Hydrothermal transformation of snse crystal to se nanorods in oxalic acid solution and the outstanding thermoelectric power factor of se/snse composite
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The present work demonstrates the synthesis of one-dimensional (1D) Se nanorods with ~50 nm diameter by hydrothermal transformation of SnSe crystals in oxalic acid solution and
suggests the reaction mechanism for this chemical transformation. SnSe particles react with oxalic acid to generate numerous Se nuclei, which crystallize into Se nanorods due to the
intrinsic character of the 1D growth of Se. The resulting Se/SnSe composite exhibits outstanding thermoelectric power factor without the aid of any rare dopants, which is higher than both
undoped polycrystalline SnSe and SnSe doped with Pb and Cu. SIMILAR CONTENT BEING VIEWED BY OTHERS THE IMPACT OF VARYING EDTA DOSAGE ON THE FLOWER-LIKE MORPHOLOGY AND THERMOELECTRIC
PROPERTIES OF CE-DOPED BI2TE₃ Article Open access 19 September 2024 OXIDATION-INDUCED THERMOPOWER INVERSION IN NANOCRYSTALLINE SNSE THIN FILM Article Open access 15 January 2021 WATER
MEDIATED GROWTH OF ORIENTED SINGLE CRYSTALLINE SRCO3 NANOROD ARRAYS ON STRONTIUM COMPOUNDS Article Open access 09 February 2021 INTRODUCTION Se is a versatile element in the chalcogenide
group that has been widely studied in various fields including chemistry, medicine, ceramics, electronics, and metallurgy. Recent advances in nanotechnology enable preparation of Se
nanostructures _via_ various synthesis techniques, and efforts to fabricate Se-based nanostructured semiconducting devices is highly essential to develop future technology because these
low-dimensional nanostructures can replace bulk materials in various applications due to their outstanding properties1,2,3,4,5. Zhang _et al_. and Liu _et al_. reported the synthesis of Se
nanoparticles that are applied to high-performance Li-Se batteries6,7. Nanosized Se particles used as a sensor for the effective detection of materials were fabricated by Zapp _et al_. and
Ahmed _et al_.8,9. In another study, Chang _et al_. prepared Se nanospheres combined with Au nanorods for the efficient application of cancer radiochemotherapy10. Se nanostructures can be
prepared by various synthetic techniques like hydrothermal synthesis, photocatalytic process, electrochemical method, vapor-phase growth, template-assisted synthesis, etc.11. Chemical
transformation is one of the strategies to achieve Se nanostructures with desired composition, dimension, and morphology. Transformations based on ion exchange12,13 and Kirkendall
effect14,15 are popular strategies used by researchers to successfully obtain target materials. However, unlike these transformations, a stabilizer-depleted transformation enables the
fabrication of unary nanostructures from binary compounds. Tang _et al_. fabricated variously shaped Te and Se nanocrystals with highly monodisperse sizes from cadmium telluride (CdTe) and
cadmium selenide (CdSe) nanoplates using ethylenediaminetetraacetate (EDTA) and L-cysteine as stabilizing agents16,17. Zhang _et al_. reported the transformation of antimony telluride
(Sb2Te3) nanoplates to Te nanoplates using tartaric acid with O2 18. However till date, only a few attempts are made to obtain pure and unary nanostructures using stabilizer-depleted
chemical transformation procedure. This article reports a prospective strategy to synthesize unary Se nanostructures from tin selenide (SnSe) bulk crystals by chemical transformation
reaction in oxalic acid solution. The formation of an intermediate complex between SnSe and oxalic acid, followed by its oxidation, yields Se nanostructures. One-dimensional (1D) growth of
Se nanostructures is observed after the nucleation because of the intrinsic anisotropy of Se, resulting in the fabrication of Se nanorods (NRs). The thermoelectric properties of the product
are also investigated and compared to those of other chalcogenide-based high-performance thermoelectric materials to confirm the potential use of the Se-based material in thermoelectric
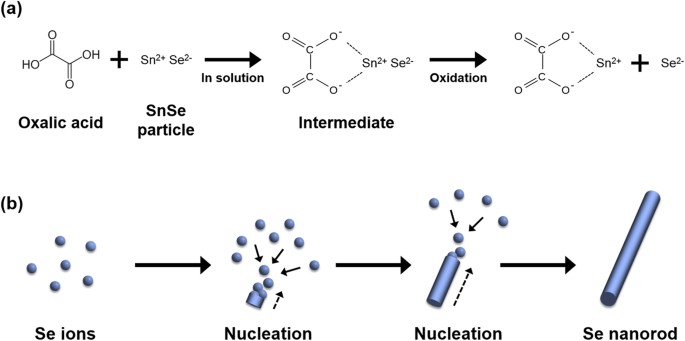
applications. RESULTS AND DISCUSSION The mechanism for the transformation procedure of SnSe to Se NR is schematically shown in Fig. 1. The reaction between ball-milled SnSe particles and
oxalic acid in aqueous solution is triggered under hydrothermal condition, leading to the formation of a complex intermediate, as represented in equation (1). $${C}_{2}{H}_{2}{O}_{4}+SnSe\to
SnSe{({C}_{2}{O}_{4})}^{2-}+2\,{H}^{+}$$ (1) This step is followed by oxidation of the metastable intermediate according to equation (2), rendering many Se nuclei in solution.
$$2\,SnSe{({C}_{2}{O}_{4})}^{2-}+{O}_{2}+2\,{H}_{2}O\to 2\,Sn{({C}_{2}{O}_{4})}^{2-}+2\,Se+4\,O{H}^{-}$$ (2) Figure 1b shows the schematic for fabrication of Se NRs from Se ions through
nucleation and subsequent 1D growth owing to the intrinsic nature of Se to grow anisotropic 1D structure11. Fourier-transform infrared (FT-IR) spectroscopic analysis was performed to
characterize the structure of oxalic acid in solution during the transformation to prove the proposed mechanism. Figure 2a shows the FT-IR spectra of aqueous solution before and after the
reaction. A large peak at ~3400 cm−1 for the solution before the reaction indicates the O-H vibrations of oxalic acid19,20. However, the intensity of the peak at ~3400 cm−1 reduces after the
transformation reaction because the O-H bonds of oxalic acid are broken during the course of the reaction, resulting in the formation of an intermediate complex, as represented in Fig. 1a.
X-ray diffraction (XRD) and thermogravimetric analysis (TGA) results further confirm the transformation process of SnSe. Figure 2b shows the XRD patterns of the SnSe powders before (pristine
SnSe) and after the reaction (Se/SnSe). Pristine SnSe powder shows diffraction patterns typical of the orthorhombic _Pnma_ crystal structure (JCPDS #48-1224) with no additional XRD
peak21,22,23, indicating the existence of pure phase of SnSe. In contrast, the sample collected after the reaction exhibits a second pattern of XRD peaks in addition to the primary
diffraction pattern of _Pnma_ crystal, originating from the pristine Se (shown in Fig. S1 in Electronic Supplementary Information), which confirms the presence of transformed Se particles in
the product. Figure 2c shows the TGA thermograms for the pristine SnSe and Se/SnSe samples. The pristine SnSe, being a single component, exhibits outstanding thermal stability up to a
temperature of 900 K while the Se/SnSe sample shows a thermal degradation at ~700 K, which is attributed to the transformed Se crystals. This observation is in line with the results of XRD
analysis. Field-emission scanning electron microscopy (FE-SEM) images for the samples before and after the reaction further prove the chemical transformation of SnSe to Se NRs. The low- and
high-magnification FE-SEM images (Fig. 2d and e) of the SnSe powder show the presence of ball-milled SnSe nanoparticles with sizes ranging from ~200 to 500 nm. Both low- and
high-magnification FE-SEM images of the chemically transformed sample display the coexistence of randomly distributed nanostructures comprising SnSe nanoparticles and 1D NRs (Figs 2f,g, and
S2 in Electronic Supplementary Information). The NRs synthesized _via_ chemical transformation were further identified by field-emission transmission electron microscopy (FE-TEM). Figures S3
and 3a and d (in Electronic Supplementary Information) show the FE-SEM and low-magnification FE-TEM images of single NR, exhibiting 1D structure with a diameter of ~50 nm. The
high-magnification FE-TEM image and the corresponding selected area electron diffraction (SAED) patterns shown in Fig. 3b and e indicate that the distances between the lattice fringes are
~0.5 and ~0.38 nm, corresponding to the (0 0 1) and (1 0 0) planes of the Se NR (Fig. S1 in Electronic Supplementary Information). The direction of (0 0 1) in the SAED is parallel to the
axis of NR, signifying the unidirectional growth of the NR crystal along the (0 0 1) plane, owing to the highly anisotropic structure of Se demonstrated in Fig. 1. The hexagonal crystal
structures of individual Se NR can be schematically illustrated by symmetric facets represented in Fig. 3c and f; this is in agreement with the previous discussion. Furthermore, Fig. 3g–i
display FE-SEM images and the corresponding energy-dispersive X-ray spectroscopy (EDS) mapping of Sn and Se atoms of Se NR, that validates the unary composition of the fabricated Se NR. The
EDS spectrum of the single NR shown in Fig. S4 (in Electronic Supplementary Information) also exhibits strong characteristic peaks of Se, and only weak peaks of Sn, which is in line with
earlier observations. These results confirm that the fabricated 1D NRs originating from SnSe are Se NRs and support the proposed chemical transformation mechanism described in Fig. 1. The
SnSe-based materials are known to exhibit outstanding thermoelectric properties24,25,26,27. In order to demonstrate the potential use of the fabricated Se/SnSe nanostructures as a
thermoelectric material, Se/SnSe sample was pelletized to examine its thermoelectric properties, and the results were compared with those of previously reported high-performance SnSe-based
thermoelectric materials. FE-SEM analysis can provide the microstructural morphology of the Se/SnSe pellet. Figure S5a shows the surface FE-SEM image of the pressed Se/SnSe pellet, revealing
flat surface of the sample. High-magnification cross-sectional FE-SEM image of the Se/SnSe pellet (Fig. S5b) demonstrates that the fabricated Se nanorods are randomly distributed in the
SnSe matrix, as indicated by the white arrows. Figure 4a shows the measured electrical resistivity (_ρ_) of the Se/SnSe sample and the reported data25,26,27 for SnSe-based materials as a
function of temperature. The _ρ_ of Se/SnSe exhibits an initial drop with increasing temperature with a subsequent increase in _ρ_ value beyond the temperature of ~400 K, similar to the
trends displayed by typical semiconductors. Additionally, it might be noted that the _ρ_ value of Se/SnSe is lower than that of un-doped polycrystalline SnSe25, but higher than that of Pb,
Cu, and Ag doped SnSe26,27. Figure 4b displays positive Seebeck coefficients (_S_) for the SnSe based materials indicating p-type semiconductor behaviors. The _S_ value of Se/SnSe increases
from ~550 μV/K to a maximum of ~610 μV/K at about 500 K, higher than those of the other undoped and doped SnSe-based materials. The high Seebeck coefficients in the Se/SnSe sample may be due
to the potential barrier scattering of carriers at interfaces between Se and SnSe particles. Generally, the low-energy carriers cause to reduce the Seebeck coefficient, hence, their
filtering at the interfaces could contribute to improve the Seebeck coefficient28,29,30. The maximum thermoelectric power factor (_S_ 2/_ρ_) of the Se/SnSe sample is ~233 μW/m·K2 at 400 K
(Fig. 4c), which is less than that of the optimized Ag-doped SnSe because of the relatively lower electrical resistivity of Ag-doped SnSe originating from the electrically conductive Ag
atoms. However, the power factor of Se/SnSe nanomaterial is higher than both undoped polycrystalline SnSe, and SnSe doped with Pb and Cu. Measurements on five different samples prepared
independently further demonstrate the experimental reproducibility of this outstanding power factor value exhibited by the fabricated Se/SnSe nanomaterial (Fig. S6 in Electronic
Supplementary Information). Therefore, this work describes a hydrothermal synthesis method to transform SnSe crystals to 1D Se NRs that displays remarkable thermoelectric properties. This
study has two-fold importance as it demonstrates a fabrication procedure of 1D Se NRs from SnSe crystals _via_ a chemical transformation, and emphasizes the potential use of resulting
Se/SnSe nanostructure as an outstanding thermoelectric material. Se NRs were chemically transformed by treating milled SnSe crystals with oxalic acid solution. The oxalic acid reacts with
the SnSe particle under a hydrothermal condition, resulting in the formation of a complex intermediate, which upon oxidation, renders Se nuclei in the solution that combine together to
crystallize into Se NRs due to their intrinsic nature of 1D growth. FE-TEM images confirm that the Se NRs exhibit 1D structure with a diameter of ~50 nm. Thermoelectric properties of the
Se/SnSe sample were examined and compared with those of previously reported SnSe-based thermoelectric materials. The outstanding thermoelectric power factor value of Se/SnSe sample is higher
than both undoped polycrystalline SnSe, and SnSe doped with Pb and Cu, which could be attributed to the interfacial carrier scattering effect of low-energy carriers between Se and SnSe
particles. This fabrication method and remarkable thermoelectric property of product can have potential application in various research areas and development of novel semiconducting devices.
METHODS PREPARATION OF SAMPLES SnSe crystals (99.999%, Alfa Aesar) were ball-milled into small particles using zirconia balls in an inert atmosphere. The rotation speed of the planetary
mill was set to 150 rpm to generate a rolling action of the balls, which applied shearing forces to the materials. 29 mg of SnSe powder was added to an aqueous solution containing 180 mg of
oxalic acid (C2H2O4) and 100 mL of DI water. After vigorous stirring, the mixture was transferred into a Teflon-lined autoclave and sealed. The vessel was then heated to 443 K for 2 h to
carry out the chemical transformation. The final product was collected and washed several times with dilute HCl solution and ethanol followed by vacuum drying in the oven. The dried product
was pressed for 10 min at 823 K under 50 MPa to obtain Se/SnSe samples. CHARACTERIZATION Fourier-transform infrared (FT-IR, Bio-rad FTS-1465) spectra of the samples were obtained with an
average of 32 scans in the 500–4000 cm−1 radiation region. X-ray diffraction (XRD, New D8-Advance/Bruker-AXS) at 40 mA and 40 kV, with Cu K_α_ radiation (0.154056 nm) and a scan rate of 1°/s
for 2θ ranging from 5–70°, was used to characterize the crystal structure of the materials. Thermogravimetric analysis (TGA, TGA-2050, TA Instruments) was used to investigate the thermal
degradation of the samples that were heated at a rate of 10 K·min−1 under N2 atmosphere. The morphology of the materials was characterized by field-emission scanning electron microscopy
(FE-SEM, SIGMA) and field-emission transmission electron microscopy (FE-TEM, JEM-2100F). The elemental mappings of the samples were performed by energy-dispersive X-ray spectroscopy (EDS,
NORAN system 7, Thermo Scientific). A four-point probe method with disk-shaped compressed pellets was used to investigate the electrical resistivity. A homemade device containing a pair of
thermocouples and voltmeters was used to measure the Seebeck coefficient. Five samples of the final product were prepared for the reproducibility of experiments, and the average values were
reported in the manuscript. DATA AVAILABILITY All data generated or analyzed during this study are included in this paper including Supplementary Information. Raw datasets are available from
the corresponding author on reasonable request. REFERENCES * Cademartiri, L. & Ozin, G. A. Ultrathin nanowires—a materials chemistry perspective. _Adv. Mater._ 21, 1013–1020 (2009).
Article CAS Google Scholar * Sannicolo, T. _et al_. Metallic nanowire‐based transparent electrodes for next generation flexible devices: a Review. _Small_ 12, 6052–6075 (2016). Article
CAS PubMed Google Scholar * Li, Z., Sun, Q., Yao, X. D., Zhu, Z. H. & Lu, G. Q. M. Semiconductor nanowires for thermoelectrics. _J. Mater. Chem._ 22, 22821–22831 (2012). Article CAS
Google Scholar * Fang, X.-Q., Liu, J.-X. & Gupta, V. Fundamental formulations and recent achievements in piezoelectric nano-structures: a review. _Nanoscale_ 5, 1716–1726 (2013).
Article ADS CAS PubMed Google Scholar * Liu, Y. _et al_. Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and
supercapacitor applications. _ACS Appl. Mater. Interfaces_ 6, 2174–2184 (2014). Article ADS CAS PubMed Google Scholar * Zhang, J. _et al_. Graphene–encapsulated selenium/polyaniline
core–shell nanowires with enhanced electrochemical performance for Li–Se batteries. _Nano Energy_ 13, 592–600 (2015). Article CAS Google Scholar * Liu, L. _et al_. Nanoporous selenium as
a cathode material for rechargeable lithium–selenium batteries. _Chem. Commun._ 49, 11515–11517 (2013). Article CAS Google Scholar * Zapp, E., Nascimento, V., Dambrowski, D., Braga, A. L.
& Vieira, I. C. Bio-inspired sensor based on glutathione peroxidase mimetic for hydrogen peroxide detection. _Sens. Actuators, B_ 176, 782–788 (2013). Article CAS Google Scholar *
Ahmed, S., Brockgreitens, J., Xu, K. & Abbas, A. A Nanoselenium Sponge for Instantaneous Mercury Removal to Undetectable Levels. _Adv. Funct. Mater._ 27, 1606572 (2017). Article Google
Scholar * Chang, Y. _et al_. Designing core–shell gold and selenium nanocomposites for cancer radiochemotherapy. _ACS Nano_ 11, 4848–4858 (2017). Article CAS PubMed Google Scholar *
Chaudhary, S., Umar, A. & Mehta, S. Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. _Prog. Mater. Sci._ 83, 270–329
(2016). Article CAS Google Scholar * Son, D. H., Hughes, S. M., Yin, Y. & Alivisatos, A. P. Cation exchange reactions in ionic nanocrystals. _Science_ 306, 1009–1012 (2004). Article
ADS CAS PubMed Google Scholar * Sines, I. T. _et al_. Engineering porosity into single-crystal colloidal nanosheets using epitaxial nucleation and chalcogenide anion exchange reactions:
The conversion of SnSe to SnTe. _Chem. Mater._ 24, 3088–3093 (2012). Article CAS Google Scholar * Knez, M. _et al_. Monocrystalline spinel nanotube fabrication based on the Kirkendall
effect. _Nat. Mater._ 5, 627–631 (2006). Article ADS PubMed Google Scholar * Henkes, A. E., Vasquez, Y. & Schaak, R. E. Converting metals into phosphides: a general strategy for the
synthesis of metal phosphide nanocrystals. _J. Am. Chem. Soc._ 129, 1896–1897 (2007). Article CAS PubMed Google Scholar * Tang, Z., Wang, Y., Sun, K. & Kotov, N. A. Spontaneous
transformation of stabilizer‐depleted binary semiconductor nanoparticles into selenium and tellurium nanowires. _Adv. Mater._ 17, 358–363 (2005). Article CAS Google Scholar * Tang, Z.,
Wang, Y., Shanbhag, S., Giersig, M. & Kotov, N. A. Spontaneous transformation of CdTe nanoparticles into angled Te nanocrystals: from particles and rods to checkmarks, X-marks, and other
unusual shapes. _J. Am. Chem. Soc._ 128, 6730–6736 (2006). Article CAS PubMed Google Scholar * Zhang, H. _et al_. Conversion of Sb2Te3 hexagonal nanoplates into three‐dimensional porous
single‐crystal‐like network‐structured Te plates using oxygen and tartaric acid. _Angew. Chem. Int. Ed._ 51, 1459–1463 (2012). Article CAS Google Scholar * Socrates, G. _Infrared and
Raman Characteristic Group Frequencies: Tables and Charts_. John Wiley & Sons, New York, USA (2004). * Lin-Vien, D., Colthup, N. B., Fateley, W. G. & Grasselli, J. G. _The Handbook
of Infrared and Raman Characteristic Frequencies of Organic Molecules_. Elsevier, Boston, USA (1991). * Butt, F. K. _et al_. Synthesis of mid-infrared SnSe nanowires and their optoelectronic
properties. _CrystEngComm_ 16, 3470–3473 (2014). Article CAS Google Scholar * Ju, H. & Kim, J. Chemically exfoliated SnSe nanosheets and their
SnSe/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) composite films for polymer based thermoelectric applications. _ACS Nano_ 10, 5730–5739 (2016). Article CAS PubMed Google
Scholar * Asfandiyar _et al_. Thermoelectric SnS and SnS-SnSe solid solutions prepared by mechanical alloying and spark plasma sintering: Anisotropic thermoelectric properties. _Sci. Rep._
7, 43262 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Zhao, L.-D. _et al_. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals.
_Nature_ 508, 373–377 (2014). Article ADS CAS PubMed Google Scholar * Han, Y.-M. _et al_. Thermoelectric performance of SnS and SnS–SnSe solid solution. _J. Mater. Chem. A_ 3, 4555–4559
(2015). Article CAS Google Scholar * Singh, N. K. _et al_. The effect of doping on thermoelectric performance of p-type SnSe: Promising thermoelectric material. _J. Alloys Compd._ 668,
152–158 (2016). Article CAS Google Scholar * Chen, C.-L., Wang, H., Chen, Y.-Y., Day, T. & Snyder, G. J. Thermoelectric properties of p-type polycrystalline SnSe doped with Ag. _J.
Mater. Chem. A_ 2, 11171–11176 (2014). Article CAS Google Scholar * Martin, J., Wang, L., Chen, L. & Nolas, G. Enhanced Seebeck coefficient through energy-barrier scattering in PbTe
nanocomposites. _Phys. Rev. B_ 79, 115311 (2009). Article ADS Google Scholar * Ko, D.-K., Kang, Y. & Murray, C. B. Enhanced thermopower via carrier energy filtering in
solution-processable Pt–Sb2Te3 nanocomposites. _Nano Lett._ 11, 2841–2844 (2011). Article ADS CAS PubMed Google Scholar * Mao, J. _et al_. High thermoelectric power factor in Cu–Ni
alloy originate from potential barrier scattering of twin boundaries. _Nano Energy_ 17, 279–289 (2015). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported by NRF (National Research Foundation of Korea) Grant funded by the Korean Government (NRF-2016H1A2A1908830-Fostering Core Leaders of the Future Basic Science Program/Global Ph.D.
Fellowship Program) and also supported by the NRF Grant funded by the Ministry of Education (2017R1D1A1B03029212). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Chemical
Engineering & Materials Science, Chung-Ang University, Seoul, 06974, Republic of Korea Hyun Ju, Dabin Park & Jooheon Kim Authors * Hyun Ju View author publications You can also
search for this author inPubMed Google Scholar * Dabin Park View author publications You can also search for this author inPubMed Google Scholar * Jooheon Kim View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.J. designed the study. D.P. synthesized samples. H.J. characterized the prepared samples and measured thermoelectric
properties. H.J. analyzed the investigated thermoelectric properties and wrote the manuscript. J.K. supervised the project. CORRESPONDING AUTHOR Correspondence to Jooheon Kim. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ju, H., Park, D. & Kim, J. Hydrothermal
transformation of SnSe crystal to Se nanorods in oxalic acid solution and the outstanding thermoelectric power factor of Se/SnSe composite. _Sci Rep_ 7, 18051 (2017).
https://doi.org/10.1038/s41598-017-18508-2 Download citation * Received: 18 October 2017 * Accepted: 13 December 2017 * Published: 22 December 2017 * DOI:
https://doi.org/10.1038/s41598-017-18508-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative