetal umbilical artery flow velocity waveforms and placental
resistance: clinical significance. _Br J Obstet Gynaecol_ 92, 23–30 (1985). Article CAS PubMed Google Scholar * Thompson, R. S. & Trudinger, B. J. Doppler waveform pulsatility index
and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. _Ultrasound Med Biol_ 16, 449–458 (1990). Article CAS PubMed Google
Scholar * Fox, S. B. & Khong, T. Y. Lack of innervation of human umbilical cord. _An immunohistological and histochemical study. Placenta_ 11, 59–62 (1990). CAS PubMed Google Scholar
* Riesenberg, R. _et al_. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. _Clin Cancer Res_
13, 6993–7002, https://doi.org/10.1158/1078-0432.ccr-07-0942 (2007). Article CAS PubMed Google Scholar * Changsirivathanathamrong, D. _et al_. Tryptophan metabolism to kynurenine is a
potential novel contributor to hypotension in human sepsis. _Crit Care Med_ 39, 2678–2683, https://doi.org/10.1097/CCM.0b013e31822827f2 (2011). Article CAS PubMed Google Scholar *
Chadha, P. S. _et al_. In _Proceedings of the BPS Winter Meeting 2013_. 197P. http://www.pa192online.org/abstract/abstract.jsp?abid=31322 (2013). * Pantoja, L. G., Miller, R. D., Ramirez, J.
A., Molestina, R. E. & Summersgill, J. T. Inhibition of Chlamydia pneumoniae replication in human aortic smooth muscle cells by gamma interferon-induced indoleamine 2, 3-dioxygenase
activity. _Infect Immun_ 68, 6478–6481 (2000). Article CAS PubMed PubMed Central Google Scholar * Okamoto, T. _et al_. Transcriptional regulation of indoleamine 2,3-dioxygenase (IDO) by
tryptophan and its analogue: Down-regulation of the indoleamine 2,3-dioxygenase (IDO) transcription by tryptophan and its analogue. _Cytotechnology_ 54, 107–113,
https://doi.org/10.1007/s10616-007-9081-4 (2007). Article CAS PubMed PubMed Central Google Scholar * Metz, R. _et al_. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR:
A novel IDO effector pathway targeted by D-1-methyl-tryptophan. _Oncoimmunology_ 1, 1460–1468, https://doi.org/10.4161/onci.21716 (2012). Article PubMed PubMed Central Google Scholar *
Liu, X. _et al_. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. _Blood_ 115, 3520–3530, https://doi.org/10.1182/blood-2009-09-246124 (2010). Article CAS
PubMed Google Scholar * Pilotte, L. _et al_. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. _Proc Natl Acad Sci USA_ 109, 2497–2502,
https://doi.org/10.1073/pnas.1113873109 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Yuasa, H. J., Ball, H. J., Austin, C. J. & Hunt, N. H. 1-L-methyltryptophan
is a more effective inhibitor of vertebrate IDO2 enzymes than 1-D-methyltryptophan. _Comp Biochem Physiol B Biochem Mol Biol_ 157, 10–15, https://doi.org/10.1016/j.cbpb.2010.04.006 (2010).
Article PubMed Google Scholar * Metz, R. _et al_. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory
compound D-1-methyl-tryptophan. _Cancer Res_ 67, 7082–7087, https://doi.org/10.1158/0008-5472.can-07-1872 (2007). Article CAS PubMed Google Scholar * Cruz, M. A., Gallardo, V., Miguel,
P., Carrasco, G. & Gonzalez, C. Serotonin-induced vasoconstriction is mediated by thromboxane release and action in the human fetal-placental circulation. _Placenta_ 18, 197–204 (1997).
Article CAS PubMed Google Scholar * Silk, J. D. _et al_. IDO induces expression of a novel tryptophan transporter in mouse and human tumor cells. _J Immunol_ 187, 1617–1625,
https://doi.org/10.4049/jimmunol.1000815 (2011). Article CAS PubMed PubMed Central Google Scholar * Bhutia, Y. D., Babu, E. & Ganapathy, V. Interferon-gamma induces a
tryptophan-selective amino acid transporter in human colonic epithelial cells and mouse dendritic cells. _Biochim Biophys Acta_ 1848, 453–462, https://doi.org/10.1016/j.bbamem.2014.10.021
(2015). Article CAS PubMed Google Scholar * Kudo, Y. & Boyd, C. A. Characterisation of L-tryptophan transporters in human placenta: a comparison of brush border and basal membrane
vesicles. _J Physiol_ 531, 405–416 (2001). Article CAS PubMed PubMed Central Google Scholar * Drenzek, J. G. _et al_. Expression of indoleamine 2,3-dioxygenase in the rhesus monkey and
common marmoset. _J Reprod Immunol_ 78, 125–133, https://doi.org/10.1016/j.jri.2008.03.005 (2008). Article CAS PubMed Google Scholar * Honig, A. _et al_. Indoleamine 2,3-dioxygenase
(IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. _J Reprod Immunol_ 61, 79–86, https://doi.org/10.1016/j.jri.2003.11.002 (2004).
Article CAS PubMed Google Scholar * Kudo, Y. _et al_. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. _J Reprod Immunol_ 61, 87–98,
https://doi.org/10.1016/j.jri.2003.11.004 (2004). Article CAS PubMed Google Scholar * Ligam, P., Manuelpillai, U., Wallace, E. M. & Walker, D. Localisation of indoleamine
2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy. _Placenta_ 26, 498–504,
https://doi.org/10.1016/j.placenta.2004.08.009 (2005). Article CAS PubMed Google Scholar * Kudo, Y., Boyd, C. A. R., Sargent, I. L. & Redman, C. W. G. Decreased tryptophan catabolism
by placental indoleamine 2,3-dioxygenase in preeclampsia. _American Journal of Obstetrics and Gynecology_ 188, 719–726, https://doi.org/10.1067/mob.2003.156 (2003). Article CAS PubMed
Google Scholar * Iwahashi, N. _et al_. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. _J Reprod Immunol_
119, 54–60, https://doi.org/10.1016/j.jri.2017.01.003 (2017). Article CAS PubMed Google Scholar * Evans, R. W. _et al_. Maternal and fetal amino acid concentrations and fetal outcomes
during pre-eclampsia. _Reproduction_ 125, 785–790 (2003). Article CAS PubMed Google Scholar * Fujigaki, H., Seishima, M. & Saito, K. Posttranslational modification of indoleamine
2,3-dioxygenase. _Anal Bioanal Chem_ 403, 1777–1782, https://doi.org/10.1007/s00216-012-5946-2 (2012). Article CAS PubMed Google Scholar * Thomas, S. R. _et al_. Post-translational
regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. _J Biol Chem_ 282, 23778–23787, https://doi.org/10.1074/jbc.M700669200 (2007). Article CAS PubMed Google Scholar
* Yuan, W., Collado-Hidalgo, A., Yufit, T., Taylor, M. & Varga, J. Modulation of cellular tryptophan metabolism in human fibroblasts by transforming growth factor-beta: selective
inhibition of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase gene expression. _J Cell Physiol_ 177, 174–186, https://doi.org/10.1002/(SICI)1097-4652(199810)177:1 174::AID-JCP18
3.0.CO;2-D (1998). Article CAS PubMed Google Scholar * Figueras, F. & Gratacos, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a
stage-based management protocol. _Fetal Diagn Ther_ 36, 86–98, https://doi.org/10.1159/000357592 (2014). Article PubMed Google Scholar * Herlihy, J. T. & Murphy, R. A. Length-tension
relationship of smooth muscle of the hog carotid artery. _Circ Res_ 33, 275–283 (1973). Article CAS PubMed Google Scholar * Wareing, M., Crocker, I. P., Warren, A. Y., Taggart, M. J.
& Baker, P. N. Characterization of small arteries isolated from the human placental chorionic plate. _Placenta_ 23, 400–409, https://doi.org/10.1053/plac.2002.0825 (2002). Article CAS
PubMed Google Scholar * Schneider, H. & Huch, A. Dual _in vitro_ perfusion of an isolated lobe of human placenta: method and instrumentation. _Contrib Gynecol Obstet_ 13, 40–47 (1985).
CAS PubMed Google Scholar * Finch, A. M. _et al_. The effect of C5a and U46619 on the isolated, perfused human placental lobule: development of a method for the online estimation of
tissue fluid accumulation. _J Pharmacol Toxicol Methods_ 34, 133–141 (1995). Article CAS PubMed Google Scholar * Mathiesen, L. _et al_. Quality assessment of a placental perfusion
protocol. _Reprod Toxicol_ 30, 138–146, https://doi.org/10.1016/j.reprotox.2010.01.006 (2010). Article CAS PubMed Google Scholar * Lang, I. _et al_. Human fetal placental endothelial
cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. _Differentiation_ 76, 1031–1043,
https://doi.org/10.1111/j.1432-0436.2008.00302.x (2008). Article CAS PubMed Google Scholar * Thomas, S. R., Mohr, D. & Stocker, R. Nitric oxide inhibits indoleamine 2,3-dioxygenase
activity in interferon-gamma primed mononuclear phagocytes. _J Biol Chem_ 269, 14457–14464 (1994). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was funded in
part by the Oesterreichische Nationalbank (Oesterreichische Nationalbank, Anniversary Fund, project number: 15671, to P.S.) and by the Austrian Science Foundation (FWF, P27166-B23 to S.F.).
PhD candidate Pablo Zardoya Laguardia received funding from the Medical University of Graz within the PhD Program Molecular Medicine. R.S. is supported by the National Health & Medical
Research Council of Australia (Grants 1020400 and 1052616 and Fellowship 1111632). We are grateful to Amin El-Heliebi (Biobank of the Medical University of Graz) for providing the tissues,
Osamu Takikawa for donating anti-IDO1 antibody, and to Bettina Amtmann and Petra Winkler for collecting the placentae. We are indebted to Gernot Desoye, Mark Wareing and Paul Brownbill for
helpful discussions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Gottfried Schatz Research Centre for Cell Signalling, Metabolism and Ageing, Department of Cell Biology, Histology and
Embryology, Medical University of Graz, Graz, 8010, Austria Pablo Zardoya-Laguardia, Astrid Blaschitz, Ingrid Lang, Liudmila Nikitina, Martin Gauster & Peter Sedlmayr * Medical
University of Graz, Department of Obstetrics and Gynaecology, Graz, 8036, Austria Birgit Hirschmugl, Martin Häusler, Mila Cervar-Zivkovic & Christian Wadsack * Medical University of
Graz, Institute for Medical Informatics, Statistics and Documentation, Graz, 8036, Austria Sereina A. Herzog * Medical University of Graz, Institute of Pathology, Graz, 8036, Austria Eva
Karpf * Victor Chang Cardiac Research Institute, Darlinghurst, NSW 2010, Australia Ghassan J. Maghzal, Chris P. Stanley & Roland Stocker * St Vincent’s Clinical School, UNSW Medicine,
Sydney, NSW 2052, Australia Ghassan J. Maghzal & Roland Stocker * Gottfried Schatz Research Centre for Cell Signalling, Metabolism and Ageing, Department of Molecular Biology and
Biochemistry, Medical University of Graz, Graz, 8010, Austria Saša Frank Authors * Pablo Zardoya-Laguardia View author publications You can also search for this author inPubMed Google
Scholar * Astrid Blaschitz View author publications You can also search for this author inPubMed Google Scholar * Birgit Hirschmugl View author publications You can also search for this
author inPubMed Google Scholar * Ingrid Lang View author publications You can also search for this author inPubMed Google Scholar * Sereina A. Herzog View author publications You can also
search for this author inPubMed Google Scholar * Liudmila Nikitina View author publications You can also search for this author inPubMed Google Scholar * Martin Gauster View author
publications You can also search for this author inPubMed Google Scholar * Martin Häusler View author publications You can also search for this author inPubMed Google Scholar * Mila
Cervar-Zivkovic View author publications You can also search for this author inPubMed Google Scholar * Eva Karpf View author publications You can also search for this author inPubMed Google
Scholar * Ghassan J. Maghzal View author publications You can also search for this author inPubMed Google Scholar * Chris P. Stanley View author publications You can also search for this
author inPubMed Google Scholar * Roland Stocker View author publications You can also search for this author inPubMed Google Scholar * Christian Wadsack View author publications You can also
search for this author inPubMed Google Scholar * Saša Frank View author publications You can also search for this author inPubMed Google Scholar * Peter Sedlmayr View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.Z.L. was involved in design and sample collection, performed the majority of the experiments, acquisition and
interpretation of the data and wrote the first draft of the article. A.B. contributed immunohistochemistry staining, B.H. conducted the placental perfusion experiments, I.L. supported with
knowledge in primary cell isolation, S.A.H. performed part of the statistical data analysis, L.N. did a quantitative PCR experiment, M.G. contributed with RT-qPCR expertise and mRNA data
analysis, M.H. and M.C. were in charge of the diagnosis of the samples obtained. E.K. provided the expertise in pathology, G.J.M. and R.S. were responsible for the LC-MS/MS experiments, and
R.S. provided useful advice including unpublished data suggestive of the decrease in IDO1 expression, activity and L-Trp -mediated arterial relaxation in IUGR and PE. C.P.S. helped towards
performing myography experiments, C.W. and S.F. contributed to the experimental planning and critical discussion. P.S. coordinated the research and revised the manuscript. All authors read
and approved the final version of the paper. CORRESPONDING AUTHOR Correspondence to Peter Sedlmayr. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY FIGURES ONLINE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Zardoya-Laguardia, P., Blaschitz, A., Hirschmugl, B. _et al._ Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient
in intrauterine growth restriction and pre-eclampsia. _Sci Rep_ 8, 5488 (2018). https://doi.org/10.1038/s41598-018-23896-0 Download citation * Received: 08 May 2017 * Accepted: 21 March 2018
* Published: 03 April 2018 * DOI: https://doi.org/10.1038/s41598-018-23896-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative