Arabidopsis transporter abcg37/pdr9 contributes primarily highly oxygenated coumarins to root exudation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The chemical composition of root exudates strongly impacts the interactions of plants with microorganisms in the rhizosphere and the efficiency of nutrient acquisition. Exudation of
metabolites is in part mediated by ATP-binding cassette (ABC) transporters. In order to assess the contribution of individual ABC transporters to root exudation, we performed an LC-MS based
non-targeted metabolite profiling of semi-polar metabolites accumulating in root exudates of _Arabidopsis thaliana_ plants and mutants deficient in the expression of ABCG36 (PDR8/PEN3),
ABCG37 (PDR9) or both transporters. Comparison of the metabolite profiles indicated distinct roles for each ABC transporter in root exudation. Thymidine exudation could be attributed to
ABCG36 function, whereas coumarin exudation was strongly reduced only in ABCG37 deficient plants. However, coumarin exudation was compromised in _abcg37_ mutants only with respect to certain
metabolites of this substance class. The specificity of ABCG37 for individual coumarins was further verified by a targeted LC-MS based coumarin profiling method. The response to iron
deficiency, which is known to strongly induce coumarin exudation, was also investigated. In either treatment, the distribution of individual coumarins between roots and exudates in the
investigated genotypes suggested the involvement of ABCG37 in the exudation specifically of highly oxygenated rather than monohydroxylated coumarins. SIMILAR CONTENT BEING VIEWED BY OTHERS
TISSUE-SPECIFIC SIGNATURES OF METABOLITES AND PROTEINS IN ASPARAGUS ROOTS AND EXUDATES Article Open access 01 April 2021 ELEVATED CO2 AND NITRATE LEVELS INCREASE WHEAT ROOT-ASSOCIATED
BACTERIAL ABUNDANCE AND IMPACT RHIZOSPHERE MICROBIAL COMMUNITY COMPOSITION AND FUNCTION Article Open access 18 November 2020 A CYBDOM PROTEIN IMPACTS IRON HOMEOSTASIS AND PRIMARY ROOT GROWTH
UNDER PHOSPHATE DEFICIENCY IN ARABIDOPSIS Article Open access 11 January 2024 INTRODUCTION Root exudation is essential for plant growth and performance as the excreted compounds play
critical roles in the establishment of mutualistic interactions with microorganisms, in pathogen defense, or in nutrient acquisition1. Among other transport systems, ATP-binding cassette
(ABC)-type transporters often efflux these compounds into the rhizosphere2,3,4,5,6,7. In _Arabidopsis thaliana_, the ABC transporter family consists of 130 members, which are assorted into
eight groups (A-H) according to overall size and arrangement of transmembrane domains (TMD) and nucleotide binding domains (NBD)8, 9. Members of subfamilies B, C, and G, which are also known
as multidrug resistance (MDR), multidrug resistance associated proteins (MRP), and pleiotropic drug resistance (PDR) proteins, respectively, are full-size ABC transporters possessing each
two TMD and two NBD. Specific functions have mainly been assigned to these transporters based on phenotypic and metabolic changes in the respective mutants. For example, ABC transporters of
subfamily B are involved in auxin transport and distribution10, and members of group C have been implicated in the regulation of stomatal aperture and guard cell ion flux, as well as in the
transport of glutathione conjugates11,12,13,14,15. However, some of these members also transport chlorophyll catabolites12, 13 and phytochelatins16,17,18 or are responsible for phytate
accumulation19, which indicates a certain level of substrate promiscuity. Functional promiscuity has also been described for ABCG36/PDR8/PEN3, a transporter involved in resistance of
Arabidopsis to nonadapted and/or host-adapted pathogens20,21,22,23,24. It also acts as a cadmium extrusion pump in roots, thereby conferring resistance to heavy metal stress25. Furthermore,
ABCG36 mediates efflux of the auxin precursor indole 3-butyric acid (IBA) from roots, as evidenced by hypersensitive root growth phenotypes of _abcg36_ mutants in the presence of IBA or
precursors of synthetic auxin analogues26, 27. Despite its multiple roles for root physiology, it has not yet been investigated whether ABCG36 contributes to root exudation. A function as a
transporter of IBA and various auxinic compounds has also been assigned to ABCG37/PDR9, as evidenced by altered responsiveness of _abcg37_ mutant plants to synthetic auxins and inhibitors of
auxin transport, but not to indole-3-acetic acid (IAA), the endogenous auxin27,28,29. Interestingly, _abcg37_ knockout lines also displayed hypersensitivity to iron (Fe) deficiency, which
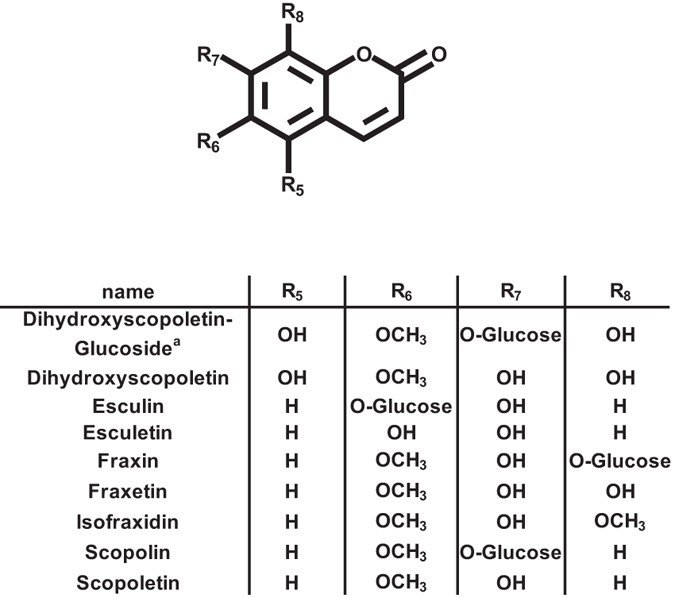
has been attributed to impaired coumarin excretion, especially of scopoletin7. Coumarins are a group of phenylpropanoid derived 1,2 benzopyrones exhibiting various patterns of methoxy and
hydroxyl functions (Fig. 1) and predominantly occur as glucosylated forms in root tissue, whereas the respective aglycones are mainly detectable in root exudates. It has been reported that
increased coumarin biosynthesis and exudation promotes plant Fe acquisition by chelating Fe3+ from insoluble Fe complexes, particularly in calcareous growth substrates7,
30,31,32,33,34,35,36. As such, _abcg37_ mutants and _feruloyl-CoA 6′ hydroxylase1_ (_f6′h1_) knockout lines that are incapable of coumarin biosynthesis show more severe Fe deficiency
symptoms and accumulate less Fe when compared to wild type plants under Fe limiting conditions7, 33, 34. Decreased abundance of all analyzed coumarins in root exudates of _abcg37_ mutants
suggested broad substrate specificity of ABCG37 with respect to the substitution pattern of coumarins7. Similarily, exudates of _f6′h1_ knockout lines do not contain any coumarins as they
are unable to synthesize coumarins33. As such, both studies did not provide hints about specificities towards individual coumarins with respect to exudation. Recently, we reported that
coumarin profiles are also profoundly altered in root exudates of phosphate (Pi) deprived plants37. We observed higher accumulation of a subset of coumarin derivatives, whereas the abundance
of others, mainly highly oxygenated coumarins, was decreased when compared to exudates of Pi replete roots. This is in contrast to Fe deficient growth conditions, which promote accumulation
of all coumarins, foremost of highly oxygenated coumarins or methylated derivatives there of 7, 33, 34. The different coumarin profiles indicate differential regulation of biosynthesis
and/or exudation of individual coumarins, which might also be reflected by the existence of transport systems with selectivity for coumarin compounds. In this study, we compared root
exudation of wild-type (Col-0) plants, of _abcg36_ and _abcg37_ knockout lines as well as of the _abcg36;abcg37_ double mutant by liquid chromatography mass spectrometry (LC-MS)-based
metabolite profiling of semi-polar metabolites. Non-targeted metabolite profiling indicated that both ABC transporters mediate the exudation of specific as well as common metabolites based
on the absence of _m/z_-retention time pairs (features) in root exudates of the mutants compared to the wild-type. The unexpected observation of similar scopoletin levels in root exudates of
_abcg37_ and wild-type seedlings prompted us to explore in more detail coumarin profiles in root exudates by a targeted LC-MS approach. Our experiments suggest selectivity of ABCG37 for the
exudation of individual, mainly highly oxygenated coumarins. RESULTS COMPARATIVE NON-TARGETED PROFILING OF SEMI-POLAR METABOLITES IN ROOT EXUDATES To investigate the contribution of
ABCG36/PDR8 and ABCG37/PDR9 transporters to root exudation, we cultivated wild-type (Col-0) seedlings, _pdr8-1_ and _pdr9-2_ knockout lines, as well as the _pdr8-1;pdr9-2_ double mutant in
the 96-well hydroponic system as described previously37. After germination and 4 days of growth, seedlings were transferred to fresh media and exudates were collected after additional 7
days. At the time of harvest (day 11), plant growth parameters such as root length and fresh weight of the entire seedlings, shoots, and roots were indistinguishable between the wild-type
and single mutants, whereas the _pdr8-1;pdr9-2_ double mutant exhibited reduced seedling (75%, _P_ = 0.02), shoot (71%, _P_ = 0.03) and root (65%, _P_ = 0.03) fresh weights as well as
shorter primary root length (77%, _P_ = 0.0004) when compared to the wild-type (Supplementary Fig. S1). The germination rate of _pdr8-1;pdr9-2_ seeds was about half compared to the other
genotypes. For root exudate analysis, we collected from 3 independent experimental setups 13 samples each for wild-type and _pdr9-2_, 15 samples for _pdr8-1_, and 8 samples for _pdr8-1;9-2_
(each sample comprised the media pooled from 8 single seedlings). For controls, we included 15 samples consisting of the media collected from blank wells. Two samples of _pdr8-1;pdr9-2_ had
to be omitted from subsequent analysis because of low data quality (retention time shift > 5 s and intensity deviation > 30% of the internal standard, 2,4 dichlorophenoxy acetic acid),
resulting in total of 124 datasets (all replicate samples, positive and negative ionization, see Supplementary Table S1). After data processing, we obtained 1,452 features (_m/z_-retention
time pairs), which comprised 854 and 598 features for positive and negative ionization modes, respectively (Supplementary Data S1). We detected 154 features with differences in abundance
(>1.5 fold difference, _P_ < 0.01) between wild-type and _pdr8-1_, 136 features between wild-type and _pdr9-2_, and 326 features between wild-type and _pdr8-1;pdr9-2_ (Fig. 2).
Compared to wild-type, 16, 1, and 7 features were of higher abundance in _pdr8-1_, _pdr9-2_ and the double mutant, respectively. The feature with higher intensity in _pdr9-2_ exudates was
absent in wild-type exudates (i.e. <1.5 fold, _P_ > 0.01 compared to blank samples). Three differential features of the wild-type vs. _pdr8-1_ comparison were not detectable in root
exudates of _pdr8-1_. This was the case for 16 and two features in the double mutant and _pdr9-2_, respectively. Almost all differential features between wild-type and _pdr9-2_ (130/136,
96%) were found among the differential signals between wild-type and the double mutant, but only 55% (88/154) of the wild-type vs. _pdr8-1_ comparison (Fig. 2). Interestingly, the comparison
between wild-type and the double mutants yielded decidedly more differential features (138) than the sum of the differential features in the comparison with the single mutants (70). As
such, the abundance of 138 features was different only between root exudates of wild-type and _pdr8-1;pdr9-2_. Both single mutants shared 32 differential features when compared to wild-type,
of which almost all (30) were contained in the wild-type vs. double mutant comparison (Fig. 2). IDENTIFICATION OF FEATURES SPECIFIC FOR ABCG36 AND ABCG37 To identify features that are
related to ABCG36/PDR8 transport substrates, we first determined the number of differential features between wild-type and _pdr8-1_ exudates. These features should also be among the
differential features between wild-type and the double mutant as well as between _pdr8-1_ and _pdr9-2_. Conversely, the remaining features should not exhibit differential abundance between
root exudates of wild-type and _pdr9-2_ or between _pdr8-1_ and the double mutant. This filtering resulted in 27 _pdr8_-specific features, 22 of which were more abundant in wild-type root
exudates. Applying the same procedure to the respective _pdr9-2_ comparisons yielded 64 _pdr9_-pecific features, which were all more abundant in root exudates of wild-type plants and may
thus be derived from ABCG37/PDR9 substrates (Supplementary Data S1). In order to obtain hints about the identity of the specific features related to ABCG36/PDR8 and ABCG37/PDR9 transport
substrates, we compared those with the more than 100 characterized or identified features occurring in Arabidopsis root exudates38. In the entire dataset, several, previously annotated
features could be detected, among them features annotated as amino acids, indolic compounds, oligolignols, phenylpropanoids and fatty acid derivatives (highlighted in green in Supplementary
Data S1). However, none of the ABCG37-related features and only one feature related to ABCG36 (thymidine _m/z_ 241.0821 at 74 s) are present in this list. Interestingly, we did not detect
scopoletin or derivatives thereof among the 64 ABCG37-related features although scopoletin was reported to be a substrate of ABCG377 and several scopoletin derivatives are listed by Strehmel
_et al_.38. To exclude the possibility that the absence of scopoletin as an ABCG37-related signal was due to the applied filtering procedure, we analyzed the raw data for signal intensities
of the scopoletin feature ([M + H]+ _m/z_ 193.0491 at 265 s). As shown in Fig. 3b, the scopoletin signal was clearly detectable in root exudates of wild-type plants, however, its intensity
was similar for all such samples. There were also no differences for the scopolin signals ([M + H + Na]+ _m/z_ 377.084 at 193 s; [M−H + formic acid]− _m/z_ 399.0893 at 193 s) between
wild-type and _pdr9-2_ exudates (Fig. 3c). The data rather indicate a decrease of scopolin in root exudates of _pdr8-1_ plants. Since the scopolin signals were different in the comparison of
_pdr8-1_ and the double mutant they were not listed as _pdr8_-specific features. However, we noticed the presence of two features at 199 s ([M−H + NaFA + KFA]_−_ _m/z_ 374. 963 and [M + H]+
_m/z_ 210.015) in the list of the _pdr9-2_ specific signals, which we previously annotated as the sodium formate and potassium formate adduct of the coumarin dihydroxyscopoletin and as
dihydroxyscopoletin after loss of a •CH3 radical37. As shown in Fig. 3a, the intensities of these signal were clearly lower in root exudates of _pdr9-2_ (_P_ < 0.01) and the double mutant
(_P_ < 0.0006) compared to wild-type, whereas no change could be observed for root exudates of _pdr8-1_. In order to further confirm the identity of the tentative dihydroxyscopoletin, we
compared its MS spectra with those of fraxetin and scopoletin (Supplementary Fig. S2). Collision induced dissociation in the positive ionization mode yielded mainly neutral losses of CO,
CH3OH, as well as ·CH3 radical elimination, generating fragments of _m/z_ of 178, 150, 133, and 122 from the [M + H]+ ion of scopoletin (_m/z_ 193). Fragmentation of fraxetin (_m/z_ 209 [M +
H]+) yielded an analogous series of fragments exhibiting an increase by 16 mass units (_m/z_ 194, 166, 149, 138). The mass spectrum of the tentative dihydroxyscopoletin (_m/z_ 225 [M + H]+)
showed several fragments with an increased mass by 16 mass units compared to fraxetin, and by 32 mass units compared to scopoletin (_m/z_ 210, 182, 165, 154). The strong similarity of the
mass spectra with a shift in fragment sizes of 16 and 32 mass units compared to authenticated fraxetin and scopoletin, as well as the strongly increased intensities of the tentative
dihydroxyscopoletin signal after Fe deficiency, and its absence in the coumarin deficient _f6′h1-1_ mutant (Fig. 4), strongly suggests its identity as dihydroxyscopoletin. However, the
parent ion of _m/z_ 225 ([M + H]+) was not present among the _pdr9-2_ specific features, although the signal intensities of this ion were also strongly decreased in exudates of _pdr9-2_ and
double mutant plants, but unchanged in exudates of _pdr8-1_ plants (Fig. 3a). Since the difference in signal intensities between _pdr9-2_ and double mutants plants was more than 1.5 fold,
the parent ion of _m/z_ 225 escaped our filtering procedure. We did not detect differences in signal intensities between the genotypes for two features which were previously annotated as
coumarinolignans38. Despite similar masses (_m/z_ 387.1075 [M + H]+ and _m/z_ 385.0937 [M-H]−) and the identical empirical formular (C20H18O8), the mass spectra showed that these are not
cleomiscosin A and B, which were recently identified in Arabidopsis root exudates36, but rather cross coupling products of syringyl alcohol and esculetin. COMPARATIVE TARGETED PROFILING OF
COUMARINS IN ROOTS AND ROOT EXUDATES Because our non-targeted metabolite profiling approach did not detect all coumarin compounds in root exudates that were reported as ABCG37/PDR9 transport
substrates7, we used a more sensitive targeted profiling approach to analyze coumarin levels in root exudates and root extracts. Additionally, we subjected the seedlings to Fe deficiency,
which increases the synthesis and exudation of most coumarins in wild-type plants7, 33, 34. We included the coumarin-deficient _f6′h1-1_ mutant in this study in order to discriminate
coumarin specific signals from signals derived from unrelated substances. With the exception of esculetin in root exudates (Fig. 4a), all coumarin signals are significantly lower in
_f6′h1-1_ plants compared to wild type, confirming the specificity of the targeted analysis. Scopolin exhibits the highest level of all coumarins, predominantly in roots, whereas its
aglycone, scopoletin, is evenly distributed between roots and root exudates (Figs 4a and 5). Similar to scopolin, esculin was mainly present in roots and only a small fraction of the esculin
pool was detected in root exudates. Esculetin, the aglycone, was barely detectable in wild-type. Dihydroxyscopoletin was exclusively detected in root exudates, whereas the occurrence of its
glucoside was restricted to roots (Fig. 4a). Consistent with the result of the non-targeted profiling approach, scopoletin levels in root exudates of the wild-type and the three ABC
transporter mutants, _pdr8-1_, _pdr9-2_, and _pdr8-1;pdr9-2_,were similar, as was its glucoside, scopolin. Likewise, we did not observe significant changes for both compounds in roots.
Esculin levels were reduced in root exudates of _pdr9-2_ and of the double mutant, but increased in roots. Esculetin exhibited a similar pattern in roots, but in exudates a change could only
be detected for _pdr8-1_. Prominent changes were observed for dihydroxyscopoletin and its glucoside. In root exudates, the level of dihydroxyscopoletin was reduced to 34% in _pdr9-2_. The
decreased (55%) and increased levels (1.8 fold) in root exudates of the double mutant and _pdr8-1_ line, respectively, were not highly statistically significant (_P_ = 0.08; _P_ = 0.07). The
decrease of the aglycone in root exudates of _pdr9-2_ and _pdr8-1;pdr9-2_ was mirrored by an increase of the glucoside in the respective roots; it strongly accumulated in roots of _pdr9-2_
(6-fold) and _pdr8-1;pdr9-2_ (9-fold) plants. COMPARATIVE TARGETED COUMARIN PROFILING UNDER FE DEFICIENCY Coumarins profoundly accumulate in root exudates during Fe deficiency, and the
contribution of ABCG37 to coumarin exudation has mainly been established after plants were subjected to Fe limiting conditions7, 33, 34. After exposure of wild-type roots to Fe-free media
for 7 days, dihydroxyscopoletin content of exudates excessively increased to levels that were almost 200-fold higher compared to +Fe control conditions, whereas esculetin and scopoletin
accumulated 6-fold and 5-fold, respectively (Fig. 4a and b). Additionally, fraxetin and isofraxidin, which escaped detection under control conditions, accumulated to appreciable levels. In
roots, dihydroxyscopoletin glycoside accumulation was almost as strong as the increase of the aglycone in exudates. We were also able to detect the glucoside in root exudates, although at a
low level compared to the endogenous root content (1%). As with fraxetin in root exudates, its glucoside, fraxin, was only detectable in roots under Fe limiting conditions. The alterations
in coumarin profiles between the transporter mutants and the wild-type under Fe deficiency were similar to the changes observed under control conditions (Fig. 4b). Dihydroxyscopoletin
glucoside and esculin contents were strongly increased in roots of _pdr9-2_ and _pdr8-1;pdr9-2_ plants whereas dihydroxyscopoletin, its glucoside, and esculin levels were strongly decreased
in root exudates. After Fe deficiency, it was possible to detect a decrease in abundance of esculetin in root exudates of _pdr9-2_ plants and of the double mutant. Furthermore we also
observed increased levels of fraxin in roots but decreased levels of fraxetin in exudates of these plants. Again, no changes in the levels of scopolin and scopoletin in roots and root
exudates could be observed for the transporter mutants. The decreased levels of isofraxidin in root exudates of the transporter mutants displayed low statistical significance (_P_ >
0.17). Based on these data we calculated the distribution of each coumarin compound between exudates and roots (Fig. 5). As expected, the glucosides esculin, scopolin, and
dihydroxyscopoletin-glucoside were more abundant in roots. Interestingly, the distribution of the aglycone esculetin between exudates and roots was similar to the distribution of its glycone
esculin under Fe sufficient conditions irrespective of the genotype. In contrast, the aglycone scopoletin was detected at slightly higher levels in exudates of Fe sufficient seedlings. Upon
Fe deficiency, the fraction of scopoletin and esculetin in exudates strongly increased, whereas the distribution of the respective glucoside remained unaltered. The ratios between agylcones
in root exudates and the corresponding glycones in roots were very low, especially for the scopoletin/scopolin pair, indicating that the majority of each coumarin compound is present as
glucoside inside roots (Fig. 6). Although we observed a strong increase in these ratios upon Fe deficiency, reflecting higher levels of aglycones in exudates, the endogenous root levels of
the respective glucosides were still higher than the respective aglycone levels in the exudates (i.e. ratios below 0.5). Only fraxetin and fraxin accumulated to almost equal levels in
exudates and roots, respectively (Fig. 6). The distribution between exudates and roots dramatically changed in _pdr9-2_ and _pdr8-1;pdr9-2_ plants. Here, the ratios between external and
internal levels of esculetin, esculin, and dihydroxyscopoletin glucoside dropped substantially (Fig. 5), as well as the ratios between aglycones in exudates and glucosides in roots for
fraxetin/fraxin, esculetin/esculin, and dihydroscopoletin/dihydroxyscopoletin glucoside (Fig. 6). However, this was not observed for scopoletin and scopolin. On the other hand, disrupted
ABCG36 function seemed to slightly (_P_ = 0.07) affect the distribution of esculetin after Fe deficiency, as indicated by the decreased ratio in _pdr8-1_ when compared to the wild-type (Fig.
6c). DISCUSSION ABC transporters are ubiquitous proteins and form a large family that greatly expanded during the evolution of plants to facilitate a wide range of physiological processes
in support of their terrestrial lifestyle. Plant genomes typically encode more than 120 ABC transporters, which exceeds the number in animals by almost 3-fold, and it remains a challenging
task to determine their transport properties and preferred substrates, which are often structurally and functionally unrelated9. In this study, we revisited the substrate specificity of two
ABC transporters, ABCG36/PDR8 and ABCG37/PDR9, which specifically localize to the outermost plasma membrane of root epidermal cells and the lateral root cap26, 27. The study of _pdr8_ and
_pdr9_ mutants as well as of recombinant ABCG37 protein indicated that both transporters redundantly regulate the accumulation of IBA and synthetic auxinic compounds in roots by facilitating
their efflux into the rhizosphere26, 27. Because ABCG37 has also been implicated to promote Fe acquisition via the exudation of Fe-chelating coumarins7, 35, we analyzed its substrate
specificity by employing a non-targeted metabolite profiling methodology of semi-polar compounds in Arabidopsis root exudates37, which we followed up by the targeted metabolite profiling of
coumarins in root extracts and exudates. Our non-targeted metabolite profiling study revealed that root exudate composition is clearly different between the ABC transporter mutants, _pdr8-1_
and _pdr9-2_, and the wild-type (Col-0). Based on the number of differential features, both transporters seem to contribute to a similar extent to root exudation in _Arabidopsis thaliana_.
However, the number of features per compound is variable and does not indicate the number of exuded metabolites. Our analysis provides only a snapshot of all possibly exuded compounds
because it was restricted to semi-polar metabolites, and differences in exudation of more polar compounds, which certainly contribute to exudate profiles, are not considered as well as low
abundant metabolites which escaped our detection. A limitation in sensitivity is likely the reason why reported, very low abundant substrates of ABCG36/PDR8 and ABCG37/PDR9, such as IBA or
auxin26,27,28,29 could not be identified in this approach. We also did not detect glucosinolates or their degradation products in root exudates of any genotype. Recently, these compounds
have been identified in root exudates harvested from older Arabidopsis plants38 using identical extraction and detection methods. It is conceivable, that glucosinolate compounds are not
exuded in young seedlings or that their levels in exudates are far less abundant compared to older plants. Furthermore, glucosinolates and their degradation products were shown to accumulate
upon infection with the root colonizing fungus _Piriformospora indica_ 39. In leaves, ABCG36 was discussed to mediate glucosinolate transport and activation after pathogen attack22. The
absence of glucosinolate signals in all investigated genotypes does not answer the question whether or not ABCG36 also mediates glucosinolate exudation by roots. It would be interesting to
investigate this aspect under glucosinolate-inducing conditions. Along this line, it should generally be considered that changes in root exudation profiles of transporter mutants may only be
robustly detectable after certain abiotic or biotic stimuli; otherwise, the capabilities of transporters may be underestimated. Despite these limitations, the changes in root exudate
profiles in comparison to the wild-type are quite specific for both _pdr8-1_ and _pdr9-2_, indicating distinct substrate specificities of ABCG36 and ABCG37. The overlap of just 32 features
between both mutants may be traced to a few compounds as one metabolite is often represented by several features; e.g., we previously showed that up to 9 features are related to
dihydroxyscopoletin37. Overlapping substrate specificity between different ABC transporters frequently occurs and has also been reported for root exudation processes. Badri _et al_.4
compared the root exudate profiles of 8 different ABC transporter mutants and recorded the absence of several excreted compounds in up to 5 genotypes. Based on the number of differential
features (138), the effect of the _pdr8-1;pdr9-2_ double mutant on root exudation is synergistic as it clearly exceeds the sum of differential features of each single mutant (70). The
strongly impaired root exudation capacity of the double mutant may be a consequence of its compromised growth, as indicated by reduced seedling, shoot, and root fresh weights as well as by
shorter primary roots. However, the number of features in root exudates that are at least 1.5 fold higher compared to the blanks (_P_ < 0.01) is similar for all genotypes, indicating that
the double mutant is not generally compromised in root exudation because of its reduced growth. This suggests partial compensations of ABCG36 function in _pdr8-1_ by ABCG37 and of ABCG37
function in _pdr9-2_ by ABCG36, which are absent in the double mutant. Interestingly, we detected several features with increased intensities in root exudates of both mutant lines when
compared to the wild type. These features may be related to precursors of ABCG36 or ABCG37 substrates that accumulate in _pdr8-1_ or _pdr9-2_ roots and are exuded by other processes.
However, this scenario remains speculative until the respective compounds have been identified. Despite the overlap and possible partial compensation of both ABCG-type transporters, we
identified by our filtering procedure several features that are specifically related to ABCG36 and ABCG37 function. Among the 27 _pdr8-_specific features, one was recently identified as
thymidine38. This compound has been detected at various levels in root exudates of different Arabidopsis accessions40 and its amount decreases upon root colonization with _Piriformospora
indica_ 39. The biological relevance of thymidine exudation in Arabidopsis is unclear; however, it has been suggested to function as an efficient signal to attract larvae in _Zea mays_ and
_Typha latifolia_ 41. Irrespective of the unknown role of thymidine exudation in Arabidopsis, its decreased level in _pdr8-1_ provides evidence that thymidine exudation is an active process
and not merely a consequence of root cell death, as suggested earlier42. Among the 64 _pdr9-_specific features, two were recently annotated as dihydroxyscopoletin37. Mass spectra37
(Supplementary Fig. S2), increased intensities of specific mass spectrometric signals in root exudates after Fe deficiency as well as the absence in exudates of _f6′h1-1_ plants33 (Fig. 4),
further support the identity of these features as the coumarin dihydroxyscopoletin. The observed accumulation of coumarins in roots and root exudates during Fe deficiency supports a role of
this compound class for promoting Fe nutrition7, 30,31,32,33,34,35,36. However, previous reports mainly focused on coumarins with a low substitution degree, such as scopolin, scopoletin,
esculetin, and esculin, whereas coumarins showing a more complex substitution pattern, such as dihydroxyscopoletin, dihydroxyscopoletin glucoside, fraxetin, fraxin, and isofraxidin have only
occasionally been taken into account7, 33, 34. Also, tools such as the use of _f6′h1_ mutants or depletion of phenolic compounds in root exudates by reverse phase columns strongly supported
the importance of coumarins for Fe acquisition, but did not discriminate between individual coumarins7, 33, 34. Our data indicate that coumarins with more elaborate substitution patterns,
especially those containing a catechol moiety, accumulate more strongly in root exudates, suggesting that these compounds might contribute considerably to Fe acquisition. Fourcroy _et al_.7
reported strongly impaired Fe nutrition in ABCG37 deficient _pdr9-2_ and _pdr9-3_ mutants, which was correlated with decreased coumarin exudation. Our results show ABCG37-dependent changes
in coumarin profiles for a subset of coumarins, foremost dihydroxyscopoletin, fraxetin, esculetin, and their glucosides. Thus, our data and published work7 suggest that the exudation of
coumarins containing a catechol moiety (esculin, fraxetin, and dihydroxyscopoletin) facilitates Fe nutrition rather than the release of monohydroxylated coumarins (scopoletin, isofraxidin).
This is also supported by the work by Sisó-Terraza _et al_.36, where coumarins containing a catechol moiety, such as fraxetin, or coumarinolignols containing a catechol moiety, such as
5′hydroxycleomiscosin strongly accumulate upon Fe deficiency. Consistent with previous publications33, 34, 43, the authors also show that coumarins containing a catechol moiety chelate and
mobilize Fe more efficiently. Surprisingly, despite their comprehensive analytical approach using highly sensitive and selective methods, Sisó-Terraza _et al_.36 did not detect
dihydroxyscopoletin. Exposure of roots to light in our hydroponic system might be a reason. However, light only poorly penetrates the outer wall of our hydroponic containers (Supplementary
Fig. S3), and it remains to be investigated, whether the low light intensities reaching the roots are actually responsible for the generation of dihydroxyscopoletin. Also, oxygen
availability could account for differences in the detection of compounds. This seems to be unlikely, however, since aerated nutrient solutions, which were weekly changed, were used in both
systems. Whether accumulation of dihydroxyscopoletin depends on the age of plants remains to be investigated as well. In our study, plants and exudates were harvested 11 days after
germination, in contrast to 35 days36. If this was the case, it would be interesting to elucidate whether the lack of dihydroxyscopoletin at later stages of plant growth might be due to
decreased biosynthesis or increased metabolization. There is a strong prevalence of coumarin glucosides in roots and of aglycones in exudates33 (Figs 3–5), suggesting that coumarins are
transported in their free form. However, the presence of glucosides in exudates and the occurrence of coumarin glucoside specific hydrolyzing ß-glucosidases with predicted apoplastic
localization44, 45 indicate glucoside transport and subsequent hydrolysis. The decreased and increased levels of esculin and dihydroxyscopoletin glucoside in _pdr9-2_ root exudates and
roots, respectively, also imply that coumarin glucosides might constitute the transported compounds. Whether this is also true for fraxin cannot be concluded from our experiments because it
escaped detection in root exudates. The _bglu42_ mutant, containing a T-DNA insertion in a gene coding for a root-epidermis localized ß-glucosidases is impaired in the Fe deficiency-induced
secretion of fluorescent compounds to the rhizosphere46. This result suggests that glucoside hydrolysis precedes exudation. However, the chemical identities of the fluorescent compounds were
not investigated in this study, so that it still remains an open question, whether or not BGLU42 mediated hydrolysis is a prerequisite of coumarin transport, and if so, whether there is a
specificity with respect to the substitution patterns of the coumarin core structure. Whatever form is transported, our results clearly show selectivity of ABCG37 _in vivo_ with respect to
the substitution pattern of the coumarin core structure. As such, ABCG37 preferentially mediates the transport of coumarins with a catechol moiety, such as dihydroxyscopoletin, esculetin,
and fraxetin or their glucosides, whereas coumarins with one hydroxyl group, such as scopoletin or its glucoside and isofraxidin are not accepted as substrate. However, it should be
considered that additional factors, such as coumarin metabolism or presence and absence of competing compounds, might have an impact on the observed coumarin profiles in the two ABCG
transporter mutants. For example, Fourcoy _et al_.7 observed strongly reduced levels also of isofraxidin and scopoletin in root exudates of _pdr9-2_ plants. Compared to our experimental
setup, their plants were 14 days older with a more developed root system at the onset of Fe deficiency. Also, Fe deficiency induced scopoletin exudation seemed to be much stronger compared
to our results. Thus, it is possible that the different observations regarding _pdr9-2_ dependent coumarin profiles are due to additional factors, which do not affect ABCG37 transport
selectivity per se. Therefore, considering these inevitable drawbacks, the transport specificity observed in _in vivo_ experiments using transporter mutants might differ from the actual
selectivity of the transporter proteins for distinct compounds, which ultimately can only be assessed using _in vitro_ transport assays. METHODS REAGENTS AND STANDARDS All buffer and media
components and solvents were of reagent or HPLC grade and obtained from Sigma-Aldrich (St. Louis, MO, USA), J.T. Baker (Deventer, The Netherlands), and Roth (Karlsruhe, Germany). Fraxetin,
fraxin, and isofraxidin were purchased from PhytoLab (Vestenbergsgreuth, Germany) and Sigma-Aldrich. PLANT LINES AND GROWTH MEDIA _Arabidopsis thaliana_ accession Columbia (Col-0) and the
Col-0 mutant lines _pdr9-2_ (At3g53480; SALK_050885), _pen3-4_, here referred to as _pdr8-1_ (At1g59870; SALK_000578), the _pdr8-1;pdr9-2_ double mutant as well as _f6′h1-1_ (At3g13610;
SALK_132418 C) have been described previously20, 27, 28, 33, 37. Seeds were surface sterilized with chlorine gas and placed individually into the wells of a 96-well PCR plate filled with
agar according to the recently developed sterile hydroponic system37(Supplementary Fig. S3). The aerated growth medium contained 5 mM KNO3, 2.5 mM KH2PO4, 2 mM MgSO4, 2 mM Ca(NO3)2, 50 µM
Fe3+-EDTA, 70 µM H3BO3, 14 µM MnCl2, 0.5 µM CuSO4, 1 µM ZnSO4, 0.2 µM Na2MoO4, 10 µM CoCl2 and 5 g l−1 sucrose, pH 5.6. Agar (Duchefa, Haarlem, The Netherlands) was routinely purified47 and
added at a concentration of 0.75% (w/v). After two days of stratification at 4 °C in the dark, the hydroponic systems were placed on a rotary shaker (80 rpm) in a growth chamber at 22 °C
under illumination for 16 h daily (170 µmol s−1 m−2; Osram LumiluxDeLuxe Cool Daylight L58W/965, Osram, Augsburg, Germany). After 4 days, the PCR plates containing the germinated seedlings
were transferred to fresh medium without sucrose. For Fe deficiency treatment, Fe3+-EDTA was omitted from the fresh medium. After additional 7 days of growth, plants and exudates were
harvested. NON-TARGETED METABOLITE PROFILING OF SEMI-POLAR COMPOUNDS One experiment consisted of five replicates per genotype, in which one replicate represented the exudates of 8 plants,
and 3 experiments were performed in a 3 month interval. In total, the number of replicates was 13 for wild-type and _pdr9-2_, 15 for _pdr8-1_, and 8 for _pdr8-1;pdr9-2_. Additionally, we
collected the media of 15 blank samples, i.e. positions in the 96 well hydroponic system that did not receive seeds. Per replicate, the exudates from 8 roots were combined, spiked with 100
nmol 2,4 dichlorophenoxy acetic acid (Duchefa) and all replicates were processed simultaneously as described37. Non-targeted metabolite profiling was performed according to Strehmel _et
al_.38. Only datasets passing the quality check (retention time shift < 5 s and intensity deviation < 30% of the internal standard, 2,4 dichlorophenoxy acetic acid) were considered for
further analysis. Replicates for each genotype were grouped, and data were processed using the R package XCMS48 with an intensity threshold of 1,000 and a minfrac value of 1. Features
(_m/z_ retention time pair) were regarded as different between genotypes if the median intensities differed by more than 1.5 fold at _P_ < 0.01 (Student’s _t_-test, two-tailed, equal
variance). TARGETED COUMARIN PROFILING Extraction, measurement, and quantification of coumarins were performed as described37 using 4-methyl-umbelliferon as standard. Quantifier and
qualifier transition as well as compound specific parameters for fraxin, fraxetin, and isofraxidin are listed in Supplementary Table S2. REFERENCES * Walker, T. S., Bais, H. P., Grotewold,
E. & Vivanco, J. M. Root exudation and rhizosphere biology. _Plant Physiol._ 132, 44–51 (2003). Article CAS PubMed PubMed Central Google Scholar * Loyola-Vargas, V. M., Broeckling,
C. D., Badri, D. & Vivanco, J. M. Effect of transporters on the secretion of phytochemicals by the roots of _Arabidopsis thaliana_. _Planta_ 225, 301–310 (2007). Article CAS PubMed
Google Scholar * Sugiyama, A., Shitan, N. & Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in
legume-Rhizobium symbiosis. _Plant Physiol._ 144, 2000–2008 (2007). Article CAS PubMed PubMed Central Google Scholar * Badri, D. V. _et al_. Altered profile of secondary metabolites in
the root exudates of _Arabidopsis_ ATP-binding cassette transporter mutants. _Plant Physiol._ 146, 762–771 (2008). Article CAS PubMed PubMed Central Google Scholar * Badri, D. V. _et
al_. An ABC transporter mutation alters root exudation of phytochemicals that provoke an overhaul of natural soil microbiota. _Plant Physiol._ 151, 2006–2017 (2009). Article CAS PubMed
PubMed Central Google Scholar * Kretzschmar, T. _et al_. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. _Nature_ 483, 341–344 (2012). Article
ADS CAS PubMed Google Scholar * Fourcroy, P. _et al_. Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by _Arabidopsis_ roots in response to iron
deficiency. _New Phytol._ 201, 155–167 (2014). Article CAS PubMed Google Scholar * Verrier, P. J. _et al_. Plant ABC proteins–a unified nomenclature and updated inventory. _Trends Plant
Sci._ 13, 151–159 (2008). Article CAS PubMed Google Scholar * Hwang, J. U. _et al_. Plant ABC Transporters Enable Many Unique Aspects of a Terrestrial Plant’s Lifestyle. _Mol. Plant_ 9,
338–355 (2016). Article CAS PubMed Google Scholar * Geisler, M. & Murphy, A. S. The ABC of auxin transport: the role of p-glycoproteins in plant development. _FEBS Lett._ 580,
1094–1102 (2006). Article CAS PubMed Google Scholar * Lu, Y. P., Li, Z. S. & Rea, P. A. AtMRP1 gene of _Arabidopsis_ encodes a glutathione _S_-conjugate pump: isolation and
functional definition of a plant ATP-binding cassette transporter gene. _Proc. Natl. Acad. Sci. USA._ 94, 8243–8248 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Lu,
Y. P. _et al_. AtMRP2, an _Arabidopsis_ ATP binding cassette transporter able to transport glutathione _S_-conjugates and chlorophyll catabolites: functional comparisons with Atmrp1. _Plant
Cell._ 10, 267–282 (1998). CAS PubMed PubMed Central Google Scholar * Tommasini, R. _et al_. An ABC-transporter of _Arabidopsis thaliana_ has both glutathione-conjugate and chlorophyll
catabolite transport activity. _Plant J._ 13, 773–780 (1998). Article CAS PubMed Google Scholar * Klein, M. _et al_. The plant multidrug resistance ABC transporter AtMRP5 is involved in
guard cell hormonal signalling and water use. _Plant J._ 33, 119–129 (2003). Article CAS PubMed Google Scholar * Klein, M. _et al_. Disruption of AtMRP4, a guard cell plasma membrane
ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. _Plant J._ 39, 219–236 (2004). Article CAS PubMed Google Scholar * Song, W.-Y.
_et al_. Arsenic tolerance in _Arabidopsis_ is mediated by two ABCC-type phytochelatin transporters. _Proc. Natl. Acad. Sci. USA_ 107, 21187–21192 (2010). Article ADS CAS PubMed PubMed
Central Google Scholar * Park, J. _et al_. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. _Plant J_ 69, 278–288 (2012). Article CAS PubMed
Google Scholar * Brunetti, P. _et al_. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in _Arabidopsis_. _J. Exp. Bot._
66, 3815–3829 (2015). Article CAS PubMed PubMed Central Google Scholar * Nagy, R. _et al_. The _Arabidopsis_ ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol
hexa_kis_phosphate transporter involved in guard cell signaling and phytate storage. _J. Biol. Chem._ 284, 33614–33622 (2009). Article CAS PubMed PubMed Central Google Scholar * Kobae,
Y. _et al_. Loss of AtPDR8, a plasma membrane ABC transporter of _Arabidopsis thaliana_, causes hypersensitive cell death upon pathogen infection. _Plant Cell Physiol_. 47 (2006). * Stein,
M. _et al_. _Arabidopsis_ PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. _Plant Cell_ 18,
731–746 (2006). Article CAS PubMed PubMed Central Google Scholar * Clay, N. K., Adio, A. M., Denoux, C., Jander, G. & Ausubel, F. M. Glucosinolate metabolites required for an
_Arabidopsis_ innate immune response. _Science_ 323, 95–101 (2009). Article ADS CAS PubMed Google Scholar * Bednarek, P. _et al_. A glucosinolate metabolism pathway in living plant
cells mediates broad-spectrum antifungal defense. _Science_ 323, 101–106 (2009). Article ADS CAS PubMed Google Scholar * Lu, X. _et al_. Mutant allele-specific uncoupling of
PENETRATION3 functions reveals engagement of the ATP-Binding cassette transporter in distinct tryptophan metabolic pathways. _Plant Physiol._ 168, 814–827 (2015). Article ADS CAS PubMed
PubMed Central Google Scholar * Kim, D. Y., Bovet, L., Maeshima, M., Martinoia, E. & Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance.
_Plant J._ 50, 207–218 (2007). Article CAS PubMed Google Scholar * Strader, L. C. & Bartel, B. The Arabidopsis PLEIOTROPIC DRUG RESISTANCE8/ABCG36 ATP binding cassette transporter
modulates sensitivity to the auxin precursor indole-3-butyric acid. _Plant Cell_ 21, 1992–2007 (2009). Article CAS PubMed PubMed Central Google Scholar * Růžička, K. _et al_.
_Arabidopsis_ PIS1 encodes the ABCG37 transporter of auxinic compounds including the auxin precursor indole-3-butyric acid. _Proc. Natl. Acad. Sci. USA_ 107, 10749–10753 (2010). Article
PubMed PubMed Central Google Scholar * Ito, H. & Gray, W. M. A gain-of-function mutation in the _Arabidopsis_ pleiotropic drug resistance transporter PDR9 confers resistance to
auxinic herbicides. _Plant Physiol._ 142, 63–74 (2006). Article CAS PubMed PubMed Central Google Scholar * Strader, L. C., Monroe-Augustus, M., Rogers, K. C., Lin, G. L. & Bartel,
B. _Arabidopsis_ iba response5 suppressors separate responses to various hormones. _Genetics_ 180, 2019–2031 (2008). Article CAS PubMed PubMed Central Google Scholar * Fourcroy, P.,
Tissot, N., Gaymard, F., Briat, J. F. & Dubos, C. Facilitated Fe nutrition by phenolic compounds excreted by the _Arabidopsis_ ABCG37/PDR9 transporter requires the IRT1/FRO2
high-affinity root Fe(2+) transport system. _Mol. Plant_ 9, 485–488 (2016). Article CAS PubMed Google Scholar * Lan, P. _et al_. iTRAQ protein profile analysis of _Arabidopsis_ roots
reveals new aspects critical for iron homeostasis. _Plant Physiol._ 155, 821–834 (2011). Article CAS PubMed Google Scholar * Rodríguez-Celma, J. _et al_. Mutually exclusive alterations
in secondary metabolism are critical for the uptake of insoluble iron compounds by _Arabidopsis_ and _Medicago truncatula_. _Plant Physiol._ 162, 1473–1485 (2013). Article PubMed PubMed
Central Google Scholar * Schmid, N. B. _et al_. Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in _Arabidopsis_. _Plant Physiol._ 164,
160–172 (2014). Article CAS PubMed Google Scholar * Schmidt, H. _et al_. Metabolome analysis of _Arabidopsis thaliana_ roots identifies a key metabolic pathway for iron acquisition.
_PloS One_ 9, e102444 (2014). Article ADS PubMed PubMed Central Google Scholar * Clemens, S. & Weber, M. The essential role of coumarin secretion for Fe acquisition from alkaline
soil. _Plant Signal. Behav._ 11, e1114197 (2016). Article PubMed Google Scholar * Sisó-Terraza, P. _et al_. Accumulation and secretion of coumarinolignans and other coumarins in
_Arabidopsis thaliana_ roots in response to iron deficiency at high pH. _Front. Plant Sci._ 7, 1711 (2016). Article PubMed PubMed Central Google Scholar * Ziegler, J. _et al_.
Non-targeted profiling of semi-polar metabolites in _Arabidopsis_ root exudates uncovers a role for coumarin secretion and lignification during the local response to phosphate limitation.
_J. Exp. Bot._ 67, 1421–1432 (2016). Article CAS PubMed Google Scholar * Strehmel, N., Böttcher, C., Schmidt, S. & Scheel, D. Profiling of secondary metabolites in root exudates of
_Arabidopsis thaliana_. _Phytochem._ 108, 35–46 (2014). Article CAS Google Scholar * Strehmel, N. _et al_. Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. _Int.
J. Mol. Sci_. 17 (2016). * Mönchgesang, S. _et al_. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. _Sci. Rep._ 6, 29033 (2016). Article ADS
PubMed PubMed Central Google Scholar * Sérandour, J. _et al_. Ubiquitous water-soluble molecules in aquatic plant exudates determine specific insect attraction. _PloS One_ 3, e3350
(2008). Article ADS PubMed PubMed Central Google Scholar * Kumar, R., Pandey, S. & Pandey, A. Plant roots and carbon sequestration. _Curr. Sci._ 91, 885–890 (2006). CAS Google
Scholar * Mladěnka, P. _et al_. _In vitro_ interactions of coumarins with iron. _Biochimie_ 92, 1108–1114 (2010). Article PubMed Google Scholar * Ahn, Y. O. _et al_. Scopolin-hydrolyzing
β-glucosidases in roots of _Arabidopsis_. _Plant Cell Physiol_. 51, 132–143 (2010). * Xu, Z. _et al_. Functional genomic analysis of _Arabidopsis thaliana_ glycoside hydrolase family 1.
_Plant Mol. Biol._ 55, 343–367 (2004). Article CAS PubMed Google Scholar * Zamioudis, C., Hanson, J. & Pieterse, C. M. J. β-Glucosidase BGLU42 is a MYB72-dependent key regulator of
rhizobacteria-induced systemic resistance and modulates iron deficiency responses in _Arabidopsis_ roots. _New Phytol._ 204, 368–379 (2014). Article CAS PubMed Google Scholar * Ward, J.
T., Lahner, B., Yakubova, E., Salt, D. E. & Raghothama, K. G. The effect of iron on the primary root elongation of _Arabidopsis_ during phosphate deficiency. _Plant Physiol_. 147 (2008).
* Smith, C. A., Want, E. J., O’Maille, G., Abagyan, R. & Siuzdak, G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and
identification. _Anal. Chem._ 78, 779–787 (2006). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Birgit Ortel for technical assistance. This work was
supported by a competitive grant from the Leibniz-Association (SAW-PAKT) “Chemical Communication in the Rhizosphere”, and by core funding to the Leibniz Institute of Plant Biochemistry from
the state of Saxony-Anhalt and the federal Republic of Germany. The authors declare no conflict of interest. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Molecular Signal
Processing, Leibniz Institute of Plant Biochemistry, D-06120, Halle (Saale), Germany Jörg Ziegler & Steffen Abel * Department of Stress and Developmental Biology, Leibniz Institute of
Plant Biochemistry, D-06120, Halle (Saale), Germany Stephan Schmidt, Nadine Strehmel & Dierk Scheel * Institute of Biochemistry and Biotechnology, Martin Luther University Halle
Wittenberg, D-06120, Halle (Saale), Germany Steffen Abel * Department of Plant Sciences, University of California-Davis, Davis, CA, 95616, USA Steffen Abel Authors * Jörg Ziegler View author
publications You can also search for this author inPubMed Google Scholar * Stephan Schmidt View author publications You can also search for this author inPubMed Google Scholar * Nadine
Strehmel View author publications You can also search for this author inPubMed Google Scholar * Dierk Scheel View author publications You can also search for this author inPubMed Google
Scholar * Steffen Abel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.Z. designed and performed the experiments, analyzed coumarins, and
wrote the paper. S.S and N.S recorded the non-targeted metabolite profiling data. D.S and S.A. supervised the project and/or commented on the manuscript. CORRESPONDING AUTHOR Correspondence
to Jörg Ziegler. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFO DATASET RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ziegler, J., Schmidt, S., Strehmel,
N. _et al._ Arabidopsis Transporter ABCG37/PDR9 contributes primarily highly oxygenated Coumarins to Root Exudation. _Sci Rep_ 7, 3704 (2017). https://doi.org/10.1038/s41598-017-03250-6
Download citation * Received: 21 February 2017 * Accepted: 25 April 2017 * Published: 16 June 2017 * DOI: https://doi.org/10.1038/s41598-017-03250-6 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative