Mycobacterium smegmatis pafbc is involved in regulation of dna damage response
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Two genes, _pafB_ and _pafC_, are organized in an operon with the Pup-ligase gene _pafA_, which is part of the Pup-proteasome system (PPS) present in mycobacteria and other
actinobacteria. The PPS is crucial for _Mycobacterium tuberculosis_ resistance towards reactive nitrogen intermediates (RNI). However, _pafB_ and _pafC_ apparently play only a minor role in
RNI resistance. To characterize their function, we generated a _pafBC_ deletion in _Mycobacterium smegmatis (Msm)_. Proteome analysis of the mutant strain revealed decreased cellular levels
of various proteins involved in DNA damage repair, including recombinase A (RecA). In agreement with this finding, _Msm_ Δ_pafBC_ displayed increased sensitivity to DNA damaging agents. In
mycobacteria two pathways regulate DNA repair genes: the LexA/RecA-dependent SOS response and a predominant pathway that controls gene expression via a LexA/RecA-independent promoter, termed
P1. PafB and PafC feature winged helix-turn-helix DNA binding motifs and we demonstrate that together they form a stable heterodimer _in vitro_, implying a function as a heterodimeric
transcriptional regulator. Indeed, P1-driven transcription of _recA_ was decreased in _Msm_ Δ_pafBC_ under standard conditions and induction of _recA_ expression upon DNA damage was strongly
impaired. Taken together, our data indicate an important regulatory function of PafBC in the mycobacterial DNA damage response. SIMILAR CONTENT BEING VIEWED BY OTHERS NOVEL WYL
DOMAIN-CONTAINING TRANSCRIPTIONAL ACTIVATOR ACTS IN RESPONSE TO GENOTOXIC STRESS IN RAPIDLY GROWING MYCOBACTERIA Article Open access 02 December 2023 AN RNA-BINDING PROTEIN ACTS AS A MAJOR
POST-TRANSCRIPTIONAL MODULATOR IN _BACILLUS ANTHRACIS_ Article Open access 21 March 2022 INTRACELLULAR LOCALIZATION OF THE MYCOBACTERIAL STRESSOSOME COMPLEX Article Open access 12 May 2021
INTRODUCTION _Mycobacterium tuberculosis_ (_Mtb_), the causative agent of tuberculosis, is one of the most successful human pathogens and represents a major global health problem. _Mtb_
features a multitude of mechanisms that allow the phagocytozed bacteria to withstand the host’s innate and adaptive immune response and enable them to persist inside the host macrophages1,2.
After successful immune evasion the pathogen can persist in the human for decades. A hallmark of activated macrophages is the production of reactive oxygen species and nitric oxide, which
damage bacterial proteins, lipids and nucleic acids3,4. Thus, in order to maintain the integrity of its genome, _Mtb_ relies on robust mechanisms of DNA damage repair. Whereas in most
bacteria the genes involved in these processes are regulated as part of the so-called SOS response, mycobacteria were shown to make use of an additional, independent regulatory
mechanism5,6,7,8. In the SOS response pathway the key regulators are LexA and RecA. Under standard growth conditions the repressor LexA prevents transcription of DNA repair genes by binding
to a specific sequence within their promoter, the SOS box9. RecA senses DNA damage by binding to single-strand DNA that is generated as a result, thereby forming nucleoprotein filaments.
This in turn activates RecA to promote auto-catalytic cleavage of the repressor LexA9. As a consequence LexA levels initially decrease and transcription of DNA damage repair genes is
de-repressed10,11. With only few exceptions, _Mtb_ harbours the genes found in _Escherichia coli_ to mediate a fully functional SOS response12,13. Early studies demonstrated that the _Mtb
recA_ gene, which itself is regulated by LexA, contains a second, LexA-independent promoter (usually referred to as P1) located more proximal to the _recA_ start codon5,6,7. Mutations in the
Lex-dependent promoter P2 that abolish its activity do not abrogate basal _recA_ transcription, and RecA expression remains inducible by the DNA damaging agent mitomycin C6. Strikingly, the
majority of DNA damage inducible genes in _Mtb_ are controlled in a LexA/RecA-independent manner, including also many members of the LexA-regulon8. In an _Mtb recA_ deletion strain,
induction of many DNA repair genes upon exposure to mitomycin C is either the same as in wild type or is only partly decreased. Bioinformatic analysis identified a consensus sequence in the
upstream region of these genes (tTGTCRgtg - 8 nt - TannnT) that resembles the _recA_ P1 promoter and is responsible for the observed LexA-independent regulation14. A recent study in _Msm_
showed that the _clp_ gene regulator (ClgR; MSMEG_2694/Rv2745c) recognizes the P1 promoter region15. Moreover, deletion of _clgR_ in _Msm_ results in impaired induction of various genes
related to DNA repair mechanisms under DNA damaging conditions. This indicates a regulatory function of ClgR in the LexA/RecA-independent DNA damage repair pathway in mycobacteria. A
post-translational protein modification pathway akin to ubiquitination contributes to the survival of _Mtb_ in host macrophages. In this modification pathway termed pupylation, mycobacteria
(and other actinobacteria) make use of the small ubiquitin-like protein Pup to target proteins for degradation by the bacterial proteasome16,17. Pup is covalently attached via its C-terminal
glutamate to lysine side chains of proteasomal substrates by action of the Pup ligase PafA18,19. Before ligation can occur, Pup, which in all mycobacteria is encoded with a C-terminal
glutamine, must be rendered competent for ligation through deamidation of its C-terminal glutamine residue by the enzyme Dop (deamidase of Pup)19,20. Toward pupylated substrates Dop can act
as a depupylase21,22, thereby counteracting the ligase activity. Pupylated proteins are recognized by the proteasomal ATPase ring, termed Mpa (mycobacterial proteasomal ATPase) in
mycobacteria, which unfolds the substrate and translocates it into the proteolytic chamber of the proteasome complex23,24,25. The genes encoding the core proteins involved in pupylation and
proteasomal degradation are clustered together in the so-called Pup-proteasome gene locus. In all mycobacterial species the gene _pafA_ encoding the Pup ligase is organized in an operon
together with two other genes, _pafB_ and _pafC_ 26. _Mtb_ PafB and PafC were shown to copurify, suggesting that they might form a complex26. Deletion of the coding genes does not affect
pupylation in _Mtb_ and therefore has no influence on the degradation of proteasomal substrates. Unlike an _Mtb pafA_ deletion mutant, a strain lacking _pafBC_ displays only a slight
decrease in resistance against nitrosative stress _in vitro_ 26,27. However, PafB and PafC were linked to conjugal DNA transfer in _Msm_ 28. Deletion of the corresponding genes does not
impair the donor strain’s ability to transfer DNA. In contrast, the corresponding recipient strain is strongly compromised in DNA uptake. A recent study indicates that PafC contributes to
the intrinsic resistance of mycobacteria to fluoroquinolone antibiotics29. However, the underlying mechanism for any of the observed phenotypes remained elusive. In order to understand the
biological function of PafB and PafC, we generated a _pafBC_ deletion mutant of _Msm SMR5_ and employed a comparative proteomic approach using iTRAQ (Isobaric Tags for Relative and Absolute
Quantitation in mass spectrometry). Notably, a large part of the proteins that displayed decreased cellular levels in the deletion mutant are associated with DNA repair, including RecA.
Accordingly, deletion of _pafBC_ resulted in increased sensitivity towards DNA damaging agents, and induction of _recA_ transcription in _Msm_ Δ_pafBC_ was strongly impaired under DNA
damaging growth conditions. Strikingly, whereas the LexA/RecA-dependent _recA_ promoter P2 remained inducible to wild type levels, induction of P1-driven transcription was completely
blocked. Our findings establish _pafBC_ as a new regulatory element in the LexA/RecA-independent DNA damage response and provide new insights into RecA regulation as well as the control of
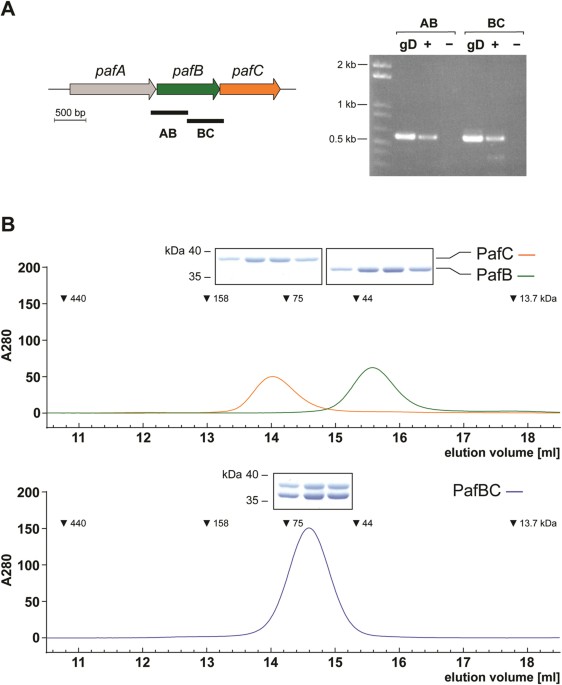
DNA repair mechanisms in mycobacteria. RESULTS _M. SMEGMATIS_ PAFB AND PAFC FORM A STABLE HETERODIMER AND FEATURE DNA BINDING MOTIFS The genes encoding PafB and PafC are located downstream
of the Pup ligase gene _pafA_. Like in its pathogenic relative _Mtb_ 26, these three genes are co-transcribed in _M_. _smegmatis_ as evidenced by the presence of a single mRNA encoding both
PafC and PafB in the _Msm_ wild type strain (Fig. 1A). Sequence analyses of PafB and PafC showed that they exhibit similarities to a multitude of bacterial transcription factors featuring a
so-called winged helix-turn-helix (wHTH) DNA-binding motif in their N-terminal region (Supplementray Fig. S1). The wHTH motif is typically comprised of three helices forming a compact
helical bundle, which is capped by an adjacent hairpin (“wing”) (Supplementray Fig. S1)30. Binding to DNA is usually mediated by insertion of helix α3 into the major groove (Supplementray
Fig. S1). Furthermore, PafB and PafC contain a C-terminal WYL domain. This domain is often found in association with wHTH motifs and is possibly involved in protein dimerization and/or the
binding of ligands, which thereby trigger a specific transcriptional response31. Indeed, our _in vitro_ analysis of the assembly state of _Msm_ PafB and PafC demonstrates that the two
proteins form a heterodimer (Fig. 1B). Analytical gel filtration profiles of PafB and PafC expressed and purified separately (Fig. 1B, upper panel) indicate that PafB (36 kDa) with an
apparent molecular weight of 40 kDa runs as a monomer, while PafC (34 kDa) runs at an apparent molecular weight of 84 kD, possibly due to homodimer formation in absence of PafB. However,
when PafB and PafC are co-expressed and co-purified (Fig. 1B, lower panel), a single main elution peak shifted away from either of the positions of the individual proteins is detected at an
apparent molecular weight in agreement with a PafBC heterodimer. Consistent with this interpretation, analysis of the elution peak in SDS-PAGE reveals that it contains equal amounts of PafB
and PafC. Taken together, bioinformatic analysis along with the observed stable heterodimer formation between PafB and PafC imply that the two proteins might act as a heterodimeric
transcriptional regulator in mycobacteria. GENERATION OF A _M. SMEGMATIS PAFBC_ DELETION STRAIN As an initial step towards understanding the role of PafBC in mycobacteria we generated a
markerless deletion of the _pafBC_ coding genes in the model organism _Mycobacterium smegmatis_ (_Msm_). The mutant was obtained by transformation of _Msm SMR5_, the streptomycin resistant
parent strain carrying a point mutation in the ribosomal protein S12 coding gene (_rpsL_) with the suicide plasmid _pMCS_Δ_pafBC-hyg-rpsL_ carrying the _rpsL_ + gene and subsequent
application of the _rpsL_ counterselection strategy (Fig. 2A)32,33. Disruption of _pafBC_ was confirmed by Southern blot analysis, and the parent strain is hereafter referred to as _Msm_
wild type, the deletion strain as _Msm_ Δ_pafBC_. The used probe hybridized to a 2.6-kbp-fragment in the wild type strain and to a 6.5-kbp-fragment in the _pafBC_ deletion mutant (Fig. 2B).
A complemented strain was generated by transforming _Msm_ Δ_pafBC_ with the integrative plasmid _pMV361_-_hyg_-_pafBC_ carrying a fragment consisting of the presumable promoter region of the
_pafABC_ operon fused to _Msm pafBC_. The complementation vector re-established cellular levels of PafBC similar to wild type (Fig. 2C). _In vitro_ growth of the _pafBC_ deletion mutant did
not differ from _Msm_ wild type when grown under standard conditions at 37 °C (Supplementray Fig. S2). Since _pafB_ and _pafC_ form an operon with the Pup-ligase encoding gene _pafA_ (Fig.
1A) we investigated whether pupylation is affected by the absence of PafBC. Anti-Pup immunoblot analysis of cell-free lysates deriving from _Msm_ wild type or _Msm_ Δ_pafBC_ grown under
standard conditions revealed no significant differences in the band pattern or band intensities of the pupylated proteome (pupylome), suggesting that PafA pupylation activity remains
unaffected (Fig. 3A). In accordance with this, we found that deletion of _pafB_ and _pafC_ did not alter the relative cellular amount of _pafA_ mRNA (Fig. 3B). Similar findings were made for
other proteins involved in the Pup-proteasome pathway, namely Dop, Mpa, PrcA and PrcB, which all displayed unchanged cellular protein levels in the _Msm_ Δ_pafBC_ mutant strain
(Supplementray Fig. S3). DELETION OF _PAFBC_ AFFECTS THE CELLULAR LEVELS OF PROTEINS INVOLVED IN DNA REPAIR PATHWAYS, TRANSCRIPTION AND RECOMBINATION In order to gain insights into the
cellular function of PafBC, a quantitative proteomic analysis of the _Msm_ Δ_pafBC_ mutant in comparison to the wild type strain was performed using iTRAQ. We identified a total of 2554
proteins (corresponding to 38.2% of all annotated _Msm_ proteins) in three biological replicates deriving from _Msm_ wild type and Δ_pafBC_ cultures grown to late exponential phase. In _Msm_
Δ_pafBC_ 54 proteins were found to be down-regulated and 17 proteins to be up-regulated at least 1.5-fold compared to _Msm_ wild type (Table 1). Among the affected proteins, 11 can be
assigned to DNA-related functions, namely DNA repair, recombination and transcription. The strongest decrease was observed for RecA, with a relative reduction of about 2.5 fold in the
_pafBC_ deletion mutant. Accordingly, when cell-free lysates deriving from cells grown under the conditions of the iTRAQ experiment were analysed by an anti-RecA immunoblot, the signal for
RecA, in the Δ_pafBC_ background was significantly reduced compared to either _Msm_ wild type or the complemented deletion strain (Fig. 4). Further analysis showed that RecA levels are
controlled by concomitant action of PafB and PafC. Expression of either _pafB_ or _pafC_ alone in _Msm_ Δ_pafBC_ did not affect RecA expression as compared to the mutant strain, while
co-expression of _pafB_ and _pafC_ resulted in a significant increase of cellular RecA levels (Supplementray Fig. S4). DOWN-REGULATION OF RECA IN _MSM_ Δ_PAFBC_ OCCURS AT THE TRANSCRIPTIONAL
LEVEL The changes in cellular levels of various proteins in _Msm_ Δ_pafBC_ implied a transcriptional regulation of the corresponding coding genes. It should be noted, however, that some of
the down-regulated proteins found in our iTRAQ analysis were previously identified as possible or, like RecA, genuine targets of the pupylation pathway. In addition, we find that RecA
accumulates in a proteasome-deficient strain of _Msm_ (Fig. 5A, bottom blot), also indicating that RecA is degraded via the Pup-proteasome pathway in _Msm_. However, the significantly
reduced levels of RecA in the _Msm_ Δ_pafBC_ mutant strain (Fig. 4, second lane) cannot be due to increased degradation of the protein, since _Msm_ wild type and _Msm_ Δ_pafBC_ exhibit
similar turn-over rates for a C-terminally His6-tagged RecA variant expressed in either of the strains (Fig. 5A, upper and middle blot). This result strongly supports the notion that
transcription of _recA_ in _Msm_ is either directly or indirectly regulated by PafBC. Carrying out relative quantification by qRT-PCR, we found _recA_ transcript levels decreased by about
36% in the _Msm_ Δ_pafBC_ mutant strain as compared to the parental strain (Fig. 5B, upper panel). In a concomitant control, we checked the transcriptional levels of another player involved
in DNA damage repair, namely _uvrB_ 34, whose corresponding gene product was not covered by the analysed iTRAQ samples. In analogy to _recA_, expression _uvrB_ decreased by 37% in _Msm_
Δ_pafBC_ compared to wild type (Fig. 5B, lower panel). Next, we examined whether PafBC influences transcription of _recA_ by binding to its promoter region. To this end, an electrophoretic
mobility shift assay (EMSA) was performed using purified _Msm_ PafBC and a DNA fragment comprising the 300 bp upstream of the _recA_ gene. Indeed, PafBC was found to bind to the _recA_
promoter region (Supplementray Fig. S5). However, PafBC displays a rather promiscuous binding behaviour _in vitro_ and comparable interaction with different promoter regions used as negative
controls in the assay was observed (Supplementray Fig. S5). Various attempts to increase specificity by testing different buffer conditions were not successful. Therefore, the EMSA did not
allow for a final conclusion concerning the binding of PafBC to the _recA_ promoter region. DELETION OF _PAFBC_ RENDERS _M. SMEGMATIS_ MORE SUSCEPTIBLE TO DNA DAMAGING CONDITIONS Like in
other prokaryotes, RecA in mycobacteria holds a key role in homologous recombination and was shown to be a crucial element in regulating the SOS response upon DNA damage10,35,36. Thus, the
observed decrease of RecA prompted us to perform a phenotypical analysis of the _Msm_ Δ_pafBC_ mutant strain under DNA-damaging conditions. In the growth experiments DNA stress was emulated
by exposing the bacterial cultures to the DNA interstrand crosslinking agent mitomycin C. In both, _Mtb_ and _Msm_, _recA_ was shown to be upregulated upon exposure to mitomycin C5,37,38. In
an initial approach we determined the minimal inhibitory concentration (MIC) of mitomycin C for _Msm_ wild type to be 160 ng/ml and 4 ng/ml for the _pafBC_ deletion strain. We subsequently
performed a survival assay using 50% of the MIC for _Msm_ wild type. The _Msm_ wild type strain, _Msm_ Δ_pafBC_ and the complemented strain were grown in shaking cultures and mitomycin C was
added at an OD of 0.1. After 8 h, serial dilutions of the respective cultures were spotted onto LB agar plates without mitomycin C. _Msm_ wild type and the complemented strain were only
mildly affected by mitomycin C, when compared to the controls grown in absence of the agent (Fig. 6A). In contrast, the number of viable cells in cultures of the _pafBC_ deletion mutant
decreased by about two orders of magnitude upon exposure to mitomycin C. Similar observations were made when _Msm_ Δ_pafBC_ was exposed to UV irradiation. After a UV dose of 25 mJ/cm2
viability of the deletion strain decreased by approximately two orders of magnitude as compared to the _Msm SMR5_ parental strain (Fig. 6B). INDUCTION OF _RECA_ UNDER DNA-DAMAGING CONDITIONS
IS IMPAIRED IN _MSM_ Δ_PAFBC_ The increased sensitivity of the _Msm_ Δ_pafBC_ mutant towards mitomycin C and UV radiation strongly implied that the induction of _recA_, which is usually
observed under DNA damaging conditions5, is hampered in the _Msm_ Δ_pafBC_ mutant. Indeed, when we performed an anti-RecA immunoblot analysis, levels of RecA increased significantly in _Msm_
wild type after the exposure to mitomycin C (Fig. 7). In contrast, the _pafBC_ deletion mutant exhibited significantly impaired upregulation of RecA levels under the same DNA damaging
growth conditions (Fig. 7A), which could be rescued by complementation with _pafBC_. In addition, we addressed the question whether induction of _recA_ under DNA damaging conditions is
accompanied by upregulation of PafBC. Therefore, _Msm_ wild type grown in the presence of mitomycin C was analysed by an anti-PafBC Western Blot (Fig. 7B). However, no alterations in PafBC
levels were observed compared to cells grown under standard conditions. PAFBC AFFECTS TRANSCRIPTIONAL CONTROL OF _RECA_ VIA THE LEXA/RECA-INDEPENDENT PROMOTER In contrast to other bacteria,
_recA_ is controlled by two promoters in mycobacteria: a LexA/RecA-dependent promoter (P2), which is activated as part of the classical SOS response, and a LexA/RecA-independent promoter
(P1). Transcriptional activity of both, the P1 and the P2 promoter, was previously shown to be inducible by mitomycin C6,7. As we observed a decrease of RecA levels in _Msm_ Δ_pafBC_ under
both standard and DNA damaging conditions, we addressed the question through which of the two promoters PafBC affects transcription of the corresponding gene. We constructed reporter strains
of _Msm_ wild type and _Msm_ Δ_pafBC_ that allowed us to monitor transcriptional activities of P1 and P2 independently. A DNA fragment encompassing the entire _recA_ promoter region and the
first 11 codons of _recA_ was fused to GFP (Fig. 8A). Either P1 or P2 was subsequently mutated in order to determine the individual contributions of the two promoter sites with respect to
the presence of PafBC. The constructs were introduced into the genome of the respective strain by an integrative vector. In the _Msm_ wild type strain reporting on the activity of the entire
P1/P2 promoter region, GFP fluorescence was observed to be about 6-fold higher in presence of mitomycin C than in the absence of the agent (Fig. 8B). In the Δ_pafBC_ strain the mitomycin
C-induced response was markedly reduced and resulted only in an approximately 3-fold increase reaching less than half of the wild type fluorescence value. When P2 was inactivated, the
response to mitomycin C stress could only be observed for the wild type strain. In contrast, mutation of P1 retained the response to mitomycin C in both strains, although the Δ_pafBC_ strain
displayed only about three-fourths of the wild type level. The weaker P2 response of the Δ_pafBC_ strain may be explained by the fact that P2 activity is dependent on RecA6, whose level was
shown is significantly reduced in the Δ_pafBC_ strain (Figs 4A and 7A). In absence of mitomycin C, GFP fluorescence in the Δ_pafBC_ strain was approximately half of the wild type level for
the reporter constructs exhibiting an intact P1 promoter, while GFP fluorescence reached the same level under standard conditions when P1 was mutated. Together, these findings clearly
demonstrate that P1-driven transcription of recA is absent in the pafBC deletion mutant while P2-driven transcription remains largely unaffected. DISCUSSION The response to DNA damage in
mycobacteria is regulated by two independent mechanisms. Besides the ubiquitous LexA/RecA-dependent SOS response, most DNA damage-inducible genes in mycobacterial species are controlled in a
LexA/RecA-independent manner via the P1 promoter5,6,7. Notably, some genes, like e.g. _recA_, are regulated by both mechanisms8,14. The presented study broadens the scope of P1-driven
transcription of DNA repair genes by adding PafBC as a novel regulatory player to the DNA damage response in mycobacteria. The goal of our study was to gain insights into the physiological
function of _pafB_ and _pafC_ in mycobacteria. Since these genes form an operon with the Pup-ligase coding gene _pafA_ (Fig. 1A)26 in mycobacteria, they were generally considered to be
associated with the Pup-proteasome gene locus. The formation of a heterodimeric complex (Fig. 1B) and the presence of a winged helix-turn-helix DNA-binding motif at the N-termini of PafB and
PafC (Fig. S1) indicated a regulatory function at the transcriptional level. However, under the conditions tested we found no influence of PafBC on any of the components known to be
involved in pupylation or proteasomal degradation (Fig. 3, Supplementray Fig. S3). Rather, quantitative proteome analyses revealed that levels of various proteins related to DNA damage
repair were altered in a PafBC-dependent manner (Table 1). Accordingly, _Msm_ Δ_pafBC_ displayed an increased susceptibility to DNA damaging agents (Fig. 6). Finally, using the example of
RecA, we demonstrated that PafBC exerts its influence on transcription of DNA-damage inducible genes at the level of the LexA/RecA-independent P1-promoter (Figs 5B and 8). To our knowledge,
this is the first study showing a role of PafBC in DNA damage response. Disruption of _pafB_ or _pafC_ in _Msm_ was previously shown to hamper a recipient strain’s ability to take up and/or
integrate DNA via homologous recombination (HR) into its genome during conjugation28. RecA is a key element in HR, as it binds to the DNA strands and catalyzes strand exchange, which
eventually results in recombination. In the iTRAQ analysis RecA displayed the highest decrease in cellular levels of those proteins that could be identified in the _Msm pafBC_ deletion
strain (Table 1). Thus, against the background of this observation the impact of disrupted _pafB_ or _pafC_ on conjugation can be attributed to impaired RecA-dependent HR rather than
impaired DNA uptake. Very recently, a _M. smegmatis_ mutant that carries a transposon insertion in _pafC_ was shown to be hypersensitive to various fluoroquinolones, like moxifloxacin or
ciprofloxacin29. The data presented here provide an explanation for the increased sensitivity. Quinolones target topoisomerase IV and DNA gyrase and their action on these enzymes eventually
results, amongst others, in multiple DNA strand breaks39. Thus, while _Msm_ wild type is able to at least partially compensate the action of quinolones by a functional DNA damage repair, the
impaired response of Δ_pafBC_ to DNA damaging conditions (Figs 6 and 7) leads to increased sensitivity to these drugs. At least under _in vitro_ conditions PafBC binding lacks specificity
as reflected by the results of our EMSA approach (Supplementray Fig. S4). It is possible that additional mechanisms might exist _in vivo_ to bring about specificity. Many transcription
factors are targeted by post-translational modifications like phosphorylation or acetylation that modulate their binding affinity40,41. For example, VirS, the transcription factor that
regulates the mycobacterial monooxygenase (_mymA_) operon, is phosphorylated by the kinase PknK42. Phosphorylation increases the affinity of VirS for the _mymA_ promoter. Furthermore, PafB
and PafC feature a WYL domain in their C-terminal half. WYL domains were previously postulated to act in ligand sensing and binding31. It is therefore conceivable that PafBC relies on an
additional interaction partner in order to specifically bind its genuine target, which is absent in the _in vitro_ EMSA experiment. It could be argued that PafBC does not act as a specific
transcriptional regulator but might function analogous to members of the Dps (DNA binding protein from starved cells) protein family43. Dps proteins are upregulated under various stress
conditions and in several species were shown to bind DNA without apparent sequence specificity, thereby shielding the DNA molecule from damaging agents44,45,46. The fact that PafBC did not
exhibit a change in cellular levels under DNA damaging conditions, makes such a mode of function very unlikely (Fig. 7B). Furthermore, most factors with wHTH motifs interact with specific
DNA sequences, while DNA binding of Dps is mediated simply by the positive charge of N-terminal lysine residues47,48. The involvement of PafBC in regulating the DNA damage response in _Msm_
is clearly supported by our data. However, it remains elusive how the signal of DNA damage is eventually translated into PafBC-dependent transcriptional control. Regulation of DNA
repair-related genes by PafBC could occur directly by binding to the P1 promoter, or indirectly by e.g. affecting the transcription of other regulators. Recently, the _clp_ gene regulator
ClgR was shown to recognize the LexA/RecA-independent P1 promoter15. Analogous to our observation with the _Msm_ Δ_pafBC_ mutant, upregulation of _recA_ and other genes involved in DNA
repair is impaired in an _Msm_ Δ_clgR_ mutant upon exposure to DNA damaging conditions15. The coding genes of 15 proteins that displayed altered levels in _Msm_ upon deletion of _pafBC_
either harbour the P1 consensus motif recognized by ClgR in the promoter region or are part of operons which bear the motif in their promoter region (Table S1). Based on these observations
different scenarios regarding P1-driven transcription are conceivable: i) PafBC and ClgR act independently on the P1 promoter, but share certain targets, like e.g. _recA_, which are
regulated by one or the other protein, depending on distinct cellular signals and/or additional transcriptional factors ii) transcription is regulated by concerted binding to the P1 promoter
by both, PafBC and ClgR iii) the impact of PafBC could be indirect by affecting transcriptional activity of _clgR_. The function of PafBC is apparently not restricted to DNA damage. This is
clearly indicated by the large number of proteins (about 80% of the proteins identified in our iTRAQ analysis) whose expression is influenced by PafBC independent of the P1 promoter and
that are not related to DNA repair (Table 1).This points towards a more global impact of PafBC in the cell’s regulatory networks. In accordance with an earlier study we did not find any
influence of PafBC on pupylation or proteasomal degradation at least under the conditions tested (Fig. 3 and Supplementray Fig. S3)26. Nevertheless, the organization of _pafB_ and _pafC_ in
an operon with _pafA_ implies functional coupling and therefore a role for PafBC in the Pup-proteasome system (PPS). Interestingly, conditional expression of ClgR in _Mtb_ results in the
induction of _prcB_ and _prcA_ 49. Given the possible link of PafBC and ClgR indicated above, it is therefore tempting to speculate that these two regulators together might modulate levels
of the PPS upon a particular cellular signal. In summary, our study assigns the first known function to the genes _pafB_ and _pafC_ in _Msm_. The elucidation of the function of PafBC as a
heterodimeric transcriptional regulator involved in the LexA/RecA-independent mycobacterial DNA damage response adds a new facet to the intricate system of DNA damage control in
mycobacteria. Yet, the co-occurrence of _pafBC_ with the Pup-proteasome gene locus remains elusive. This association could have evolved since both systems - DNA repair and
Pup-proteasome-dependent protein degradation - might play a role under specific stress conditions and are therefore regulated by overlapping regulatory mechanisms. Whether PafBC and the
Pup-proteasome system indeed share also a more direct functional link remains to be further scrutinized. METHODS BACTERIAL STRAINS, OLIGONUCLEOTIDES, MEDIA AND GROWTH CONDITIONS The
oligonucleotides used in this study are summarized in table S2. Strains used were _Mycobacterium smegmatis_ (_Msm_) SMR5, _Msm_ Δ_pafBC_ and the complemented strain _Msm_ Δ_pafBC_-_pafBC_
(see following section). Bacteria were grown in LB medium supplemented with 0.05% (v/v) TWEEN-80 (LB-T) or 7H9 medium (Difco Laboratories) without OADC supplement. When appropriate,
hygromycin B was added to a final concentration of 100 µg ml−1. Growth curves of _Msm_ SMR5, _Msm_ Δ_pafBC_ and _Msm_ Δ_pafBC_-_pafBC_, were measured in 24-well plates in a total volume of
0.5 ml. OD600 measurements were carried out in a Synergy H1 Hybrid Multi-Mode Microplate Reader (Biotek). For proteomic analyses, strains were grown in 7H9 medium without OADC. Cells were
routinely grown at 37 °C in a shaking incubator at 140 rpm. DISRUPTION OF PAFBC IN M. SMEGMATIS To generate a markerless deletion of _pafBC_ in _Msm_ the knock-out plasmid
pMCS-_hyg-rpsL_-Δ_pafBC_ was generated as follows: a 1.5 kbp _Nco_I/_Hind_III fragment encompassing the 5’ _pafBC_ flanking sequence and a 1.5 kbp _Hind_III/_Nde_I fragment encompassing the
3’ flanking sequence were amplified from _Msm_ chromosomal DNA. The PCR products were cut with the respective restriction enzymes and were cloned into the vector pMCS-_hyg-rpsL_. Competent
cells of streptomycin-resistant _Msm_ mc2155 SMR5 were transformed with the knock-out plasmid and the _rpsL_ counterselection strategy32,33 was applied to delete a 1.9 kbp fragment
comprising _pafB_ and _pafC_. Deletion of the genes was confirmed by PCR and Southern blot analysis, using a _pafA_ specific 0.5 kbp probe. _Msm_ Δ_pafBC_ was complemented with the
integrative plasmid pMV361-_hyg-amp_ containing the genes encoding wild type PafB and PafC fused to 300 bp upstream sequence of the _pafABC_ operon, encompassing the native promoter.
PREPARATION OF CELL-FREE LYSATES AND IMMUNOBLOT ANALYSES Cells were harvested by centrifugation and were washed once with 1 Vol. PBS supplemented with 0.05% (v/v) TWEEN-80. Pellets were then
resuspended in lysis buffer containing 100 mM Tris-HCl pH 8, 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 1 mM MgCl2, 1 mM PMSF, supplemented with 1x protease inhibitor cocktail (Sigma-Aldrich), DNase
and RNase. Cells were lysed using a FastPrep-24 Bead Beater (MP Biomedicals; 2 × 30 s at 6 m/s, 0.15mm zirconia beads). Cell debris and insoluble material was removed by centrifugation
(16,000 × g, 5 min, 4 °C). Protein concentration of cell-free lysates was determined by the Bradford assay. Lysates were routinely separated by SDS-PAGE and were subjected to immunoblot
analyses, using polyclonal anti-PafBC antiserum (1:1,000) raised against purified _Msm_ PafBC (Eurogentec), anti-Pup antiserum (1:10,000)19 or anti-His6-tag antibody (1:10,000; Bethyl
Laboratory, Montgomery, TX, USA). For detection a goat anti-rabbit IgG alkaline phosphatase antibody-horseradish peroxidase (HRP) (BioRad) or a rabbit anti-mouse IgG alkaline phosphatase
antibody-HRP (Promega) was used together with Amersham ECL Prime Western Blotting Detection Reagent (1:1 ratio; GE Healthcare Life Sciences) as a substrate for the horseradish peroxidase. To
control for equal loading, membranes were stripped and control immunoblots were performed using a monoclonal antibody that was raised against the RNA polymerase subunit β (RpoB) of _E.
coli_ (1:7,500; GeneTex). For immunoblotting of RecA, cells were harvested by centrifugation and were washed once with 700 µl PBS. Pellets were then resuspended in lysis buffer (PBS
supplemented with 1 mM PMSF, 1x c0mplete (Roche) protease inhibitor), bead beaten and centrifuged as above. Lysates were separated by SDS-PAGE and were subjected to immunoblot analyses,
using commercially available anti-RecA antibody (1:1,000, MBL International, clone ARM414) raised in mouse and commercially available anti-RpoB antibody (1:1,000, BioLegend, clone 8RB13)
raised in mouse. For detection, HRP-conjugated anti-mouse IgG antibody (1:250,000, abcam #ab6789) together with Amersham ECL Select Western Blotting Detection Reagent (GE Healthcare Life
Sciences) was used. DETERMINATION OF THE MINIMAL INHIBITORY CONCENTRATION (MIC) MICs were determined as described50. Briefly, cultures of _Msm_ SMR5 and _Msm_ Δ_pafBC_ were grown to mid-log
phase in LB-T. Cultures were diluted to an OD600 of 0.01 and aliquots of 50 µl were transferred to 96-well plates containing two-fold serial dilutions of mitomycin C, resulting in a final
concentration ranging from 500 to 1 ng ml−1. Each strain was tested in triplicates in two independent experiments. Plates were incubated at 37 °C. The MIC was defined as the mitomycin C
concentration at which no visible growth was observed after three days of incubation. ASSESSING SURVIVAL AFTER MITOMYCIN C AND UV EXPOSURE _Msm_ SMR5, _Msm_ Δ_pafBC_ (both carrying the empty
plasmid pMV361-_hyg-amp_) and the complemented strain _Msm_ Δ_pafBC_-_pafBC_ were grown in LB-T to OD600 of 1.0 and were subsequently diluted to an OD600 of 0.1. Cultures were supplemented
with 80 ng ml−1 mitomycin C. Controls were grown in the absence of mitomycin C. After 8 h of incubation, serial dilutions of the cultures were spotted onto LB agar containing 100 µg ml−1
hygromycin B. Plates were incubated at 37 °C for 3 days and survival was assessed by comparing control samples to the samples deriving from mitomycin C-treated cultures. For the UV
irradiation assay, serial dilutions of freshly grown cultures (OD600 = 1.0) were spotted onto LB agar plates containing 100 µg ml−1 hygromycin B. Plates were exposed to a UV dose of 25
mJ/cm2 using a Stratalinker UV Crosslinker 2400 (Stratagene). Control samples were not exposed to UV. Plates were incubated for 3 days at 37 °C. Survival was assessed by comparing colonies
grown on the respective treated and untreated plates. GFP-REPORTER ASSAY TO MONITOR RECA P1 AND P2 TRANSCRIPTIONAL ACTIVITY To monitor activities of the _recA_ P1 and P2 promoter, a 419 bp
DNA fragment, comprising 386 bp upstream sequence and the first 11 codons of _recA_ was fused to the GFP gene and was inserted in a pMyNT-derived integrative plasmid. pMyNT was kindly
provided by A. Geerlof (EMBL Hamburg). To assess the individual activity of each promoter, the P1 or P2 promoter motif was replaced with a stretch of the coding sequence of mouse histone
H2Ab2 of identical size and similar GC content. The vectors were transformed into _Msm_ SMR5 and _Msm_ Δ_pafBC_. Three individual colonies of each reporter strain were grown in 7H9
supplemented with hygromycin B (50 µg ml−1) to an OD600 of 0.7 at 37 °C. Cultures were exposed to DNA-damaging stress by the addition of mitomycin C (80 ng/ml) and were grown for an
additional 4 h. Control cultures were grown in the absence of mitomycin C. Cells were harvested by centrifugation and cell-free lysates were prepared by resuspension of the pellet in 700 µl
ice-cold lysis buffer (PBS supplemented with 1 mM PMSF, 1x c0mplete EDTA-free (Roche) protease inhibitor, 1 mM EDTA) and bead beating as described above for the RecA immunoblots. Protein
content was determined by the Bradford assay. GFP fluorescence was measured in a black 96-well half-area plate (30 µl lysate/well) with a Synergy2 microplate reader (BioTek) instrument
(excitation filter 485/20, emission filter 528/20). RECA DEGRADATION ASSAY The gene encoding an N-terminally His6-tagged variant of _Msm recA_ was cloned into the pMyC plasmid under the
control of an acetamide-inducible promoter. _Msm_ SMR5, _Msm_ Δ_pafBC_ and _Msm_ ΔprcB carrying pMyC-His6-_recA_ were grown in 7H9 medium to an OD600 of 1.0. Acetamide (final concentration
0.02% w/v) was added to the cultures to induce _recA_ expression for 1 h. Cells were washed three times with PBS supplemented with 0.05% TWEEN-80 (v/v). Then cells were resuspended in 7H9
medium without acetamide and incubation of cultures continued for 9 h. Samples were withdrawn at the given time points. Cell-free lysates were prepared as described above. Equal protein
concentrations were loaded and separated by SDS-PAGE for subsequent immunoblot analysis with an anti-His6-tag antibody in order to monitor His6-RecA degradation. RNA ISOLATION AND
QUANTITATIVE REAL-TIME PCR For RNA isolation strains were grown to an OD600 of 2.6 in 7H9 medium without OADC. Cells were pelleted by centrifugation and were resuspended in an equal volume
of Trizol-reagent (Life Technologies). The samples were subsequently lysed by bead beating using Lysing MatrixB tubes and a Fast Prep-24 device (MP Biomedicals). Supernatants were used for
isolation of RNA with the RNA mini kit (Bio & Sell) according to the manufacturer’s instructions. DNA contaminations were removed by treating isolated RNA with DNase I (Thermo Fisher
Scientifc). Total RNA concentration was determined by using a NanoDrop spectrophotometer (Thermo Fisher Scientific). To synthesize cDNA from _Msm_ RNA the High Capacity RNA-to-DNA Kit
(Applied Biosystems) was used according to the manufacturer’s instructions. Primers for qRT-PCR were designed with the Primer3 software51. An equivalent of ~10 ng cDNA was analysed by
qRT-PCR using 5x QPCR MIX EvaGreen (Bio&Sell) in a 7500 Fast System (Applied Biosystems). Cycling conditions were as follows: 1 cycle of 95 °C for 15 min, 40 cycles of 95 °C for 15 s, 60
°C for 20 s, and 72 °C for 30 s, followed by a melting curve for each sample. cDNA derived from at least three independent biological replicates and was measured in technical triplicates,
respectively. The relative expression levels were calculated according to Pfaffl52 using the 16 S rRNA gene as the reference. PROTEOMIC ANALYSIS BY ITRAQ Cell lysates were prepared as
described above from cultures of _Msm_ SMR5 and _Msm_ Δ_pafBC_ grown to an OD600 of 2.5. 100 µg of proteins were digested and labelled according to the iTRAQTM manufacturing protocol (AB
Sciex). A detailed description of the iTRAQ analysis can be found in the Supplementary information. REFERENCES * Abdallah, A. M. _et al_. Type VII secretion–mycobacteria show the way. _Nat
Rev Microbiol_ 5, 883–891 (2007). Article CAS PubMed Google Scholar * Gorna, A. E., Bowater, R. P. & Dziadek, J. DNA repair systems and the pathogenesis of _Mycobacterium
tuberculosis_: varying activities at different stages of infection. _Clin Sci (Lond)_ 119, 187–202 (2010). Article CAS Google Scholar * MacMicking, J., Xie, Q. W. & Nathan, C. Nitric
oxide and macrophage function. _Annu Rev Immunol_ 15, 323–350 (1997). Article CAS PubMed Google Scholar * Flynn, J. L. & Chan, J. Immunology of tuberculosis. _Annu Rev Immunol_ 19,
93–129 (2001). Article CAS PubMed Google Scholar * Movahedzadeh, F., Colston, M. J. & Davis, E. O. Determination of DNA sequences required for regulated _Mycobacterium tuberculosis_
RecA expression in response to DNA-damaging agents suggests that two modes of regulation exist. _J Bacteriol_ 179, 3509–3518 (1997). Article CAS PubMed PubMed Central Google Scholar *
Davis, E. O. _et al_. DNA damage induction of recA in _Mycobacterium tuberculosis_ independently of RecA and LexA. _Mol Microbiol_ 46, 791–800 (2002). Article CAS PubMed Google Scholar *
Gopaul, K. K., Brooks, P. C., Prost, J. F. & Davis, E. O. Characterization of the two _Mycobacterium tuberculosis recA_ promoters. _J Bacteriol_ 185, 6005–6015 (2003). Article CAS
PubMed PubMed Central Google Scholar * Rand, L. _et al_. The majority of inducible DNA repair genes in _Mycobacterium tuberculosis_ are induced independently of RecA. _Mol Microbiol_ 50,
1031–1042 (2003). Article CAS PubMed Google Scholar * Little, J. W., Mount, D. W. & Yanisch-Perron, C. R. Purified lexA protein is a repressor of the _recA_ and _lexA_ genes. _Proc
Natl Acad Sci USA_ 78, 4199–4203 (1981). Article CAS PubMed PubMed Central ADS Google Scholar * Little, J. W. & Mount, D. W. The SOS regulatory system of _Escherichia coli_. _Cell_
29, 11–22 (1982). Article CAS PubMed Google Scholar * Bertrand-Burggraf, E., Hurstel, S., Daune, M. & Schnarr, M. Promoter properties and negative regulation of the _uvrA_ gene by
the LexA repressor and its amino-terminal DNA binding domain. _J Mol Biol_ 193, 293–302 (1987). Article CAS PubMed Google Scholar * Cole, S. T. & Barrell, B. G. Analysis of the
genome of _Mycobacterium tuberculosis_ H37Rv. _Novartis Found Symp_ 217, 160–172; discussion 172–167 (1998). * Mizrahi, V. & Andersen, S. J. DNA repair in _Mycobacterium tuberculosis_.
What have we learnt from the genome sequence? _Mol Microbiol_ 29, 1331–1339 (1998). Article CAS PubMed Google Scholar * Gamulin, V., Cetkovic, H. & Ahel, I. Identification of a
promoter motif regulating the major DNA damage response mechanism of _Mycobacterium tuberculosis_. _FEMS Microbiol Lett_ 238, 57–63 (2004). CAS PubMed Google Scholar * Wang, Y., Huang,
Y., Xue, C., He, Y. & He, Z. G. ClpR protein-like regulator specifically recognizes RecA protein-independent promoter motif and broadly regulates expression of DNA damage-inducible genes
in mycobacteria. _J Biol Chem_ 286, 31159–31167 (2011). Article CAS PubMed PubMed Central Google Scholar * Striebel, F., Imkamp, F., Özcelik, D. & Weber-Ban, E. Pupylation as a
signal for proteasomal degradation in bacteria. _Biochim Biophys Acta_ 1843, 103–113 (2013). Article PubMed CAS Google Scholar * Imkamp, F., Ziemski, M. & Weber-Ban, E.
Pupylation-dependent and -independent proteasomal degradation in mycobacteria. _Biomol Concepts_ 6, 285–301 (2015). Article CAS PubMed Google Scholar * Pearce, M. J., Mintseris, J.,
Ferreyra, J., Gygi, S. P. & Darwin, K. H. Ubiquitin-like protein involved in the proteasome pathway of _Mycobacterium tuberculosis_. _Science_ 322, 1104–1107 (2008). Article CAS PubMed
PubMed Central ADS Google Scholar * Striebel, F. _et al_. Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. _Nat
Struct Mol Biol_ 16, 647–651 (2009). Article CAS PubMed Google Scholar * Imkamp, F. _et al_. Deletion of _dop_ in _Mycobacterium smegmatis_ abolishes pupylation of protein substrates _in
vivo_. _Mol Microbiol_ 75, 744–754 (2010). Article CAS PubMed Google Scholar * Burns, K. E. _et al_. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome
substrates. _Mol Cell_ 39, 821–827 (2010). Article CAS PubMed PubMed Central Google Scholar * Imkamp, F. _et al_. Dop functions as a depupylase in the prokaryotic ubiquitin-like
modification pathway. _EMBO Rep_ 11, 791–797 (2010). Article CAS PubMed PubMed Central Google Scholar * Pearce, M. J. _et al_. Identification of substrates of the _Mycobacterium
tuberculosis_ proteasome. _EMBO J_ 25, 5423–5432 (2006). Article CAS PubMed PubMed Central Google Scholar * Sutter, M., Striebel, F., Damberger, F. F., Allain, F. H. & Weber-Ban, E.
A distinct structural region of the prokaryotic ubiquitin-like protein (Pup) is recognized by the N-terminal domain of the proteasomal ATPase Mpa. _FEBS Lett_ 583, 3151–3157 (2009). Article
CAS PubMed Google Scholar * Striebel, F., Hunkeler, M., Summer, H. & Weber-Ban, E. The mycobacterial Mpa-proteasome unfolds and degrades pupylated substrates by engaging Pup’s
N-terminus. _EMBO J_ 29, 1262–1271 (2010). Article CAS PubMed PubMed Central Google Scholar * Festa, R. A., Pearce, M. J. & Darwin, K. H. Characterization of the proteasome
accessory factor (paf) operon in _Mycobacterium tuberculosis_. _J Bacteriol_ 189, 3044–3050 (2007). Article CAS PubMed PubMed Central Google Scholar * Darwin, K. H., Ehrt, S.,
Gutierrez-Ramos, J. C., Weich, N. & Nathan, C. F. The proteasome of _Mycobacterium tuberculosis_ is required for resistance to nitric oxide. _Science_ 302, 1963–1966 (2003). Article CAS
PubMed ADS Google Scholar * Coros, A., Callahan, B., Battaglioli, E. & Derbyshire, K. M. The specialized secretory apparatus ESX-1 is essential for DNA transfer in _Mycobacterium
smegmatis_. _Mol Microbiol_ 69, 794–808 (2008). CAS PubMed PubMed Central Google Scholar * Li, Q. _et al_. Proteasome Accessory Factor C (_pafC_) is a novel gene involved in
_Mycobacterium_ Intrinsic Resistance to broad-spectrum antibiotics - Fluoroquinolones. _Sci Rep_ 5, 11910, srep11910 [pii] https://doi.org/10.1038/srep11910 (2015). * Aravind, L.,
Anantharaman, V., Balaji, S., Babu, M. M. & Iyer, L. M. The many faces of the helix-turn-helix domain: transcription regulation and beyond. _FEMS Microbiol Rev_ 29, 231–262 (2005).
Article CAS PubMed Google Scholar * Makarova, K. S., Anantharaman, V., Grishin, N. V., Koonin, E. V. & Aravind, L. CARF and WYL domains: ligand-binding regulators of prokaryotic
defense systems. _Front Genet_ 5, 102, https://doi.org/10.3389/fgene.2014.00102 (2014). Article PubMed PubMed Central CAS Google Scholar * Sander, P., Springer, B. & Böttger, E. C.
Gene replacement in _Mycobacterium tuberculosis_ and _Mycobacterium bovis_ BCG Using _rpsL_ as a dominant negative selectable marker. _Methods Mol Med_ 54, 93–104 (2001). CAS PubMed Google
Scholar * Sander, P., Meier, A. & Böttger, E. C. _rpsL_ +: a dominant selectable marker for gene replacement in mycobacteria. _Mol Microbiol_ 16, 991–1000 (1995). Article CAS PubMed
Google Scholar * Guthlein, C. _et al_. Characterization of the mycobacterial NER system reveals novel functions of the _uvrD1_ helicase. _J Bacteriol_ 191, 555–562 (2009). Article CAS
PubMed Google Scholar * Kowalczykowski, S. C. Biochemical and biological function of _Escherichia coli_ RecA protein: behavior of mutant RecA proteins. _Biochimie_ 73, 289–304 (1991).
Article CAS PubMed Google Scholar * Kowalczykowski, S. C., Dixon, D. A., Eggleston, A. K., Lauder, S. D. & Rehrauer, W. M. Biochemistry of homologous recombination in _Escherichia
coli_. _Microbiol Rev_ 58, 401–465 (1994). CAS PubMed PubMed Central Google Scholar * Durbach, S. I., Andersen, S. J. & Mizrahi, V. SOS induction in mycobacteria: analysis of the
DNA-binding activity of a LexA-like repressor and its role in DNA damage induction of the _recA_ gene from _Mycobacterium smegmatis_. _Mol Microbiol_ 26, 643–653 (1997). Article CAS PubMed
Google Scholar * Papavinasasundaram, K. G. _et al_. Slow induction of RecA by DNA damage in _Mycobacterium tuberculosis_. _Microbiology_ 147, 3271–3279 (2001). Article CAS PubMed
Google Scholar * Drlica, K., Malik, M., Kerns, R. J. & Zhao, X. Quinolone-mediated bacterial death. _Antimicrob Agents Chemother_ 52, 385–392 (2008). Article CAS PubMed Google
Scholar * Hu, L. I. _et al_. Acetylation of the response regulator RcsB controls transcription from a small RNA promoter. _J Bacteriol_ 195, 4174–4186 (2013). Article CAS PubMed PubMed
Central Google Scholar * Zebre, A. C. _et al_. Interaction with enzyme IIBMpo (EIIBMpo) and phosphorylation by phosphorylated EIIBMpo exert antagonistic effects on the transcriptional
activator ManR of _Listeria monocytogenes_. _J Bacteriol_ 197, 1559–1572 (2015). Article CAS PubMed PubMed Central Google Scholar * Kumar, P. _et al_. The _Mycobacterium tuberculosis_
protein kinase K modulates activation of transcription from the promoter of mycobacterial monooxygenase operon through phosphorylation of the transcriptional regulator VirS. _J Biol Chem_
284, 11090–11099 (2009). Article CAS PubMed PubMed Central Google Scholar * Chiancone, E. & Ceci, P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions:
Detoxification of iron and hydrogen peroxide and DNA binding. _Biochim Biophys Acta_ 1800, 798–805 (2010). Article CAS PubMed Google Scholar * Almiron, M., Link, A. J., Furlong, D.
& Kolter, R. A novel DNA-binding protein with regulatory and protective roles in starved _Escherichia coli. Genes Dev_ 6, 2646–2654 (1992). Article CAS PubMed Google Scholar *
Martinez, A. & Kolter, R. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. _J Bacteriol_ 179, 5188–5194 (1997). Article CAS PubMed PubMed Central
Google Scholar * Chowdhury, R. P. & Chatterji, D. Estimation of Forster’s distance between two ends of Dps protein from mycobacteria: distance heterogeneity as a function of
oligomerization and DNA binding. _Biophys Chem_ 128, 19–29 (2007). Article CAS PubMed Google Scholar * Grant, R. A., Filman, D. J., Finkel, S. E., Kolter, R. & Hogle, J. M. The
crystal structure of Dps, a ferritin homolog that binds and protects DNA. _Nat Struct Biol_ 5, 294–303 (1998). Article CAS PubMed Google Scholar * Ceci, P. _et al_. DNA condensation and
self-aggregation of _Escherichia coli_ Dps are coupled phenomena related to the properties of the N-terminus. _Nucleic Acids Res_ 32, 5935–5944 (2004). Article CAS PubMed PubMed Central
Google Scholar * Ehira, S., Teramoto, H., Inui, M. & Yukawa, H. Regulation of _Corynebacterium glutamicum_ heat shock response by the extracytoplasmic-function sigma factor SigH and
transcriptional regulators HspR and HrcA. _J Bacteriol_ 191, 2964–2972 (2009). Article CAS PubMed PubMed Central Google Scholar * Pfister, P. _et al_. 23S rRNA base pair 2057-2611
determines ketolide susceptibility and fitness cost of the macrolide resistance mutation 2058A– >G. _Proc Natl Acad Sci USA_ 102, 5180–5185 (2005). Article CAS PubMed PubMed Central
ADS Google Scholar * Rozen, S. & Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. _Methods Mol Biol_ 132, 365–386 (2000). CAS PubMed Google Scholar
* Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. _Nucleic Acids Res_ 29, e45 (2001). Article CAS PubMed PubMed Central Google Scholar *
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. _J Mol Biol_ 215, 403–410 (1990). Article CAS PubMed Google Scholar * Altschul,
S. F. _et al_. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. _Nucleic Acids Res_ 25, 3389–3402 (1997). Article CAS PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS We would like to thank Peter Sander for fruitful discussions and critical comments on the manuscript. We thank Jonas Grossmann, Christian
Trachsel and Claudia Fortes from the FGCZ proteomics team for technical support and advice. Research in the lab of FI is supported by grants of the University of Zurich. Research in the lab
of EWB is supported by the Swiss National Science Foundation (31003A_163314). AUTHOR INFORMATION Author notes * Begonia Fudrini Olivencia and Andreas U. Müller contributed equally to this
work. AUTHORS AND AFFILIATIONS * University of Zurich, Institute of Medical Microbiology, Zurich, Switzerland Begonia Fudrini Olivencia, Sibylle Burger & Frank Imkamp * ETH Zurich,
Institute of Molecular Biology and Biophysics, Zurich, Switzerland Andreas U. Müller & Eilika Weber-Ban * Functional Genomic Center, University of Zurich/ETH, Zurich, Switzerland Bernd
Roschitzki Authors * Begonia Fudrini Olivencia View author publications You can also search for this author inPubMed Google Scholar * Andreas U. Müller View author publications You can also
search for this author inPubMed Google Scholar * Bernd Roschitzki View author publications You can also search for this author inPubMed Google Scholar * Sibylle Burger View author
publications You can also search for this author inPubMed Google Scholar * Eilika Weber-Ban View author publications You can also search for this author inPubMed Google Scholar * Frank
Imkamp View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.F.O., A.U.M., E.W.-B. and F.I. designed research; B.F.O., A.U.M., S.B., B.R.,
E.W.-B. and F.I. performed experiments and analysed data; B.F.O., A.U.M., E.W.-B. and F.I. wrote the paper. CORRESPONDING AUTHOR Correspondence to Frank Imkamp. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fudrini Olivencia, B., Müller, A.U., Roschitzki, B. _et al._ _Mycobacterium
smegmatis_ PafBC is involved in regulation of DNA damage response. _Sci Rep_ 7, 13987 (2017). https://doi.org/10.1038/s41598-017-14410-z Download citation * Received: 18 November 2016 *
Accepted: 11 October 2017 * Published: 25 October 2017 * DOI: https://doi.org/10.1038/s41598-017-14410-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative