Echinomycin inhibits adipogenesis in 3t3-l1 cells in a hif-independent manner
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Obesity is a risk factor for many diseases including diabetes, cancer, cardiovascular disease, and chronic kidney disease. Obesity is characterized by the expansion of white adipose
tissue (WAT). Hypertrophy and hyperplasia of adipocytes cause tissue hypoxia followed by inflammation and fibrosis. Its trigger, preadipocyte differentiation into mature adipocytes, is
finely regulated by transcription factors, signal molecules, and cofactors. We found that echinomycin, a potent HIF-1 inhibitor, completely inhibited adipogenesis in 3T3-L1 WAT preadipocytes
by affecting the early phase of mitotic clonal expansion. The dose required to exert the effect was surprisingly low and the time was short. Interestingly, its inhibitory effect was
independent of HIF-1 pathways. Time-course DNA microarray analysis of drug-treated and untreated preadipocytes extracted a major transcription factor, CCAAT/enhancer-protein β, as a key
target of echinomycin. Echinomycin also inhibited adipogenesis and body weight gain in high fat diet mice. These findings highlight a novel role of echinomycin in suppressing adipocyte
differentiation and offer a new therapeutic strategy against obesity and diabetes. SIMILAR CONTENT BEING VIEWED BY OTHERS GRK5 IS REQUIRED FOR ADIPOCYTE DIFFERENTIATION THROUGH ERK
ACTIVATION Article Open access 21 January 2025 IL-27 ALLEVIATES HIGH-FAT DIET-INDUCED OBESITY AND METABOLIC DISORDERS BY INHIBITING ADIPOGENESIS VIA ACTIVATING HDAC6 Article Open access 19
March 2025 HMGB2 ORCHESTRATES MITOTIC CLONAL EXPANSION BY BINDING TO THE PROMOTER OF C/EBPΒ TO FACILITATE ADIPOGENESIS Article Open access 02 July 2021 INTRODUCTION Obesity is a global
health burden and serves as a significant risk factor for many diseases such as diabetes, hypertension, cancer, cardiovascular disease, and chronic kidney disease1, 2. Expansion of white
adipose tissue (WAT), which is caused by the proliferation and differentiation of white adipocytes, is responsible for obesity development. Elucidating the underlying mechanism and
abrogating it have been of great interest in this field. Obesity exacerbates tissue hypoxia due to increased adipocyte oxygen consumption and insufficient angiogenesis3, 4. Hypoxia inducible
factor (HIF)-1, the master regulator of cellular adaptation to hypoxia consisting of α and β subunits, is induced early in the course of diet-induced obese WAT4 and contributes to glucose
intolerance and insulin resistance4, 5. Previous reports on genetic deletion of HIF-1 have both positive and negative effects on adipogenesis of WAT4, 5. Echinomycin is a cyclic peptide
belonging to a family of quinoxaline antibiotics isolated from _Streptomyces echinatus_ 6. It reversibly intercalates into double-stranded DNA sequences such as 5′-CGTACG, 5′-[d(ACGTACGT)2]
or 5′-[d(TCGATCGA)2]. The target sequence includes a hypoxia responsive element (HRE) sequence, making echinomycin a potent HIF-1 inhibitor7, 8. Antitumour activity was confirmed in several
preclinical studies, which led to a phase II clinical trial of echinomycin in metastatic soft tissue sarcoma patients9. We decided to investigate the effect and mechanism of echinomycin as
an HIF-1 inhibitor of WAT adipogenesis in 3T3-L1 cells, a preadipocyte cell line that is widely used as a model of adipocyte differentiation with a well characterized adipogenic cascade1, 2,
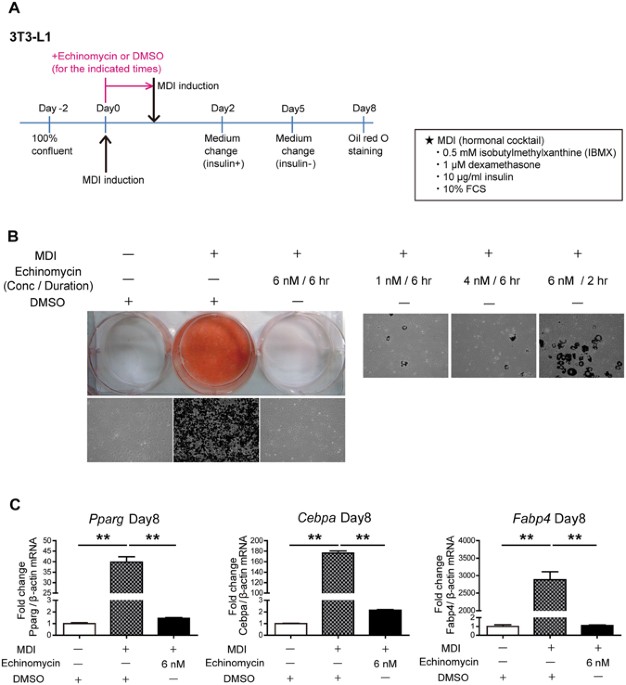
as well as in high fat diet (HFD) mice that is commonly used as an obesity model. RESULTS ECHINOMYCIN INHIBITS ADIPOGENESIS IN 3T3-L1 CELLS The conventional hormone cocktails containing MDI
lead 3T3-L1 preadipocytes to robustly differentiate into mature adipocytes as shown by a substantial amount of lipid accumulation in mature adipocytes in oil red O staining (Fig. 1A and B).
To investigate the effect of echinomycin on adipocyte differentiation, 3T3-L1 preadipocytes were treated with various concentrations and/or durations of echinomycin simultaneously with MDI
(Fig. 1B). Echinomycin inhibited adipogenesis in a time- and dose-dependent manner. Even under re-stimulation with MDI 6 hr after MDI plus echinomycin treatment, cells failed to
differentiate into mature adipocytes (Fig. 1B _left upper panel_). Treatment with 6 nM for 6 hr was sufficient to fully inhibit adipogenesis, and we used this condition in the following
studies unless otherwise indicated. This treatment concentration and time is within the range of previously reported usage of echinomycin in cancer cells (e.g., U-251 and HeLa cells)7, 8,
10. Accordingly, the altered expression levels of mature adipogenic marker genes such as fatty acid binding protein 4 (_Fabp4_), peroxisome proliferator-activated receptor γ (_Pparg_), and
CCAAT/enhancer-binding protein α (_Cebpa_) were blunted by echinomycin treatment (Fig. 1C). On the other hand, the addition of echinomycin from 24 hr after induction was insufficient to
abolish adipocyte differentiation (data not shown), which suggested that echinomycin exerts its effect on the early stage of adipogenesis. ECHINOMYCIN INHIBITS ADIPOGENESIS INDEPENDENTLY OF
HIF ACTIVITY Echinomycin intercalates DNA and binds to sequences including HRE sequences, that is, GCGTG or ACGTG, inhibiting HIF transcriptional activity. In 3T3-L1 cells, 6 nM echinomycin
treatment significantly reduced the increased HREluc reporter activity from MDI treatment (Fig. 2A), as well as the expression levels of representative HIF-1 target genes such as hexokinase
2 (_Hk2_), vascular endothelial growth factor A (_Vegfa_) (Fig. 2B), and glucose transporter 1 (GLUT1) (Fig. 2C). Although HIF-1α was induced at 2 hr after MDI treatment, which was probably
a PI3K/AKT-mediated response to insulin, this induction was very modest compared with that by hypoxic stimuli (Fig. 2D). Although echinomycin is a DNA intercalater, it also affects the
expression level of HIF-1α protein depending on the cell line11; in 3T3-L1 cells, echinomycin reduced HIF-1α protein expression (Fig. 2D _left panel_). HIF-2α was not induced at this early
time point (Fig. 2D _right panel_), which is in accordance with previous reports that HIF-2α is induced in much later phase of adipogenesis12, 13. These results demonstrate that the
conventional effect of echinomycin as a HIF-1 inhibitor both in function and quantity also applies to 3T3-L1 cells. On the other hand, hypoxia (1% O2) inhibited adipocyte differentiation,
which was demonstrated by Oil red O staining and its quantification (Fig. 2E). qRT-PCR for the mature adipocyte marker genes (e.g., _Pparg_, _Fabp4_, and _Cebpa_) at day 8 after MDI exposure
also remained low under hypoxic condition (Fig. 2F). Previous studies have also reported that 3T3-L1 cells with knockdown of HIF-1α undergo adipogenic differentiation even under hypoxia,
which implies that HIF-1 functions as an inhibitory signal in the cascade12, 14. Thus, if an effect of echinomycin on adipogenesis is mediated by the HIF pathway, echinomycin should have
stimulated adipogenesis. Our results suggest that echinomycin inhibits adipogenesis independently of HIF pathways. TRANSCRIPTOME ANALYSIS OF EARLY ADIPOGENESIS WITH OR WITHOUT ECHINOMYCIN To
investigate the molecular mechanism that underlies the effect of echinomycin on adipogenesis, gene expression profiling of 3T3-L1 cells during adipogenesis was performed with DNA
microarrays at times 0, 2, 6, and 24 hr following treatment either with MDI plus DMSO or MDI plus echinomycin (6 nM for 6 hr). First, the 2,204 gene set probes that increased more than
2-fold compared with 0 hr among the time-course DMSO-treated control groups were selected and clustered into three groups (Fig. 3A and Table S1). Different subsets of genes responded at
different time points to MDI stimulation, consistent with previous reports15; cluster 1 with genes responded as early as 2 hr after MDI treatment [e.g., CCAAT/enhancer-binding protein
(_Cebp_) _b_, _Cebpd_, Kruppel like factor (_Klf_) _4_, _Klf5_, and _Klf9_]; cluster 2 with genes such as fos-like antigen 1 (_Fosl1_) and integrin subunit alpha 5 (_Itga5_); and cluster 3
with genes such as a disintegrin, metallopeptidase domain 8 (_Adam8_) and hepatocyte growth factor (_Hgf_). To focus on the gene expression profiles and their changes at the very early phase
of adipogenesis, we compared gene expression profiles of cells treated with MDI plus DMSO and MDI plus echinomycin at 2 hr and 6 hr after induction. The 14,005 probes were distributed in
all of the four quadrants at either 2 hr or 6 hr, which signified that some of the genes are even upregulated under echinomycin treatment, ruling out the possibility that echinomycin
globally suppresses gene transcription (Fig. 3B). The microarray analysis was validated by qRT-PCR for representative genes in adipogenesis (e.g., _Cebpb_, _Cebpd_, _Klf4_, and _Klf5_) (Fig.
3C). The probes induced in both the 2 hr- and 6 hr- MDI plus DMSO treated groups more than 2-fold compared with 0 hr were extracted (Fig. 3B-c). The resulting 564 probes with annotation
(out of 592 probes) were further clustered by centred average linkage (Fig. 3D and Table S2). Because a 2 hr treatment with echinomycin was insufficient to inhibit adipogenesis (Fig. 1B),
expression of the target genes of echinomycin was expected to be suppressed at both 2 hr and 6 hr. The cluster that met this criterion included 111 probes (Fig. 3D and Table S2). C/EBPΒ AS A
KEY TARGET OF ECHINOMYCIN During early differentiation (24–48 hr following MDI treatment), 3T3-L1 cells are known to re-enter the cell cycle and undergo two to three cycles of clonal
expansion before terminal differentiation1, 16, 17. The fact that re-stimulation with MDI 6 hr after echinomycin treatment failed to induce adipocyte differentiation supports the hypothesis
that echinomycin inhibited this critical phase of clonal expansion. We observed that cell proliferation was inhibited in an echinomycin-dose-dependent manner in this period, supporting our
hypothesis (Fig. 4A and B). Gene ontology analysis of the above 111 probes by DAVID also showed significant enrichment (enrichment score 4.65, _P_ < 4.5 × 10−7) in transcriptional
regulation (Fig. 4D). Representative genes included _Cebpb_, _Klf5_, _Klf9_, B-cell lymphoma 3-encoded protein (_Bcl3_), Glis Family Zinc Finger 1 (_Glis1_), _Smad6_, and _Etv6_. These
results suggest that the key transcription factors critical in the adipogenic cascade are targets of echinomycin. Three members of the C/EBP family, C/EBPβ, C/EBPδ and C/EBPα, play crucial
roles in adipocyte differentiation18, 19. In the very early phase of adipogenesis, C/EBPβ and C/EBPδ are induced, and these in turn activate PPARγ and C/EBPα, which play central roles in the
rest of the regulatory cascade in adipocyte differentiation20,21,22. Mice lacking C/EBPβ display reduced depots of WAT, a phenotype exacerbated in C/EBPβ−/−/C/EBPδ−/− mice23. Whereas
_Cebpb_ was extracted as one of the top candidate target genes of echinomycin, the expression level of _Cebpd_ did not change following echinomycin treatment (Fig. 3C and Table S2). Other
adipogenic cascade regulators such as KLF5 and KLF9, which are listed as candidate genes in Fig. 4D, are known to be downstream of C/EBPβ and thus are not expected to be a direct target of
echinomycin. In addition, genes upstream of _Cebpb_ in the cascade, such as _Creb_, _Klf4_, and _Krox20_, were not affected by echinomycin treatment either (Figs 3C and 4C, and Table S2)24.
Enhanced C/EBPβ protein expression was suppressed by echinomycin continuously from 6 hr after adipogenic induction (Fig. 5A). The result that 2 hr treatment with echinomycin was insufficient
to inhibit adipogenesis (Fig. 1B), and the fact that the expression of C/EBPβ in this period is a prerequisite for mitotic clonal expansion in adipogenic differentiation, both support the
hypothesis that C/EBPβ is the target. MDI treatment increased the activity of the mouse _Cebpb_ promoter, which was significantly decreased by echinomycin treatment, which indicated that
echinomycin binds to _Cebpb_ promoter and suppresses its transcription (Fig. 5B). To rule out the possibility that C/EBPβ induction by MDI stimuli is dependent on HIF-1, loss-of-function
mutations of HIF-1 transcriptional activity was introduced to 3T3-L1 cells through CRISPR/Cas9 system25. CRISPR/Cas9-mediated stable knockout 3T3-L1 cells of the _Hif1a_ gene was generated
by puromycin screening and selecting positive clones using the HREluc assay (Fig. 5C _left panel_). Knockout of _Hif1a_ did not suppress the induction of C/EBPβ under MDI stimuli, which
indicates that C/EBPβ induction is independent of HIF-1, and that the effect of echinomycin on C/EBPβ is not mediated by HIF-1. In addition, 3T3-L1 cells with retrovirus transduction of
C/EBPβ (Fig. 5D-a) escaped from the suppression of adipogenesis by echinomycin (Fig. 5D-b). These results strongly point to C/EBPβ as the main target of echinomycin. ECHINOMYCIN INHIBITS
ADIPOGENESIS _IN VIVO_ To further investigate the effect of echinomycin on adipogenesis _in vivo_, male C57BL/6Jcl mice were fed with high-fat diet (HFD) for 2.5 weeks. Echinomycin was
intraperitoneally injected at two doses and frequency for the entire period based on the previous reports in mice and humans26, 27: 10 μg/kg for 5-days on/2-days off (EC-Low), or 50 μg/kg
every alternate day (EC-High). These regimens are much milder than those used in human clinical trials for anti-cancer treatment. Each regimen effectively inhibited the HIF-1 activity (shown
by the qRT-PCR for _Vegfa_) in the epididymal white adipose tissue (eWAT) of both normal fat diet (NFD) and HFD mice, which demonstrated that echinomycin was effectively delivered to eWAT
(Fig. 6A). Each echinomycin treatment inhibited the body weight gain (Fig. 6B), without effects on the food intake (data not shown). eWAT/gBW and qRT-PCR of the established HFD-induced
gene28, _leptin_, were analysed among each group, which confirmed that echinomycin inhibited adipogenesis _in vivo_ (Fig. 6C and D). H&E staining of eWAT accordingly demonstrated that
echinomycin efficiently suppressed the adipocyte size in HFD mice (Fig. 6E). These results are in accordance with the _in vitro_ results. DISCUSSION Our current study demonstrates that
echinomycin, a DNA intercalater, completely inhibits adipogenesis in the 3T3-L1 adipocyte cell line of WAT, as well as in eWAT of HFD mice. A DNA transcriptome array extracted one of the
essential adipogenic transcription regulators, C/EBPβ, as its major target (Fig. 7). An advantage of this small molecule is that a relatively small dose and short-term treatment are
sufficient to exhibit its effect. Echinomycin is traditionally known as a HIF-1 inhibitor7, 8. It binds to HRE sequences and decreases HIF-1 transcriptional activity. Whether echinomycin
affects the expression of HIF-1α protein depends on the cell line; in U251, HepG2, and HeLa cells, HIF1-α protein level is not affected7, 8, 10; in MCF-7 cells, it is decreased11. In 3T3-L1
cells, echinomycin inhibited HIF-1α protein expression and HIF-1 activity. The _in vivo_ functional role of HIF-1 in adipogenesis of WAT is controversial. Some reported that
adipocyte-specific genetic deletion of HIF-1α protects obese mice from insulin resistance and inflammation, whereas constitutively active expression of HIF-1α results in increased insulin
resistance and tissue fibrosis3. In these knockout mice, white adipose tissue mass and mean adipocyte size are increased4, 12, which implies that HIF-1 in the end inhibits adipogenesis in
WAT. Others, on the other hand, reported the opposite results using the same _aP2_ promoter-driven adipocyte-specific _Hif1a_ deleted mice29, 30. HIF-1 deficiency improved HFD-induced
insulin resistance and reduced WAT adipocity. _In vitro_ studies using 3T3-L1 cells show that hypoxia inhibits adipogenesis through HIF-112, 14. Our results also demonstrate that while
HIF-1α is induced as early as 2 hr and again from 12 hr after adipogenic induction (data not shown), adipogenesis is suppressed under hypoxia. These findings suggest that although the
temporal induction of HIF-1 in the early course of adipogenesis may have some functional roles in regulating the growth arrest and differentiation of preadipocytes, it acts as an inhibitory
signal for adipogenesis. Of note, while HIF-2α is known to promote adipogenesis in 3T3-L1 cells, previous reports suggest that HIF-2α is induced at later phase of adipogenesis, from 4 day
after MDI induction12, 13. We confirmed that HIF-2α was not induced at least until 24 hr after MDI induction (data not shown), and that the inhibitory effect of echinomycin on adipogenesis
was unlikely to be mediated by HIF-2. In contrast to our original expectation, our results demonstrated that echinomycin exerted its inhibitory effect on adipogenesis independently of the
HIF pathway. Adipocyte differentiation is precisely controlled by a complex network of transcription factors, cofactors and signalling molecules. A comprehensive DNA microarray analysis was
performed, and C/EBPβ was identified as a key target of echinomycin in the adipogenic cascade. C/EBPβ is known as an indispensable adipogenic transcription factor, and ectopic overexpression
of C/EBPβ is sufficient to induce PPARγ and subsequent adipocyte differentiation. Continuous suppression of C/EBPβ was observed both at mRNA and the protein level, while C/EBPδ remained
unaffected. An _in silico_ search predicted several HRE sequences in the promoter region of _Cebpb_, which are potential binding sites for echinomycin, and the loss of this echinomycin
effect in _Cebpb_ promoters smaller than 1 kbp suggests that echinomycin inhibits the _Cebpb_ transcription and its binding site exists between 2 k and 1 k in the promoter region. None of
their mutations, however, cancelled the effect of echinomycin on the promoter activity (data not shown), which prevented us from drawing an unequivocal conclusion on its binding site. These
observations have two implications for the mechanism of action of echinomycin on C/EBPβ; Echinomycin binds to a _Cebpb_ promoter region which is yet to be identified, or, echinomycin binds
to an upstream gene of _Cebpb_, which results in the transcriptional inhibition of C/EBPβ. Although our microarray results and _Cebpb_ promoter assay favour the former story, the exact
mechanism of action of echinomycin on C/EBPβ remains to be a subject of future research. There are some other reports on inhibitory molecules against adipogenesis. Rapamycin, an inhibitor of
mammalian target of rapamycin, suppresses adipogenic differentiation through its anti-proliferative activity31. Pyridinyl imidazoles also block 3T3-L1 adipogenesis by targeting p38
mitogen-activated protein kinase32. Our study gives an additional insight into how adipogenesis could be blocked by a temporal intervention. It is also unique that it affects the very early
phase of transcriptional cascade. Taken together, our results demonstrate a novel effect of echinomycin beyond its anti-tumour activity, by acting on adipocyte differentiation. Echinomycin
inhibits adipogenesis of 3T3-L1 cells in a HIF-independent manner. Time-course DNA microarray analysis of drug-treated and untreated preadipocytes extracted a major transcription factor,
CCAAT/enhancer-protein β, as a key target of echinomycin. The effect of echinomycin also applied to eWAT of experimental obesity model, HFD mice. This study provides further molecular
insight into the spatially and temporally regulated cascade of WAT adipocytes. MATERIALS AND METHODS REAGENTS Insulin, dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), and oil red O
were purchased from Sigma (Sigma-Aldrich, St Louis, MO). Echinomycin (Calbiochem, Gibbstown, NJ) was dissolved in DMSO (Wako, Tokyo, Japan) according to the manufacturer’s instructions. CELL
CULTURE, DIFFERENTIATION, AND DRUG TREATMENT 3T3-L1 cells (American Type Culture Collection, Rockville, MD) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Nissui, Tokyo, Japan)
supplemented with 10% calf serum (CS). The differentiation protocol is as described previously33. Briefly, two days after cells reached confluence (Day 0), the medium was replaced with a
mixture consisting of 10% FCS and MDI (0. 5 mM IBMX, 1 μM DEX, and 10 μg/ml insulin). After 48 h, the medium was changed to DMEM containing 10% FCS and 10 μg/ml insulin. The medium was
replenished at 2-day intervals, and the appearance of cytoplasmic triglycerides was monitored by microscopy and confirmed by staining with oil red O. For hypoxic stimulation, cells were
exposed to 1% O2/5% CO2, with nitrogen as the balance, in a multigas incubator, APM-30D (ASTEC, Fukuoka, Japan). OIL RED O STAINING At day 8, 3T3-L1 cells were rinsed with PBS twice and
fixed with 10% formaldehyde in H2O for 10 min. The cells were treated with 60% isopropanol in H2O for 1 min and then stained in freshly diluted oil red O solution (0.18% (w/v) oil red O in
60% isopropanol) for 20–40 mins until the cells were stained. The cells were then washed with 60% isopropanol in H2O and twice with PBS. The cells were observed in PBS under a microscope and
photographed. Oil red O quantification was performed by extracting the dye by 100% isopropanol and OD 490 nm was subsequently measured in 96-well dishes. LUCIFERASE REPORTER ASSAY HIF-1
transcriptional activity (HREluc activity) was measured by dual-luciferase reporter assays (Promega, Madison, WI) in 24-well culture dishes. Five-hundred nanograms of pGL3 (Promega) vector
driven by 7× hypoxia-responsive elements (pHREluc)34 and 25 ng of _Renilla_ luciferase vector (pRL-TK or pRL-CMV) were co-transfected using Lipofectamine LTX (Invitrogen, Carlsbad, CA); 2
days after full confluence, the cells were differentiated as above and exposed to hypoxia (1% O2) for 8 hr. The cells were processed in fixed protein aliquots, and HREluc activity was
measured using a Lumat 9507 luminometer (EG and Berthold, Bad Wildbad, Germany). The relative light unit (RLU) value of firefly luciferase was divided by that of _Renilla_ luciferase to
correct for the transfection efficiency. To measure _Cebpb_ promoter activity, a similar method to the above was used. _Cebpb_ promoter fragments of 3 kb, 2 kb, 1 kb, or 0.5 kb (mCEBPB3K, 2
K, 1 K, or 0.5 K promoter) were PCR amplified from mouse genomic DNA using PrimeStar HS DNA polymerase (Takara Bio, Shiga, Japan) and primers with _KpnI_ and _XhoI_ overhangs (see Table 1
for primers). Digested and purified fragments were inserted into the pGL3basic reporter vector (Promega). PLASMIDS AND STABLE OVEREXPRESSION 3T3-L1 clones, which stably overexpress C/EBPβ,
were generated with retrovirus transduction (Platinum Retrovirus Expression System, Pantropic, Cell Biolabs, San Diego, CA). pcDNA-mC/EBPb was a gift from Jed Friedman (Addgene plasmid
#49198). The plasmid was digested with _XhoI_ and _PmeI_, and subcloned into the retroviral vector, pMXs-IRES-Puro using the _XhoI_-_SnaBI_ site. The plasmids were transfected to the
packaging cell line, and the culture supernatants were collected 24 and 48 hr later, passed through 0.22-μm filters, and added to the cells. Drug selection by 2 μg/ml puromycin was initiated
72 hr after infection. For CRISPR-Cas9 system, pX330-U6-Chimeric_BB-CBh-hSpCas9 was a gift from Feng Zhang (Addgene plasmid #42230)24. Hif1a target sgRNAs were designed by using
CRISPRdirect35, and ligated into pX330 plasmid by following the procedures from Zhang lab (http://www.genome-engineering.org/crispr). Sequences are listed in Table 1. 3T3-L1 cells were
co-transfected with the two pX330-Hif1a and a puromycin resistant plasmid which facilitates selection with puromycin (2 μg/ml). Selected colonies went further selection by HREluc activity
under hypoxia. MICROARRAY ANALYSIS A transcriptome microarray analysis of 3T3-L1 cells was conducted at 0 hr, 2 hr, 6 hr, and 24 hr of treatment with either MDI plus DMSO or MDI plus
echinomycin (6 nM). For the 24-hr samples, cells were washed and re-incubated with MDI 6 hr after induction. Total cellular RNA was isolated using RNAiso plus (Takara Bio). cRNA was
synthesized from cellular RNA and hybridized to high density Affymetrix microarray gene chips containing 45,102 probe sets. The expression value for each mRNA was obtained using the Robust
Multi-array Analysis (RMA) method. Probes with normalized intensity lower than 100 in all seven arrays (31,097 probes) were excluded from the analyses, which left 14,005 probes for further
analysis. To identify the transcripts that were increased by MDI treatment, all probes with a fold change >= 2.0 in expression (either 2, 6, or 24 hr after MDI + DMSO treatment) compared
with the control (time 0 hr) were selected (2,204 probes), and hierarchical clustering analysis was performed using the average linkage and relative correlation as a measure of similarity
for the selected genes. To identify transcripts that are significantly induced by MDI and suppressed both at 2 hr and 6 hr after echinomycin treatment, the following selection procedures
were performed. All probes that had a fold change >= 2.0 in expression compared with the control (time 0 hr) at both two time points, 2 hr and 6 hr, were first selected (592 probes).
After excluding probe sets that did not have gene annotation, the remaining 564 probes were used for further analyses. Hierarchical clustering analysis of a log 2-fold change from the
control (time 0 hr) was performed using the average linkage and relative correlation as a measure of similarity for the selected genes. The Database for Annotation, Visualization and
Integrated Discovery (DAVID)36, 37, an online gene database provided by the NIH (version 6.8; http://david.abcc.ncifcrf.gov/), was used to investigate biological functions associated with
the gene lists. RNA ISOLATION AND REAL-TIME QUANTITATIVE RT-PCR (QRT-PCR) RNA isolation and real-time quantitative (q) RT-PCR assays were performed as previously described38. The data were
calibrated to the β-actin value. The primers are described in Table 1. IMMUNOBLOTTING ANALYSIS The cells were collected for immunoblotting analysis as previously described38. The primary
antibodies were as follows: anti-HIF1α (Novus Biologicals, Littleton, CO); anti-HIF2α (Novus Biologicals, Littleton, CO); anti-GLUT1 (Abcam plc, Cambridge, UK); anti-C/EBPβ (Santa Cruz
Biotechnology, Santa Cruz, CA); and anti-α-tubulin (Cell Signaling Technology, Danvers, MA). HRP-conjugated anti-rabbit IgG (Bio-Rad Laboratories, Hercules CA) or anti-mouse IgG (Bio-Rad
Laboratories) antibodies were used as secondary antibodies. The signals were detected with the ECL Plus reagent (Thermo Fischer Scientific, Waltham, MA) using the chemiluminescence protocol.
For detecting the signals of GLUT1 and HIF-2α, SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fischer Scientific) was used. ANIMAL EXPERIMENTS Male C57BL/6NJcl mice aged 7
weeks (Nippon Seibutsu Zairyo Center, Tokyo, Japan) were fed with either normal fat diet or high fat diet (HFD 60) containing 60% of fat (Oriental Yeas Co. Ltd., Tokyo, Japan). Echinomycin
was dissolved in DMSO at 5 mg/ml and subsequently diluted with 10 mg/ml BSA/saline to a concentration of 0.0293 mg/ml. Either 1)10 μg/kg for 5-days on/2-days off, or, 2) 50 ug/kg alternate
day, was intraperitoneally injected until the day of sacrifice. At sacrifice, the mice were euthanized and the epididymal white adipose tissue (eWAT) were harvested, measured, fixed in
formalin for immunohistochemical analysis, snap-frozen in OCT, or stored at −80 °C for qRT-PCR analysis. All mice were housed in the animal care facility of the University of Tokyo under
standardized conditions (25 °C, 50% humidity, 12 hr light/dark cycle) with food and water available at libitum. All the experiments were carried out in accordance with the guidelines and
regulations of the Committee on Ethical Animal Care and Use at the University of Tokyo Graduate School of Medicine, as well as with the guidelines of the National Institutes of Health for
the use of animals in research. All experimental protocols were approved by the animal care and use committee at the University of Tokyo Graduate School of Medicine (Approval Number:
M-P16-104). For adipocyte size measurement, eWAT was processed for paraffin embedding, and 3-μm tissue sections were stained with hematoxylin and eosin. The adipocyte areas were manually
traced and analyzed using the Image J software. STATISTICAL ANALYSIS All data are reported as the mean ± SEM or the mean ± SD. The data for two groups were analysed using Student’s unpaired
_t_ test. The differences among more than two groups were analysed using one-way ANOVA with Tukey’s post-test or two-way ANOVA with Bonferroni’s post-test. Differences with a _P_ value <
0.05 were considered significant. GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA) was used for data analysis. REFERENCES * MacDougald, O. A., Cornelius, P., Liu,
R. & Lane, M. D. Insulin regulates transcription of the CCAAT/enhancer binding protein (C/EBP) alpha, beta, and delta genes in fully-differentiated 3T3-L1 adipocytes. _J Biol Chem._ 270,
647–54 (1995). Article CAS PubMed Google Scholar * Cowherd, R. M., Lyle, R. E. & McGehee, R. E. Jr. Molecular regulation of adipocyte differentiation. _Semin Cell Dev Biol._ 10,
3–10 (1999). Article CAS PubMed Google Scholar * Halberg, N. _et al_. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. _Mol Cell Biol._
29, 4467–83 (2009). Article CAS PubMed PubMed Central Google Scholar * Lee, Y. S. _et al_. Increased Adipocyte O2 Consumption Triggers HIF-1α Causing Inflammation and Insulin Resistance
in Obesity. _Cell._ 157, 1339–52 (2014). Article CAS PubMed PubMed Central Google Scholar * Lee, K. Y., Gesta, S., Boucher, J., Wang, X. L. & Kahn, C. R. The differential role of
Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. _Cell Metab._ 14, 491–503 (2011). Article CAS PubMed PubMed Central Google Scholar * Corbaz, R. _et
al_. Metabolic products of actinomycetes. VII. Echinomycin. _Helv Chim Acta._ 40, 199–204 (1957). Article CAS Google Scholar * Kong, D. _et al_. Echinomycin, a small-molecule inhibitor of
hypoxia-inducible factor-1 DNA-binding activity. _Cancer Res._ 65, 9047–55 (2005). Article CAS PubMed Google Scholar * Yu, H. _et al_. Inhibition of hypoxia-inducible factor-1 function
enhances the sensitivity of multiple myeloma cells to melphalan. _Mol Cancer Ther._ 8, 2329–38 (2009). Article Google Scholar * William, J. _et al_. A phase II clinical trial of
echinomycin in metastatic soft tissue sarcoma. An Illinois Cancer Center Study. _Investigational New Drugs._ 13, 171–4 (1995). Article Google Scholar * Vlaminck, B. _et al_. Dual effect of
echinomycin on hypoxia-inducible factor-1 activity under normoxic and hypoxic conditions. _FEBS J._ 274, 5533–42 (2007). Article CAS PubMed Google Scholar * Hattori, K. _et al_.
Solution-phase synthesis and biological evaluation of triostin A and its analogues. _Org. Biomol. Chem._ 14, 2090–111 (2016). Article CAS PubMed Google Scholar * Lin, Q., Lee, Y. J.
& Yun, Z. Differentiation arrest by hypoxia. _J Biol Chem._ 281, 30678–83 (2006). Article CAS PubMed Google Scholar * Shimba, S., Wada, T., Hara, S. & Tezuka, M. EPAS1 promotes
adipose differentiation in 3T3-L1 cells. _J Bio Chem._ 279, 40946–53 (2004). Article CAS Google Scholar * Yun, Z., Maecker, H., Johnson, R. & Giaccia, A. Inhibition of PPAR2 gene
expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. _Dev Cell._ 2, 331–41 (2002). Article CAS PubMed Google Scholar * Burton, G.
R., Nagarajan, R., Peterson, C. A. & McGehee, R. E. Jr. Microarray analysis of differentiation-specific gene expression during 3T3-L1 adipogenesis. _Gene._ 329, 167–85 (2004). Article
CAS PubMed Google Scholar * Rosen, E. D. & MacDougald, O. A. Adipocyte differentiation from the inside out. _Nat Rev Mol Cell Biol._ 7, 885–96 (2006). Article CAS PubMed Google
Scholar * Tang, Q. Q., Otto, T. C. & Lane, M. D. Mitotic clonal expansion: A synchronous process required for adipogenesis. _Proc Natl Acad Sci USA_ 100, 44–9 (2003). Article ADS CAS
PubMed Google Scholar * Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. Transcriptional regulation of adipogenesis. _Genes Dev._ 14, 1293–307 (2000). CAS PubMed
Google Scholar * Darlington, G. J., Ross, S. E. & MacDougald, O. A. The role of C/EBP genes in adipocyte differentiation. _J. Biol. Chem._ 273, 30057–60 (1998). Article CAS PubMed
Google Scholar * Farmer, S. R. Transcriptional control of adipocyte formation. _Cell Metab._ 4, 263–73 (2006). Article CAS PubMed PubMed Central Google Scholar * Lin, F. T. & Lane,
M. D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. _Proc. Natl. Acad. Sci. USA_ 91, 8757–8761 (1994). Article ADS CAS
PubMed PubMed Central Google Scholar * Tontonoz, P., Hu, E. & Spiegelman, B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor.
_Cell._ 79, 1147–56 (1994). Article CAS PubMed Google Scholar * Tanaka, T., Yoshida, N., Kishimoto, T. & Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPβ
and/or C/EBPδ gene. _EMBO J._ 16, 7432–43 (1997). Article CAS PubMed PubMed Central Google Scholar * Chen, Z., Torrens, J., Anand, A., Spiegelman, B. M. & Friedman, J. M. Krox20
stimulates adipogenesis via C/EBPβ-dependent and -independent mechanisms. _Cell Metab_. 1, 93–106 (2005). Article CAS PubMed Google Scholar * Cong, L. _et al_. Multiplex Genome
Engineering Using CRISPR/Cas Systems. _Science_. 339, 819–23 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Wang, Y., Liu, Y., Malek, S., Zheng, P. & Liu, Y.
Targeting HIF1α eliminates cancer stem cells in hematological malignancies. _Cell Stem Cell._ 8, 399–411 (2011). Article CAS PubMed PubMed Central Google Scholar * Foster, B. _et al_.
Echinomycin: The first bifunctional intercalating agent in clinical trials. _Investigational New Drugs._ 3, 403–410 (1986). Google Scholar * Koza, R. _et al_. Changes in gene expression
foreshadow diet-induced obesity in genetically identical mice. _PLoS Genetics._ 5, 769–80 (2006). Google Scholar * Jiang, C. _et al_. Disruption of Hypoxia-Inducible Factor 1 in Adipocytes
Improves Insulin Sensitivity and Decreases Adiposity in High-Fat Diet–Fed Mice. _Diabetes._ 60, 2484–95 (2011). Article CAS PubMed PubMed Central Google Scholar * Jiang, C. _et al_.
Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. _J Biol Chem._ 288, 3844–57 (2013). Article CAS PubMed Google Scholar * Yeh, W.
C., Bierer, B. E. & McKnight, S. L. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. _Proc Natl Acad Sci USA_ 92, 11086–90 (1995). Article ADS CAS
PubMed PubMed Central Google Scholar * Engelman, J. A., Lisanti, M. P. & Scherer, P. E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. _J Biol
Chem._ 273, 32111–20 (1998). Article CAS PubMed Google Scholar * Ntambi, J. M. _et al_. Differentiation-induced gene expression in 3T3–L1 preadipocytes. Characterization of a
differentially expressed gene encoding stearoyl-CoA desaturase. _J. Biol. Chem._ 263, 17291–300 (1988). CAS PubMed Google Scholar * Tanaka, T. _et al_. Hypoxia in renal disease with
proteinuria and/or glomerular hypertension. _Am J Pathol_ 165, 1979–92 (2004). Article PubMed PubMed Central Google Scholar * Naito, Y., Hino, K., Bono, H. & Ui-Tei, K. CRISPRdirect:
software for designing CRISPR/Cas guide RNA with reduced off-target sites. _Bioinformatics_ 31, 1120–1123 (2015). Article CAS PubMed Google Scholar * Huang, D. W., Sherman, B. T. &
Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. _Nature Protoc._ 4, 44–57 (2009). Article CAS Google Scholar * Huang, D. W.,
Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. _Nucleic Acids Res._ 37, 1–13 (2009). Article
Google Scholar * Yamaguchi, J., Tanaka, T., Eto, N. & Nangaku, M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein δ. _Kidney Int._ 88, 262–75 (2015).
Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a Grant-in-Aid for Scientific Research (C) 26461215 (T.T.), Grant-in-Aid
for Scientific Research on Innovative Areas 26111003 (M.N.), and Grants-in-Aid for Scientific Research (B) 15H04835 (M.N.) by Japan Society for the Promotion of Science (JSPS). The authors
thank Kahoru Amitani (The University of Tokyo) for technical support. The authors appreciate Hideko Nagasawa (Gifu Pharmaceutical University) and Kent Fukui (Japan Tobacco Inc.) for helpful
discussions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Nephrology and Endocrinology, The University of Tokyo Graduate School of Medicine, Tokyo, Japan Junna Yamaguchi,
Tetsuhiro Tanaka, Hisako Saito & Masaomi Nangaku * Genome Science laboratory, Research Center for Advanced Science and Technology, The University of Tokyo, Tokyo, Japan Seitaro Nomura
& Hiroyuki Aburatani * Department of Diabetes and Metabolic Diseases, Department of Molecular Science on Diabetes, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan
Hironori Waki * Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan Takashi Kadowaki Authors * Junna Yamaguchi View author
publications You can also search for this author inPubMed Google Scholar * Tetsuhiro Tanaka View author publications You can also search for this author inPubMed Google Scholar * Hisako
Saito View author publications You can also search for this author inPubMed Google Scholar * Seitaro Nomura View author publications You can also search for this author inPubMed Google
Scholar * Hiroyuki Aburatani View author publications You can also search for this author inPubMed Google Scholar * Hironori Waki View author publications You can also search for this author
inPubMed Google Scholar * Takashi Kadowaki View author publications You can also search for this author inPubMed Google Scholar * Masaomi Nangaku View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS J.Y., and M.N. designed research; J.Y., and S.N. conducted the _in vitro_ experiments; J.Y., and H.S. conducted the animal
experiments; J.Y., T.T., S.N., H.A., H.W., T.K., and M.N. analysed the results; J.Y., T.T., and M.N. wrote the manuscript. All authors reviewed the manuscript. CORRESPONDING AUTHOR
Correspondence to Masaomi Nangaku. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yamaguchi, J.,
Tanaka, T., Saito, H. _et al._ Echinomycin inhibits adipogenesis in 3T3-L1 cells in a HIF-independent manner. _Sci Rep_ 7, 6516 (2017). https://doi.org/10.1038/s41598-017-06761-4 Download
citation * Received: 07 October 2016 * Accepted: 19 June 2017 * Published: 26 July 2017 * DOI: https://doi.org/10.1038/s41598-017-06761-4 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative