Light guided in-vivo activation of innate immune cells with photocaged tlr 2/6 agonist
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The complexity of the immune system creates challenges in exploring its importance and robustness. To date, there have been few techniques developed to manipulate individual
components of the immune system in an _in vivo_ environment. Here we show a light-based dendritic cell (DC) activation allowing spatial and temporal control of immune activation _in vivo_.
Additionally, we show time dependent changes in RNA profiles of the draining lymph node, suggesting a change in cell profile following DC migration and indicating that the cells migrating
have been activated towards antigen presentation. SIMILAR CONTENT BEING VIEWED BY OTHERS OPTOGENETIC ENGINEERING OF STING SIGNALING ALLOWS REMOTE IMMUNOMODULATION TO ENHANCE CANCER
IMMUNOTHERAPY Article Open access 06 September 2023 LIGHT-MEDIATED DISCOVERY OF SURFACEOME NANOSCALE ORGANIZATION AND INTERCELLULAR RECEPTOR INTERACTION NETWORKS Article Open access 02
December 2021 LABEL-FREE BIOSENSOR ASSAY DECODES THE DYNAMICS OF TOLL-LIKE RECEPTOR SIGNALING Article Open access 12 November 2024 INTRODUCTION Harnessing the innate and adaptive immune
response has led to the development of vaccines and therapeutics1,2,3 . However, as the immune system “rivals the nervous system in complexity4,” understanding how to design better responses
and therapies remains a challenge. One area of complexity is the presentation of antigens by the innate system to the adaptive system – including chemical signaling, spatial migration and
cell-cell signaling. During this process, dendritic cells (DCs), activated by Toll-like receptors (TLRs) convey pathogenic information to the cells of the adaptive immune systems through the
production of cytokines and cell surface markers5, 6. This process involves the migration of activated DCs into lymphatics to present antigens to T-cells7,8,9,10. However, understanding
this complex system by manipulating sets of cells within it has been a challenge. Chemical control of various innate and adaptive immune cellular processes has been a burgeoning area of
interest11,12,13,14,15,16. Recently, we developed a method to tag and remotely induce a guided immune response (TRIGIR) with a photo-caged TLR2/6 agonist17. TRIGIR allows for selective
labeling of cells, followed by remote light activation. Here we use the TRIGIR method for _in vivo_ light-based activation to control the migration of dendritic cells. We validate our _in
vivo_ activation by monitoring DC migration using adoptively transferred bioluminescent DCs (Luc-DCs) that bear the TRIGIR compound. Further, to confirm that the migrating cells were
presenting antigens and further priming adaptive immune cells18, 19, we performed RNA analysis on the target lymph-node. Reported herein is a general procedure where adoptively transferred
immune cells can be remotely activated using a UV light source. Though this methodology calls for a TLR2/6 bearing cell type and has limited tissue penetration of UV light used to activate
the cells, it may find use in controlling activation of skin or subcutaneous DCs and for studying effects of inflammation within different spatiotemporal parameters. We expect that
improvements in both optogenetic techniques, longer wavelength photo-cages, and light delivery methods will help expand the technique to answer many different immunological questions.
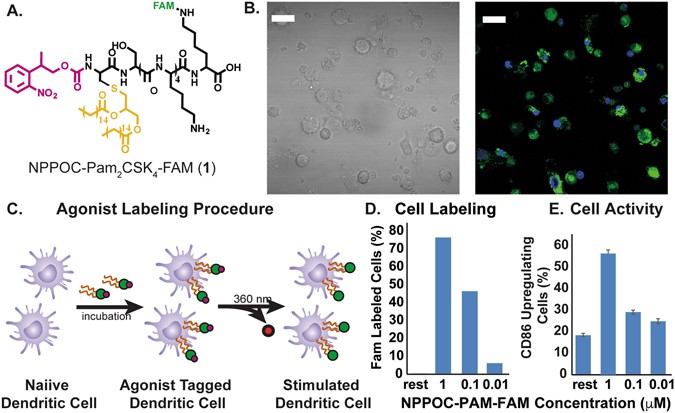
RESULTS CELL LABELING WITH NPPOC-PAM2CSK4 Previous work from our lab showed that photo-caging of the N-terminus of the TLR2/6 agonist, Pam2CSK4 20, can inhibit its activity to activate
TLR2/6. Upon light exposure and subsequent uncaging of the N-terminus, TLR2/6 is activated by the TRIGIR compound. The intercalation of the TRIGIR compound’s palmityl chains21 on the TLR2 of
DCs allows labelling of the agonists to quiescent innate immune cells without activating TLR2/6. These labelled cells can then be used in adoptive transfer experiments to achieve remote
control of inflammatory processes _via_ TLR2. We sought to adapt this technique _in vivo_ by labeling cells, performing subcutaneous injection and then activating of the cells in their local
environment. As the agonist stays co-localized, we can have the spatial control of agonist presentation and immune cell activation17. In initial experiments, we observed that high
concentration of the TRIGIR compound, NPPOC- Pam2CSK4 (1, Fig. 1A), incubation overnight resulted in higher amount of labeling of the agonist (Fig. 1D). However, this also resulted in higher
background activation of the cells (Fig. 1E). Therefore, labeling the primary DCs, harvested from transgenic luciferase expressing mice, at 0.1 μM (Fig. 1C) showed both good labeling and
did not elicit a background immune response (Fig. 1D,E). PHOTO-ACTIVATION OF TRANSFERRED DENDRITIC CELLS Before adoptive transfer, the DCs were incubated with 1 over-night. The cells were
then washed to remove excess 1 in the supernatant. The labeled cells were then injected into the footpad of mouse at 1 million cells/30 μL for the mice. To activate the cells with light, the
injected footpad of mice was then irradiated with 360 nm light (15 W) for 15 mins (Figure SI 6). To determine the limit of activity due to the limit of UV light tissue penetration, we
irradiated labelled cells with 360 nm light for 15 min _in vitro_ before injection. This experiment served as a “pre-activated” control and served as an upper limit for what might be
achieved with photo-activated DCs _in vivo_. During the imaging process, following previously reported procedures22, we blocked the bioluminescence occurring from the injected foot with
black tape to enhance the signal from the popliteal lymph node (Fig. 2). To understand the activity of mature DCs, we compared the migration of the Pam2CSK4 stimulated Luc-DCs and
non-stimulated Luc-DCs that were adoptively transferred into the footpad of a mouse over a period of 96 hrs. We found the Pam2CSK4 stimulated Luc-DCs migrate faster than the unstimulated
Luc-DCs, where we observed migration activity as early as 24 h in Pam2CSK4 stimulated Luc-DCs with a slow migration, over 96 hrs, of the unstimulated DCs into the draining lymph node at
later time points (Fig. 3A,B). Because activation of dendritic cells leads to upregulation of cell surface receptors that aid in the migration and translocation of DCs into the lymph node23,
we theorize that a shorter time is required for the activated cell to migrate into the lymph node compared to the unstimulated DCs. We sought to determine if light-activation of TLR2 _via_
1 _in vivo_ recapitulated the migration of activated DCs. We imaged the migration of the Luc-DC in mice whose footpads were exposed to UV light (+UV) or not exposed to UV (−UV). Following
the trends seen in the Pam2CSK4 stimulated cells, the footpads which were directly exposed to UV showed migration of Luc-DCs into the popliteal lymph node much sooner than that of the
non-exposed footpads (Fig. 3A,C). Additionally, the cells that were exposed to UV migrate at a similar rate as the cells that were photo-activated before being transferred into a mouse. From
this data, we conclude that TRIGIR labelled cells can be activated with light in a non-invasive manner and recapitulate the timing and quantity of their migration to the lymph node.
CONFIRMATION OF SYSTEMIC ACTIVATION _VIA_ RNA ANALYSIS OF POPLITEAL LYMPH NODE To further confirm the inflammatory state of TRIGIR activated DCs _in vivo_ by light, we harvested popliteal
lymph nodes from the mice and analyzed the RNA levels. This measurement also helped us determine if the activated DCs were enacting their antigen presenting role. If the cells were activated
following light exposure, the migrated cells will elicit a systemic response as recruitment and maturation of adaptive immune cells occurs in the lymph node. We harvested lymph nodes from
both light irradiated and non-irradiated animals which all contained TRIGIR-labeled DCs identical to our previous experiments. To determine differences, we plotted the changes as a relative
fold-change of the from irradiated:non-irradiated at each time point. Using this measurement, we determined how irradiation and TLR stimulation changed activity in the lymph node. First, we
observed that upon TRIGIR activation, there is a gradual increase in _ccr7_ which is upregulated by immune cells that enter the lymph node through recognition of CCL19 and CCL21 on the lymph
node (Fig. 4D)24,25,26. From this we conclude there are more _ccr7_ producing cells recruited into the lymph node. These cells are likely the TRIGIR activated dendritic cells which we
observed migrate to the lymph node as well as T cells that have been recruited into the lymph node within the first 72 h after UV exposure as a result of DC activation. We saw further
evidence for T cell recruitment upon TRIGIR activation with an increase of _cd34_ and _cd28_ within the same time period. CD34 is required for T cells to enter the lymph node while blocking
DC migration into the lymph node27. The downregulation of _cd34_ at early time points matches the increased migration of the stimulated DCs from the footpad into the lymph node (Fig. 4B).
The gradual increase suggests the increase of T cell trafficking into the lymph node and decrease in DC migration from the footpad. Similar to the _cd34_ trends, we saw a gradual increase in
_cd28_, a T cell receptor that recognizes CD80 and CD8628, reaching a maximum after 72hrs (Fig. 4C). This gradual rise indicates the increase in T cell population in the popliteal lymph
node. These trends follow known T cell maturation and migration following mature DC contact in the lymph node29. In comparison, there is a general upregulation of _nfkb1_ 30, 31 starting as
early as 48 hours, which could be due to the inflammatory signaling from the activated DCs that have migrated into the popliteal lymph node (Fig. 4A). DISCUSSION With our method of _in vivo_
photo-activation of immune cells, we delivered a photo-caged, TRIGIR agonist and activated it in a non-invasive manner with light. Using the TRIGIR method of tagging cells, we can overcome
the limitation of spatial control of soluble agonists as well as site-specific cell delivery. Compared to conventional adoptive transfer methods that require activation of cells prior to
transfer to the animal our method allows for less steps in preparation of the transferred cells and controls when the cells will be activated following adoptive transfer. In addition to
temporal control of cell activation, this method offers for the potential of light dosage dependent mitigation of inflammatory signals where longer irradiation times would activate more
cells, allowing for sustained activation without the increasing inflammatory response. This method can also be applied to a variety of cells to induce different responses to TLR2/6
activation. Because TRIGIR is cell specific, but requires labeling, it is compatible with many different primary cell types that can be adoptively transferred. By changing the types of cells
and cell populations, one can dissect not only autocrine signaling, but also paracrine signaling following light activation of cell subsets. The technique will not limit researchers to
adoptive transfer in the footpad but can create a depot of tagged, subcutaneous cells placed close to an area of interest and gain spatial and temporal control of elicited cellular response.
We offer the clear caveat that current photo-activation methods will limit this method to dermal or subcutaneous activation of innate immune cells. Our data suggest that this technique will
give researchers the potential to customize an innate cellular response depending on the target disease or immunological model. In conclusion, we present a method for light activation of
adoptively transferred cells _via_ TLR2/6. This technique presents a unique way to answer spatial and temporal questions about the innate immune response. METHODS All animal studies and mice
maintenance were carried out in accordance with relevant gidelines and regulations approved by the Institutional Animal Care and Use Committee at University of California, Irvine (IACUC
#2012-3048). BONE MARROW-DERIVED DENDRITIC CELL HARVEST AND CULTURE Bone marrow-derived dendritic cells (BMDCs) were harvested from 6-week-old B6;FVB-_Ptprc_ _a_ Tg(CAG-luc,-GFP)L2G85Chco
_Thy1_ _a_/J mice (Jackson Laboratory). Femur bones were removed from mice and the bone marrow was extracted into PBS buffer and pelleted. ACK Lysing Buffer (3 mL, Lonza) was added to the
cell pellet and incubated for 2 min at RT. PBS buffer (13 mL) was then added to the cell suspension, and the cell solution was centrifuged at 300 RCF for 10 min at RT. Thereafter, the cell
pellet was resuspended in BMDC complete media composed of RPMI 1640, 10% heat inactivated FBS, 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 2 mM L-glutamine (Life
Technologies), 10,000 U/mL penicillin, 10 mg/mL streptomycin, 25 μg/mL amphotericin B, and 50 μM beta-mercaptoethanol. Harvested cells were plated at 1 × 106 cells/mL in 100 mm petri dishes
(10 mL total media) and incubated at 37 °C in a CO2 incubator (day 0 of cell culture). On day 3, 10 mL of fresh BMDC primary media was added to each petri dish. On day 5, BMDCs were released
and plated in 24-well plates at 5 × 105 cells/mL for cell surface marker activation, cytokine profile flow cytometry experiments. GENERAL PROCEDURE FOR FLOW CYTOMETRY FOR CELL SURFACE
MARKER UPREGULATION BMDCs were incubated in individual wells with each agonist (9:1 BMDC:agonist) in 0.5 mL culture media for 18 h at 37 °C with 5% CO2. The cells were released from the
plate and centrifuged at 2500 RPM at 4 °C for 10 min. The cell pellet was resuspended in cold FACS (composed of PBS (1x), 10% FBS, and 0.1% sodium azide) buffer (100 μL) and incubated with
CD16/32 FcR blocking antibodies (1.0 μg/1 × 106 cells) on ice for 10 min. The cell suspension was pelleted and the supernatant was removed. The cell pellet was resuspended in cold FACS
buffer (100 μL) and incubated with PE-CD86 (1.0 μg/1 × 106 cells) on ice and removed from light for 30 min. Each sample was washed twice with 300 μL cold fluorescence-activated cell sorting
(FACS) buffer. The dendritic cells were resuspended in cold FACS buffer (150 μL) and kept on ice until being loaded onto the flow cytometer. GENERAL PROCEDURE FOR CELL LABELING BMDCs were
incubated at 3 × 106 cells in 2 mL of media in a 6 well cell culture plate with the addition of NPPOC-Pam-FAM at 100 nM overnight at 37 °C with 5% CO2. Following incubation, the cells were
collected in 15 mL conical tubes and rinsed with PBS 5 times. After the final rinse, the cells were counted and resuspended in PBS at a final cell concentration of 1 million cells/30 μL of
PBS. For the _ex vivo_ UV exposed cells, the labeled cells were deprotected with 365 nm light following the last rinse, counted, and resuspended in PBS at a final cell concentration of 1
million cells/30 μL of PBS. GENERAL PROCEDURE FOR ADOPTIVE TRANSFER Labeled Luc-BMDCs were adoptively transferred _via_ subcutaneous injection in the footpad of a C57/BL6J (Jackson Lab)
mouse. The labeled cells were loaded into a syringe (10 cc, insulin syringe) at 1 million cells/ 30 μL of PBS. UV exposed mice were put under isofluorane (2% in 1 L/min O2) and exposed to UV
light (UVP 95-01300-01 BL-15 long wave UV lamp, 15 W) for 15 mins. IVIS IMAGING PROCEDURE Luciferin was injected into each mouse (15 mg/mL in sterile PBS, 10 uL/g/mouse) _via_
intraperitoneal injection. After 10 mins following the luciferin injection, the mice were anesthetized with isoflurane (2% in 1 L/min O2). Before taking images the injected foot was taped
with black athletic tape and black electrical tape (3 M) to enhance the bioluminescent signal from the lymph node. Images were analyzed using Living Image Software. LYMPH NODE TISSUE HARVEST
AND RNA EXTRACTION Popliteal lymph nodes were harvest following each designated time point and suspended in RNAlater solution for up to 2 weeks. The harvested RNA was homogenized with
prefilled 2 mL, 1.5 mm Zirconium bead tubes at 250 G for 90 secs. The homogenized tissue solution was extracted for RNA following the procedures for RNeasy Mini Kit (Qiagen). cDNA was
reverse transcribed using the extracted RNA (KIT). Murine _ccr7, cd34, cd28, nfkb1_ expression was quantified using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Fisher) in the ABI 7300
detection system (Applied Biosystems). GAPDH gene expression was measured as endogenous reference. The relative fold change was calculated following the 2^-ddCT method32. Fold change was
normalized to the average of non-irradiated mice (non treated group) and UV mice (treated group) of triplicate of 6 different mice in each group. REFERENCES * Querec, T. _et al_. Yellow
fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. _J. Exp. Med._ 203, 413–424 (2006). Article PubMed PubMed Central
Google Scholar * Schreibelt, G. _et al_. Toll-like receptor expression and function in human dendrtiic cell subsets:implications for dendritic cell-based anti-cancer immunotherapy. _Cancer
Immunol. Immunother._ 59, 1573–1582 (2010). Article CAS PubMed Google Scholar * Melero, I. _et al_. Evolving synergistic combinations of targeted immunotherapies to combat cancer. _Nat.
Rev. Cancer_ 15, 457–472 (2015). Article CAS PubMed Google Scholar * Adler, M. E. Signaling Breakthroughs of the Year. _Sci. Signal_. 10, eaam5681 (2017). * Katsikis, P. D.,
Schoenberger, S. P. & Pulendran, B. Probing the ‘labyrinth’ linking the innate and adaptive immune systems. _Nat. Immunol._ 8, 899–901 (2007). Article CAS PubMed Google Scholar *
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. _Nat. Immunol._ 5, 987–995 (2004). Article CAS PubMed Google Scholar * Schimmelpfennig, C. H.
_et al_. _Ex Vivo_ Expanded Dendritic Cells Home to T-Cell Zones of Lymphoid Organs and Survive _in Vivo_ after Allogeneic Bone Marrow Transplantation. _Am. J. Pathol._ 167, 1321–1331
(2005). Article CAS PubMed PubMed Central Google Scholar * Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. _Nat.
Revi. Immunol._ 5, 617–628 (2005). Article CAS Google Scholar * Wu, W. _et al_. Structure-Activity Relationships in Toll-like Receptor-2 agonistic Diacylthioglycerol Lipopeptides. _J.
Med. Chem._ 53, 3198–3213 (2010). Article CAS PubMed PubMed Central Google Scholar * Buwitt-Beckmann, U. _et al_. Lipopeptide structure determines TLR2 dependent cell activation level.
_FEBS J._ 272, 6354–6364 (2005). Article CAS PubMed Google Scholar * Pawlak, J. _et al_. Bioorthogonal Deprotection on the Dendritic Cell Surface for Chemical Control of Antigen
Cross-Presentation. _Angew. Chem. Int. Ed._ 54, 5628–5631 (2015). Article CAS Google Scholar * Parasar, B. & Chang, P. V. Chemical optogenetic modulation of inflammation and immunity.
_Chem. Sci._ 8, 1450–1453 (2017). Article CAS PubMed Google Scholar * Liu, H., Kwong, B. & Irvine, D. J. Membrane Anchored Oligonucleotides for _in vivo_ Tumor Cell Modification and
Localized Cancer Immunotherapy. _Angew. Chem. Int. Ed._ 50, 7052–7055 (2011). Article CAS Google Scholar * Govan, J. M., Young, D. D., Lively, M. O. & Deiters, A. Optically Triggered
Immune Response through Photocaged Oligonucleotides. _Tetrahedron Lett._ 56, 3639–3642 (2015). Article CAS PubMed PubMed Central Google Scholar * Parker, C. G., Domaoal, R. A.,
Anderson, K. S. & Spiegel, D. A. An Antibody-Recruiting Small Molecule That Targets HIV gp120. _J. Am. Chem. Soc._ 131, 16392–16394 (2009). Article CAS PubMed PubMed Central Google
Scholar * Kim, J. _et al_. Activation of Toll-Like Receptor 2 in Acne Triggers Inflammatory Cytokine Responses. _J. Immunol._ 169, 1535–1541 (2002). Article CAS PubMed PubMed Central
Google Scholar * Mancini, R. J., Stutts, L., Moore, T. & Esser-Kahn, A. P. Controlling the Origins of Inflammation with a Photoactive Lipopeptide Immunopotentiator. _Angew. Chemie. Int.
Ed._ 54, 5962–5965 (2015). Article CAS Google Scholar * Martín-Fontecha, A., Lanzavecchia, A. & Sallusto, F. Dendritic Cell Migration to Peripheral Lymph Nodes in Dendritic Cells.
31–49 (Springer Berlin Heidelberg, 2009). * Martín-Fontecha, A. _et al_. Regulation of Dendritic Cell Migration to the Draining Lymph Node. _J. Exp. Med._ 198, 615–621 (2003). Article
PubMed PubMed Central Google Scholar * Spohn, R. _et al_. Synthetic lipopeptide adjuvants and Toll-like receptor 2—structure–activity relationships. _Vaccine_ 22, 2494–2499 (2004).
Article CAS PubMed Google Scholar * Kang, J. Y. _et al_. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. _Immunity_ 31, 873–884 (2009).
Article CAS PubMed Google Scholar * Lee, H. W. _et al_. Tracking of dendritic cell migration into lymph nodes using molecular imaging with sodium iodide symporter and enhanced firefly
luciferase genes. _Sci. Rep._ 5, 9865 (2015). Article CAS PubMed PubMed Central Google Scholar * Bertho, N. _et al_. Efficient migration of dendritic cells toward lymph node chemokines
and induction of TH1 responses require maturation stimulus and apoptotic cell interaction. _Blood_ 106, 1734–1741 (2005). Article CAS PubMed Google Scholar * Ritter, U. _et al_. Analysis
of the CCR7 expression on murine bone marrow-derived and spleen dendritic cells. _J. Leukoc. Biol._ 76, 472–476 (2004). Article CAS PubMed Google Scholar * Noor, S. _et al_.
CCR7-Dependent Immunity during Acute Toxoplasma gondii Infection. _Infect. Immun._ 78, 2257–2263 (2010). Article CAS PubMed PubMed Central Google Scholar * Clatworthy, M. R. _et al_.
Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. _Nat. Med._ 20, 1458–1463 (2014). Article CAS PubMed PubMed Central Google Scholar * Drew, E.,
Merzaban, J. S., Seo, W., Ziltener, H. J. & McNagny, K. M. CD34 and CD43 Inhibit Mast Cell Adhesion and Are Required for Optimal Mast Cell Reconstitution. _Immunity_ 22, 43–57 (2005).
Article CAS PubMed Google Scholar * Linterman, M. A. _et al_. CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. _eLife_
3, e03180 (2014). Article PubMed Central Google Scholar * Mempel, T. R., Henrickson, S. E. & von Andrian, U. H. T-cell priming by dendriticcells in lymph nodes occurs in three
distinct phases. _Nature_ 427, 154–159 (2004). Article ADS CAS PubMed Google Scholar * Baltimore, D. Discovering NF-κB. _Cold Spring Harb. Perspect. Biol._ 1, a000026 (2009). Article
PubMed PubMed Central Google Scholar * Gerondakis, S. & Siebenlist, U. Roles of the NF-κB Pathway in Lymphocyte Development and Function. _Spring Harb. Perspect. Biol._ 2, a000182
(2010). Google Scholar * Livak, K. & Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. _Methods_ 25, 402–408
(2001). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the Prescher Laboratory for help with IVIS imaging. The authors acknowledge the
financial support provided by NIH (1U01Al124286-01 and 1DP2Al112194-01), Prof. Esser-Kahn thanks the Pew Scholars Program, the Cottrell Scholars Program for generous support. This work was
supported, in part, by a grant from the Alfred P. Sloan foundation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Chemistry, University of California, Irvine, Irvine, CA,
92697, USA Keun Ah Ryu, Bethany McGonnigal, Troy Moore, Tawnya Kargupta, Rock J. Mancini & Aaron P. Esser-Kahn Authors * Keun Ah Ryu View author publications You can also search for this
author inPubMed Google Scholar * Bethany McGonnigal View author publications You can also search for this author inPubMed Google Scholar * Troy Moore View author publications You can also
search for this author inPubMed Google Scholar * Tawnya Kargupta View author publications You can also search for this author inPubMed Google Scholar * Rock J. Mancini View author
publications You can also search for this author inPubMed Google Scholar * Aaron P. Esser-Kahn View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS K.R. designed and performed experiments, analyzed data, and wrote the manuscript. R.J.M. initially synthesized the photocaged agonist. B.M., T.K., and T.M. performed mouse
experiements. A.P.E. supervised the project. All authors provided comments and contributions and have given approval to the final version of the manuscript. CORRESPONDING AUTHOR
Correspondence to Aaron P. Esser-Kahn. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ryu, K.A.,
McGonnigal, B., Moore, T. _et al._ Light Guided _In-vivo_ Activation of Innate Immune Cells with Photocaged TLR 2/6 Agonist. _Sci Rep_ 7, 8074 (2017).
https://doi.org/10.1038/s41598-017-08520-x Download citation * Received: 16 May 2017 * Accepted: 10 July 2017 * Published: 14 August 2017 * DOI: https://doi.org/10.1038/s41598-017-08520-x
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative