Sex-specific responses to winter flooding, spring waterlogging and post-flooding recovery in populus deltoides
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Winter flooding events are common in some rivers and streams due to dam constructions, and flooding and waterlogging inhibit the growth of trees in riparian zones. This study
investigated sex-specific morphological, physiological and ultrastructural responses to various durations of winter flooding and spring waterlogging stresses, and post-flooding recovery
characteristics in _Populus deltoides_. There were no significant differences in the morphological, ultrastructural and the majority of physiological traits in trees subjected to medium and
severe winter flooding stresses, suggesting that males and females of _P_. _deltoides_ were winter flooding tolerant, and insensitive to winter flooding duration. Males were more tolerant to
winter flooding stress in terms of photosynthesis and chlorophyll fluorescence than females. Females displayed greater oxidative damage due to flooding stress than males. Males developed
more efficient antioxidant enzymatic systems to control reactive oxygen species. Both sexes had similarly strong post-flooding recovery capabilities in terms of plant growth, and
physiological and ultrastructural parameters. However, Males had better recovery capabilities in terms of pigment content. These results increase the understanding of poplars’s adaptation to
winter flooding stress. They also elucidate sex-specific differences in response to flooding stress during the dormant season, and during post-flooding recovery periods. SIMILAR CONTENT
BEING VIEWED BY OTHERS EVALUATION OF SECONDARY SEXUAL DIMORPHISM OF THE DIOECIOUS _AMARANTHUS PALMERI_ UNDER ABIOTIC STRESS Article Open access 12 August 2023 EXPLORING WATER RELATIONS AND
PHENOLOGICAL TRAITS OF _BETULA UTILIS_ (D. DON) IN WESTERN HIMALAYAN TREELINE ECOTONE Article Open access 06 September 2024 MORPHOLOGICAL AND PHYSIOLOGICAL RESPONSES OF TWO WILLOW SPECIES
FROM DIFFERENT HABITATS TO SALT STRESS Article Open access 26 October 2020 INTRODUCTION Riparian forests often experience a wide range of flooding or waterlogging conditions, which can cause
declines in growth and even the death of certain plant species. Furthermore, winter flooding events are common in some rivers and streams due to the artificial water level regulation
associated with dams1, 2. For example, to operate China’s Three Gorges Dam at full capacity, the water level of the Three Gorges Reservoir was artificially regulated at a winter maximum of
175 m for energy generation, and a summer minimum of 145 m for flood control1, 2. Thus, the hydrological regimes brought about by dams can be the opposite of the river’s natural flood
rhythms. This can cause some riparian forests to suffer winter flooding stress while enduring floods of various durations and depths. Flood tolerance varies greatly with plant species and
genotype, the sex of dioecious plants, plant age, flooding duration and depth, and flooding season3,4,5,6,7,8,9. Previous studies have confirmed that _Populus deltoides_ is flood-tolerant,
and can spread widely in European and North American riparian and floodplain zones5, 6, 10,11,12. _P. deltoides_ is not naturally occurring in China, and must be imported from North America.
It is recognized as a desirable tree species for the construction of riparian-protective forests in China because of its fast growth and strong tolerance to waterlogging stress5, 6. _P.
deltoides_ belongs to Sect. _Aigeiros_. Revegetation activities in the water level fluctuation zone of the Three Gorges Reservoir have demonstrated that Sect. _Aigeiros_ poplars, including
_P. nigra_ and _P_. × _canadensis_, could be suitable for the construction of riparian-protective forests1, 2. However, the mechanisms by which _P. deltoides_ adapts to winter flooding and
recovers afterward are still unknown. Previous studies have focused on the phenotypic plasticity and adaptive plasticity of poplars in response to summer flooding or waterlogging stresses
during the plant growth stage of development4,5,6,7,8. Poplars are deciduous species. Deciduous plants’ physiological and molecular properties are markedly different during the dormant and
growing seasons13,14,15. However, few studies have investigated the responses of poplars to winter flooding stress during the dormant season. The morphological, physiological and
ultrastructural responses of poplars to winter flooding stress during dormancy, and during post-growth recovery, remain poorly understood. _Populus_ spp. is a dioecious species. Previous
studies have established the sex-specific morphological, physiological, and biochemical characteristics of poplars in response to environmental stress16,17,18,19,20,21. Different sexes of
poplars might employ different strategies to cope with abiotic stress, and that males possess a better self-protection mechanism than females. Our previous studies have also demonstrated
that _P. deltoides_ males develop better cellular defense mechanisms against waterlogging stress than females, making males less susceptible6. However, Juvany and Munné-Bosch22 reviewed
responses to abiotic and biotic stresses and suggested that general conclusions about sex-related stress tolerance in plants were not possible. For example, Nielsen _et al_.4 and Rood _et
al_.8 suggested that female _P. angustifolia_ were more flood-tolerant than males, and that females could be more successful in lower, more flood-prone sites. However, Letts _et al_.7
reported that there were no significant differences in the photosynthetic gas exchange, leaf reflectance, chlorophyll fluorescence or photosynthetic water-use efficiency of female and male
_P. angustifolia_. In addition, comparisons of the sex-dependent responses of poplars to abiotic stress have largely been conducted in summer during the fast growth stage. Little study has
occurred regarding responses to winter flooding during dormancy and post-growth recovery. Consequently, the physiological mechanisms underlying sex-related differences in winter flooding
stress responses remain poorly understood. The present study investigated sexual dimorphism in _P. deltoides_ during winter flooding, waterlogging, and post-flooding recovery. The main
research questions were as follows. (1) How does winter flooding stress affect _P. deltoides_? (2) What are the responses to different durations of winter flooding? (3) Do these responses
differ between the sexes? (4) Do winter flooding-stressed trees recover to normal levels in terms of morphological, physiological and ultrastructural traits? (5) Are there sexual differences
in recovery characteristics? To answer these questions, we measured morphological, physiological and ultrastructural variations to reveal gender-related responses to winter flooding stress
and post-flooding recovery. RESULTS COMPARATIVE ANALYSIS OF SURVIVAL RATES AND MORPHOLOGICAL TRAITS Generally, all flooding-stressed _P. deltoides_ seedlings survived, and epicormic shoot
germination from nodes and new leaf emergence occurred almost simultaneously with controls. With increasing flooding duration, significant visible damages, such as mortality, leaf chlorosis,
leaf necrosis, or leaf abscission were not observed during the plant growth stage of development. Morphological adaptations such as hypertrophied lenticels, aerenchyma tissues and
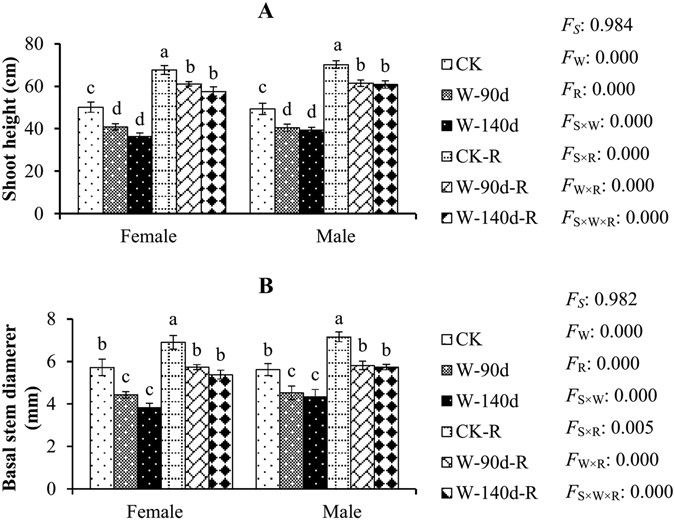
adventitious roots, which often occur when plants are exposed to summer waterlogging stress, did not appear in the present study. When flooding was combined with waterlogging, shoot height
(Fig. 1A) and basal stem diameter (Fig. 1B) were significantly inhibited in comparison to controls. Greater inhibition was observed with increasing flooding duration. Significant differences
were not found between the two flooding treatments (W-90d and W-140d). In addition, significant differences in the shoot height and basal stem diameter of females and males were not found
within each flooding treatment. During the recovery stage, the shoot heights and basal stems of flooding stressed females and males both recovered well. For example, shoot height growth
rates in females and males were 38.8% and 42.4% in CK, respectively, whereas they were 49.8% and 51.8% in W-90d, and 57.9% and 54.4% in W-140d during the recovery stage. The basal stem
growth rates in females and males were 21.0% and 27.6% in CK, respectively, whereas they were 29.3% and 28.8% in W-90d, and 40.8% and 32.0% in W-140d during the recovery stage. In addition,
significant differences were not found between the two post-flooding recovery treatments (W-90d-R and W-140d-R). In addition, there were no significant differences in shoot height and basal
stem during the recovery stage between females and males within each post-flooding recovery treatment. WINTER FLOODING STRESS AFFECTED THE PHYSIOLOGICAL TRAITS OF TWO-YEAR-OLD _P. DELTOIDES_
TREES AT AN EARLY STAGE OF DEVELOPMENT Severe flooding stress (W-140d) significantly decreased _A_ (Table 1), _Fv/Fm_ (Fig. 2A), _Yield_ (Fig. 2B), and _ETR_ (Fig. 2C), whereas it
significantly increased REL (Fig. 3A), GSH content (Fig. 4A), H2O2 levels (Fig. 5A), and activities of POD (Fig. 6A) and SOD (Fig. 6B) in both sexes of _P. deltoides_. However, significant
variations in RWC _E_ (Table 1), _WUEi_ (Table 1), _qP_ (Fig. 2D), _qN_ (Fig. 2E), (Fig. 3B), MDA (Fig. 5B), APx (Fig. 6C) were found only in either females or males, and significant
variations in _Chl a_ (Table 2), _Chl b_ (Table 2), _Caro_ (Table 2), _Total Chl_ (Table 2), _Chl a_/_Chl b_ (Table 2), _gs_ (Table 1), soluble protein (Fig. 4B), reducing sugar (Fig. 4C),
proline (Fig. 4D), ·OH (Fig. 5C), CAT (Fig. 6D), and GR (Fig. 6E) were absent in both sexes of _P. deltoides_ exposed to W-140d conditions (Supplementary Table 1). Winter flooding durations
had little effects on majority physiological parameters in both sexes of _P. deltoides_. For example, _Chl a_, _Chl b_, _Caro_, _Total Chl_, _Chl a_/_Chl b_, _qP_, _qN_, REL, RWC, GSH,
soluble protein, reducing sugar, proline, MDA, ·OH, O2 .− (Fig. 5D), POD, SOD, CAT, and GR in both sexes of _P. deltoides_ did not vary significantly between medium flooding stress (W-90d)
and severe winter flooding stress (W-140d). However, significant variations in _Ci_ (Table 1) and _WUEi_ were found in both sexes of _P. deltoides_ between W-90d and W-140d treatments. In
addition, there were significant variations in some parameters, including _A_, _gs_, _E_, _Fv/Fm_, _Yield_, _ETR_, H2O2, and APx, but only in either females or males (Supplementary Table 1).
When the flood water drained away, _Chl b, Chl a_/_Chl b_, _A_, _WUEi_, _Fv/Fm_, _Yield_, _ETR_, _qP_, _qN_, REL, RWC, GSH, soluble protein, proline, H2O2, MDA, ·OH, O2 .−, POD, SOD, CAT,
and GR in previously severe flooded females and males of _P. deltoides_ after 15 d recovery growth (W-140d-R) recovered to normal or even better levels than in individual unflooded plants
(Supplementary Table 1). In addition, previously flooded plants subjected to different flooding durations (W-90d-R and W-140d-R) exhibited insignificant differences in terms of _A_, _Chl a_,
_Caro_, _Total Chl_, _Chl a_/_Chl b_, _Fv/Fm_, _Yield_, _ETR_, _qP_, _qN_, RWC, REL, GSH, soluble protein, reducing sugar, proline, H2O2, MDA, OH, O2 .−, POD, SOD, CAT, and GR after 15 days
of recovery growth after flood water was drained away. SEXUALLY-DEPENDENT PHYSIOLOGICAL RESPONSES TO WINTER FLOODING COMBINED WITH WATERLOGGING STRESS, AND POST-FLOODING RECOVERY There were
no significant intersexual differences in terms of _A, gs, E, Chl a_, _Chl b_, _Caro_, _Total Chl_, _Chl a_ /_Chl b_, _Yield, ETR_, _qP_, REL, GSH, soluble protein, reducing sugar, proline,
·OH, POD, SOD, CAT, and GR under severe winter flooding conditions (W-140d), and severe winter flooding stresses had almost equal effects on males and females in terms of _gs, E, Chl b_,
_Caro_, _Total Chl_, _qP_, REL, GSH, reducing sugar, and POD. However, although significant declines in _A_, _Fv/Fm_, _Yield_, and _ETR_ were found in both sexes of _P. deltoides_ under
severe winter flooding stress condition (W-140d), significant variations in _A_, _Fv/Fm_, _Yield_, and _ETR_ (Supplementary Table 1) under medium winter flooding stress conditions (W-90d)
were only found in females, but not in males. Interestingly, the sexes did differ in terms of H2O2, MDA, O2 .−, OH, APx, and CAT (Supplementary Table 1). Under severe flooding conditions
(W-140d), significant increases in MDA and O2 .−, and increases in OH, were observed in females, while significant decreases in O2 .−, and decreases in ·OH, and insignificant variation in
MDA were observed in males. In addition, compared with flooded males, the flooded females had higher levels of H2O2, O2 .−, and MDA. Although both sexes of _P. deltoides_ exposed to winter
flooding stress displayed similar trends and activity levels of POD, SOD, and GR, there were differences in the trends of APx and CAT activities (Supplementary Table 1). Males showed
significant increases in APx activity but no variation in CAT activity. In contrast, female APx activity did not vary, while CAT activity decreased. In general, previously-flooded females
and males had similar recovery capabilities in terms of majority physiological parameters during the post-recovery stage. However, when the flood water drained away, males showed significant
higher levels in _Ci_, _E_, _Chl a_, _Chl b_, and _Total Chl_, and significant lower levels in _WUEi_ and MDA in comparison to females under W-90d-R conditions. In addition, compared with
females, males showed significant higher levels in _Ci_, _E_, _Chl a_, _Total Chl_, and APx, and significant lower levels in _WUEi_ and MDA under W-140d-R conditions. COMPARATIVE ANALYSIS OF
ULTRASTRUCTURAL MORPHOLOGY We next asked how the ultrastructural morphology of _P. deltoides_ females and males varied in response to winter flooding stress and post flooding recovery.
Thus, we examined their cellular ultrastructure. As shown in Fig. 7, chloroplasts of both sexes were well arranged. The majority had distinct granum regions, and some small plastoglobules
were found, especially in females. Under medium flooding stress (W-90d), plastoglobules became enlarged in both sexes, and their numbers decreased in females. However, under severe flooding
stress (W-140d), disintegrated chloroplasts and numerous tilted granal stacks were found, and plastoglobules gradually disappeared. Some large vesicles, starch grains, and swollen
mitochondria were found, especially in females. With the growth of development, more distinct granum and small starch grains developed. During the post-flooding recovery stage, the
percentage of vesicles decreased, while distinct granum regions increased. The number of mitochondria, small starch grains, and small plastoglobules increased. In addition, no significant
differences in the ultrastructural morphology of _P. deltoides_ females and males were observed. DISCUSSION Winter flooding is a special event in some rivers due to the artificial water
level regulation. Kozlowski3 suggested that flooding during the growing season can have greater negative effects on deciduous species than flooding during the dormant season. However,
information about morphological and physiological responses to winter flooding stress is still scarce. Therefore, some researchers have suggested that the mechanisms of winter flooding
adaptation in flooding tolerant species should be investigated to better understand their survival responses to reversed flooding patterns1,2,3 All plants survived 140 days of winter
flooding without mortality, leaf chlorosis, leaf necrosis, or leaf abscission, which suggests that, in terms of seedling survival, both female and male _P. deltoides_ are winter flood
tolerant. Therefore, both female and male _P. deltoides_ are superior candidates for the construction of protective riparian forests in winter flooding areas. Flooding or waterlogging during
the growing season can induce aerenchyma tissues and adventitious roots in a wide variety of both flood-intolerant and tolerant angiosperms and gymnosperms, especially in flood tolerant
species, as concluded by Kozlowski3. However, some often-visible morphological adaptations, including the formation of hypertrophied lenticels, aerenchyma tissues and adventitious roots,
were not observed in winter flooding-stressed female and male _P. deltoides_, which might be a result of low oxygen demands during the dormant season. The majority of previous studies have
demonstrated that flooding or waterlogging stresses can decrease plant growth and development, pigment content, photosynthetic capacity, stomatal conductance, and chlorophyll fluorescence.
They may also increase ROS and MDA levels, increase the contents of some cellular non-enzymatic components, and increase the activity of antioxidant enzyme systems2,3,4, 6,7,8, 23, 24.
Compared with summer waterlogging during growth and development, winter flooding stress during the dormant season caused fewer physiological dysfunctions in _P. deltoides_ 5, 6. The duration
of flooding significantly affects survival rates, plant growth and yield, and physiological responses1, 3, 9. Our results suggest that female and male of _P. deltoides_ were not very
sensitive to flooding duration. The duration of winter flooding had little effect on plant growth, physiology, and biochemistry due to the low levels metabolic activity occurring during the
dormant season. Normally, although the stomata reopen slowly and the rate of photosynthesis increases when flood water drains away, previously-flooded plants may have difficultly recovering
to normal levels of growth, photosynthetic capacity, and other physiological responses, because absorption of water by their small root systems can’t adequately replenish transpirational
losses within a short period3, 25. In present study, the ultrastructural morphology of mesophyll cells and majority physiological parameters in previously flooded plants recovered to normal
after 15 d recovery growth. These phenomena may be because the winter flooding stress caused little damage to the one-year-old root systems, and then the previously flooded plants could
absorb water and nutrients normally after flood water drained away. These results also suggest that _P. deltoides_ has strong self-repairing capabilities during post-flooding recovery. Some
studies of trees have observed sex-specific responses to a number of biotic stresses26, 27 and environmental stresses such as waterlogging stress6, water deficit16, chilling17, salinity21,
enhanced UV-B radiation28, atmospheric CO2 enrichment29, nutrient deficiency19, 30, excess manganese, and a combination of different stresses31, 32. These studies concluded that females seem
to be more sensitive to environmental stress and usually experience greater negative effects. In other cases, female plants exhibited better tolerance of adverse conditions than males,
females showed a more conservative strategy of water use or higher photosynthetic rates compared with males4, 33,34,35. For example, Male and female _P. angustifolia_ genotypes grew
similarly with favorable water levels, but males tended to be more inhibited by flooding, while females were more flood tolerant4. Females of some perennial herbs are more resistant to
drought stress than males35. Therefore, the information available thus far is insufficient for establishing generalizations about whether male and female dioecious plants show differential
abiotic stress resistance. Furthermore, most comparisons of male and female performance have mainly focused on short-term physiological measurements during the growth season. In this paper
we highlight the need for physiological studies of sexual dimorphism in dioecious plants in response to different durations of winter flooding stress during the dormancy season and during
post-flooding recovery. Both sexes had similarly strong flooding tolerances in terms of the majority of physiological parameters and ultrastructural variations at an early stage of
development. Differences between sexes do not always exist. For example, the female and male _P. angustifolia_ did not display significant differences in photosynthetic gas exchange, leaf
reflectance, chlorophyll fluorescence, and WUE in response to groundwater availability, and soil water typically declines between May and September7. The similar sexual responses of _P.
deltoides_ to winter flooding stress may be the result of low metabolic activity during the dormancy season and early growth developmental stage. The significant variations in _A_,
_Fv_/_Fm_, _Yield_, and _ETR_ (Supplementary Table 1) only in females under medium winter flooding stress conditions (W-90d) suggest that female _P. deltoides_ are more sensitive than males
to winter flooding stress in terms of photosynthesis and chlorophyll fluorescence. Our results are consistent with previous studies. For example, female _P. yunnanensis_ grown in China
during a drought exhibited gas exchange rate depression and greater damage to cell organelles than male did32. Female _P. cathayana_ grown in China were also more responsive and showed
greater negative effects on net photosynthesis than males when grown under increased drought stress and elevated temperatures16. Additionally, although the previously-flooded females and
males had similar recovery capabilities in the post-flood period, males had better recovery capabilities than females in terms of pigment content, especially _chl a_. Root oxygen deficiency
resulting from flooding stress caused photoxidative damage to leaves via increased generation of ROS2, 3. These are important signaling molecules indicative of oxidative stress. They can
directly attack membrane lipids, resulting in lipid peroxidation and oxidation of proteins and nucleic acids6, 36,37,38. The major indicators of ROS accumulation are H2O2, O2 .−, and ·OH.
One of the most frequently-used indicators of lipid peroxidation is MDA, and MDA content reflects the degree of membrane lipid peroxidation6, 37, 38. The sexes differ in terms of H2O2, O2
.−, OH, MDA, APx, and CAT in response to winter flooding stress, which suggest that female plants encountered more serious oxidative damage during winter flooding stress than males. Plants
can protect cellular and sub-cellular systems to control ROS levels and membrane lipid peroxidation with antioxidant enzymatic systems. These results indicate that males develop more
efficient antioxidant enzymatic systems to control ROS accumulation than females do. Our results indicate that, in terms of antioxidant enzymatic systems, _P. deltoides_ males are more
tolerant to winter flooding stress than females. Previous studies have shown that females of _P. yunnanensis_, _P. cathayana_, and _P. deltoides_ exhibit greater ROS accumulation and
oxidative stress damage, and have less efficient antioxidant enzymatic systems than males during environmentally stressful growth season conditions6, 17, 18, 21, 28, 30. In conclusion, both
sexes of _P. deltoides_ are winter flood tolerant, based on seedling survival, and morphological, physiological, and ultrastructural responses. Winter flooding stress differentially affected
physiological traits in _P. deltoides_ at early growth stages of development. Significant variations in terms of _chl a_, _chl b_, _caro_, _total chl_, _chl a_/_chl b_, _gs_, soluble
protein, reducing sugar, proline, ·OH, CAT, and POD were absent under severe winter flooding stress in both sexes. In addition, fatally-damaging ultrastructural responses were not found in
_P. deltoides_. In both sexes, the duration of winter flooding stress had insignificant effects on morphological, ultrastructural, and the majority of physiological responses, except for
_A_, _gs_, _E_, _Fv/Fm_, _Yield_, _ETR_, H2O2, and APx. When flood water was drained away, previously-flooded plants grew at a faster rate than unflooded plants. The majority of
physiological parameters and ultrastructural morphology of mesophyll cells in previously-flooded plants could recover to normal levels in previously-flooded plants, whose recover was better
than that of unflooded plants. Both sexes had similar responses to W-90d and W-140 conditions in terms of majortity physiological parameters. However, females were more sensitive than males
to winter flooding stress in terms of photosynthesis and chlorophyll fluorescence, based on their significant declines in _A_, _Fv_/_Fm_, _Yield_, and _ETR_ under W-90d conditions. Females
encountered more serious oxidative damage than males under flooding conditions. The results indicate that males develop more efficient antioxidant enzymatic systems to control ROS
accumulation than females. Additionally, although previously-flooded females and males had strong and similar post-flooding recovery capabilities, males recovered better in terms of pigment
content, especially _chl a_. This study provides new light on the adaptation mechanisms of _P_. _deltoides_ trees subject to winter flooding stress. It also increases the understanding of
sexually-dimorphic responses to flooding stress during the dormant season, and to post-flooding recovery. MATERIALS AND METHODS PLANT MATERIALS AND EXPERIMENTAL DESIGN One-year-old cuttings
of _P. deltoides_ were collected from 25 female and 25 male trees at Qianjiang (30°09′ N, 121°31′ E), Hubei Province, China. The cuttings were planted in March 2013. After sprouting and
growing for about 2 months, 360 cuttings (180 females and 180 males) with similar crown sizes and equal heights were selected and each was replanted into a 10 L plastic bucket filled with 10
kg homogenized soil. Plants were placed in a natural environment with 1261 mm mean annual rainfall, 1494 mm annual evaporation, 80% annual relative humidity and 16.9 °C annual temperature
at the Wuhan Botanical Garden, Chinese Academy of Sciences. After a growing season, the branches of all plants were pruned to an identical height, i.e., 10 cm above ground level, and the
leaves were removed. These plants were used for subsequent winter flooding treatments. The experimental layout was completely randomized according to the two main factors (sex and watering
rate; Fig. 8). Three watering treatment regimes were employed, being well-watered (CK), 100 days winter flooding stress combined with 40 days waterlogging (severe flooding stress, W-140d),
and 50 days winter flooding stress combined with 40 days waterlogging treatment (medium flooding stress, W-90d). In the well-watered treatment, all pots were watered excessively every three
days and excess water was allowed to drain through drainage holes into dishes placed under the buckets. In the winter flooding treatment, pots were watered every nine days to 5 cm above the
top of the plants. For 100 days and 50 days winter-flooding treatments, the plants were submerged in December and in the next February, respectively. Both flooding treatments were
simultaneously transferred to waterlogging treatments on March 29, 2014 for sprouting before the start of the growing season. During the next 40 days of waterlogging treatment, pots were
watered every nine days to 5 cm above the soil surface (W-140d and W-90d). At the end of the waterlogging treatment, each plant’s shoot height, basal stem diameter, gas exchange rate and
chlorophyll fluorescence were measured. Fresh leaves were collected for physiological analyses, and then the excess water was drained away on May 6, 2014. During following post recovery
stage, all pots were watered excessively every three days for 15 days of growth recovery (CK-R, W-140d-R, and W-90d-R) as above description. At the end of the 15 day growth recovery period,
each plant’s shoot height, basal stem, gas exchange rate and chlorophyll fluorescence were measured, and fresh leaves were collected for physiological analyses. All plants used for fresh
leaf collection were not used in the next treatment. Five replications, each with six cuttings, were used for each treatment. THE MORPHOLOGICAL TRAITS At the end of each experimental stage,
the plant height and basal stem diameter of each tested plants were measured. The comparative observations on leaf senescence and abscission, hypertrophied lenticels, aerenchyma tissue, and
adventitious roots were performed each week. GAS EXCHANGE MEASUREMENTS The net photosynthetic rate (_A_), stomatal conductance (_gs_), intercellular CO2 concentration (_Ci_) and
transpiration (_E_) were measured from 9:00 to 11:30 am on 5, 20 May with a LI-COR 6400 portable photosynthesis system (LI-COR Inc. Lincoln, Nebr.), respectively. The PAR, provided by a
6400-02 LED light source, was set to 1400 μmol m−2s−1. The flow rate of air through the sample chamber was set at 500 μmol m−2s−1, and the leaf temperature and relative humidity was
maintained at 25 ± 0.8 °C by thermoelectric coolers and 50%, respectively. The methods are modified from Yang _et al_.6 and Xu _et al_.16. Five cuttings from each treatment were selected for
measuring. A measurement was made on each of the three terminal leaflets of the uppermost fully opened leaf of each cutting. Instantaneous water use efficiency (WUEi = _A/E_) was calculated
by dividing photosynthetic rate by transpiration. DETERMINATION OF CHLOROPHYLL CONTENT Chlorophylls were extracted in 80% (v/v) chilled acetone and quantified using a spectrometer
(UV-1800PC, MAPADA, Shanghai) as described by Yang _et al_.6 and Xu _et al_.16. The absorbances of chlorophyll _a_ (_Chl a_), chlorophyll _b_ (_Chl b_), and carotenoids (_Caro_) were
determined at 663 nm, 646 nm, and 470 nm, respectively. The absorbance values were converted to concentrations as described by Lichtenthaler39. And the total chlorophyll (chlorophyll _a_ +
_b_, _Total Chl_) and _Chl a_/_Chl b_ were calculated. CHLOROPHYLL FLUORESCENCE MEASUREMENTS We selected the same five cuttings and same leaves that used for gas exchange measurements for
chlorophyll fluorescence measurements. Chlorophyll fluorescence kinetics parameters (_F_v/_F_m, maximum efficiency of PSII; _Yield_, the effective quantum yield of PSII; _qN_,
non-photochemical quenching coefficient; _qP_, photochemical quenching coefficient; _ETR_, photosynthetic electron transportation rate) were measured with a PAM chlorophyll fluorometer (PAM
2500, Walz, Effeltrich, Germany). The leaf samples were placed in darkness for 30 min by covering with aluminum foil followed by measurement of minimum fluorescence (_F_o) at 250 μmol m−2s−1
PPF and _F_m at 2400 μmol m−2s−1 PPF following a saturating pulse of actinic light6, 11. Measurements were carried out between 8:30 and 11:30 on May 6 and 21, respectively. DETERMINATION OF
RELATIVE WATER CONTENT AND RELATIVE ELECTROLYTE LEAKAGE The fourth-sixth fully expanded leaves were sampled to determine the leaf relative water content (RWC) as described by Yang _et
al_.6. Five freshly cut leaf discs (1.5 cm in diameter) from the fifth fully expanded leaves were used determined the leaf relative electrolyte leakage (REL) using a conductivity instrument
(FE38, Mettler-Toledo Instruments Co., Ltd, Shanghai, China) according to procedure of Zhang _et al_.17. DETERMINATION OF SOLUBLE PROTEIN CONTENT, GLUTATHIONE (GSH), REDUCING SUGAR, AND FREE
PROLINE CONTENT About 2 g fresh samples were ground with liquid nitrogen and then homogenized in 10 ml 100 mM universal sodium phosphate extraction buffer as described by Han _et al_.40.
The supernatant was stored in volumes of 0.5 ml at –80 °C until using for the determination of soluble protein, reactive oxygen species (ROS) level and antioxidant enzymes activities40. The
soluble protein was quantified by Bradford method41, the soluble protein content was expressed as mg/g · FW. The concentrations of GSH, reducing sugar, proline were assayed according the
procedure of Bates _et al_.42, Sairam _et al_.43 and Yang _et al_.6. The GSH concentration, reducing sugar, and proline concentration were calculated and expressed as mg/g·FW, mg/g·FW, and
μg/g·FW, respectively. DETERMINATION OF ROS LEVEL AND MALONDIALDEHYDE (MDA) CONTENT The detections of superoxide (O2 .−), hydrogen peroxide (H2O2), hydroxyl radicals (·OH), and MDA were
based on the procedures of Yang _et al_.6 and Yang _et al_.38. The concentrations of O2 .−, H2O2, ·OH, and MDA were calculated and expressed as ng/g·FW, μmol/g·FW, ng/g·FW, and μmol/g·FW,
respectively. ASSAY OF ANTIOXIDANT ENZYMES ACTIVITIES The antioxidant enzyme including guaiacol peroxidase (POD), ascorbate peroxidase (APx), catalase (CAT), superoxide dismutase (SOD), and
glutathione reductase (GR) activities were determined according to the manufacturer’s instructions as described Zhang _et al_.17 and Han _et al_.40. The activities of POD, CAT, and GR were
calculated and expressed as U/mg·protein, The APx activities were determined as described by Yang _et al_.38, and expressed as U/g·protein. The CAT activity was calculated and expressed as
U/mg · protein. TRANSMISSION ELECTRON MICROSCOPY Three small leaf sections (2 cm in length, 1 cm in width) from fifth fully expanded leaves, avoiding the midrib, were selected for the
transmission electron microscope analysis according the procedures of Zhang _et al_.17. The sections were fixed in 2.5% (v/v) glutaral pentanedial in 0.2 M of PBS (sodium phosphate buffer,
pH 7.0) for 3 h at 25 °C and postfixed in 2% osmium tetraoxide (OsO4) for 2 h. The tissues were then sequentially dehydrated in 30%, 50%, 70%, and 90% acetone, and embedded in Epon 812 for 2
h. Ultra-thin sections (80 nm) were sliced, stained with uranyl acetate and lead citrate, and mounted on copper grids for viewing in the H-7000FA TEM (Japan) at an accelerating voltage of
160 kV. STATISTICAL ANALYSES Results were expressed as means ± standard errors (n = 5). SPSS 13.0 software was used for statistical analysis. Analyses of variance (ANOVA) for variables from
measurements were used for testing the species and treatment differences. Differences were considered significant at P < 0.05. REFERENCES * Yang, F., Wang, Y. & Chan, Z. Perspectives
on screening winter-flood-tolerant woody species in the riparian protection forests of the Three Gorges Reservoir. _Plos One_ 9, e108725 (2014). Article ADS PubMed PubMed Central Google
Scholar * Yang, F., Wang, Y. & Chan, Z. Review of environmental conditions in the water level fluctuation zone: perspectives on riparian vegetation engineering in the Three Gorges
Reservoir. _Aquat Ecosyst Health_ 18, 240–249 (2015). Google Scholar * Kozlowski, T. T. Responses of woody plants to flooding and salinity. _Tree Physiol Monog_ 1, 1–29 (1997). Google
Scholar * Nielsen, J. L., Rood, S. B., Pearce, D. W., Letts, M. G. & Jiskoot, H. Streamside trees: responses of male, female and hybrid cottonwoods to flooding. _Tree Physiol_ 30,
1479–1488 (2010). Article PubMed Google Scholar * Cao, F. L. & Conner, W. H. Selection of flood-tolerant _Populus deltoides_ clones for reforestation projects in China. _Forest Ecol
Manag_ 117, 211–220 (1999). Article Google Scholar * Yang, F. _et al_. Different eco-physiological responses between male and female _Populus deltoides_ clones to waterlogging stress.
_Forest Ecol Manag_ 262, 1963–1971 (2011). Article Google Scholar * Letts, M. G., Phelan, C. A., Johnson, D. R. E. & Rood, S. B. Seasonal photosynthetic gas exchange and leaf
reflectance characteristics of male and female cottonwoods in a riparian woodland. _Tree Physiol_ 28, 1037–1048 (2008). Article CAS PubMed Google Scholar * Rood, S. B., Nielsen, J. L.,
Shenton, L., Gill, K. M. & Letts, M. G. Effects of flooding on leaf development, transpiration, and photosynthesis in narrow leaf cottonwood, a willow-like poplar. _Photosynth Res_ 104,
31–39 (2010). Article CAS PubMed Google Scholar * Kozlowski, T. T. Plant responses to flooding of soil. _Bioscience_ 34, 162–167 (1984). Article Google Scholar * Regehr, D. L., Bazzaz,
F. A. & Boggess, W. R. Photosynthesis, transpiration and leaf conductance in _Populus deltoides_ in relation to flooding and drought. _Photosynthetica_ 9, 52–61 (1975). Google Scholar
* Cooper, D. J., D’Amico, D. R. & Scott, M. L. Physiological and morphological response patterns of _Populus deltoides_ to alluvial ground water pumping. _Environ Manage_ 31, 215–226
(2003). Article PubMed Google Scholar * Rood, S. B., Braatne, J. H. & Hughes, F. M. R. Ecophysiology of riparian cottonwoods: stream flow dependency, water relations and restoration.
_Tree Physiol_ 23, 1113–1124 (2003). Article PubMed Google Scholar * Rohde, A. & Boerjan, W. Gene expression during the induction, maintenance, and release of dormancy in apical buds
of poplar. _J Exp Bot_ 58, 38–55 (2007). Article Google Scholar * Cooke, J. E. K., Eriksson, M. E. & Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and
molecular mechanisms. _Plant, Cell Environ_ 35, 1707–1728 (2012). Article CAS Google Scholar * Yordanov, Y. S., Ma, C., Strauss, S. H. & Busov, V. B. EARLY BUD-BREAK 1 (EBB1) is a
regulator of release from seasonal dormancy in poplar trees. _P Natl Acad Sci USA_ 111, 10001–10006 (2014). Article ADS CAS Google Scholar * Xu, X., Peng, G., Wu, C., Korpelainen, H.
& Li, C. Drought inhibits photosynthetic capacity more in females than in males of _Populus cathayana_. _Tree Physiol_ 28, 1751–1759 (2008). Article PubMed Google Scholar * Zhang, S.,
Jiang, H., Peng, S., Korpelainen, H. & Li, C. Sex-related differences in morphological, physiological, and ultrastructural responses of _Populus cathayana_ to chilling. _J Exp Bot_ 62,
675–686 (2011). Article CAS PubMed Google Scholar * Chen, F., Zhang, S., Zhu, G., Korpelainen, H. & Li, C. _Populus cathayana_ males are less affected than females by excess
manganese, comparative proteomic and physiological analyses. _Proteomics_ 13, 2424–2437 (2013). Article CAS PubMed Google Scholar * Randriamanana, T. R. _et al_. Sex-related differences
in growth and carbon allocation to defense in _Populus tremula_ as explained by current plant defence theories. _Tree Physiol_ 34, 471–487 (2014). Article CAS PubMed Google Scholar *
Jiang, H. _et al_. Transcriptional profiling in dioecious plant _Populus cathayana_ reveals potential and sex-related molecular adaptations to solar UV‐B radiation. _Physiol Plantarum_ 153,
105–118 (2015). Article CAS Google Scholar * Li, Y. _et al_. Males exhibit competitive advantages over females of _Populus deltoides_ under salinity stress. _Tree Physiol_ 36, 1573–1584
(2016). Article PubMed Google Scholar * Juvany, M. & Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context.
_J Exp Bot_ 66, 451–454 (2015). Article Google Scholar * Sloan, J. L., Islam, M. A. & Jacobs, D. F. Reduced translocation of current photosynthate precedes changes in gas exchange for
_Quercus rubra_ seedlings under flooding stress. _Tree Physiol_ 36, 54–62 (2016). Article PubMed Google Scholar * Kogawara, S., Yamanoshita, T., Norisada, M. & Kojima, K. Steady
sucrose degradation is a prerequisite for tolerance to root hypoxia. _Tree Physiol_ 34, 229–240 (2013). Article Google Scholar * Larson, K. D., Schaffer, B. & Davies, F. S. Flooding,
leaf gas exchange and growth of mango in containers. _J Am Soc Hortic Sci_ 116, 156–160 (1989). Google Scholar * Wang, N., Li, Z., Wu, F. & Tang, M. Comprehensive analysis of trihelix
genes and their expression under biotic and abiotic stresses in _Populus trichocarpa_. _Sci Rep_ 6, 36274 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Wu, N. _et al_.
Comparative photochemistry activity and antioxidant responses in male and female _Populus cathayana_ cuttings inoculated with arbuscular mycorrhizal fungi under salt. _Sci Rep_ 6, 37663
(2016). Article ADS CAS PubMed PubMed Central Google Scholar * Xu, X. _et al_. Different growth sensitivity to enhanced UV-B radiation between male and female _Populus cathayana_.
_Tree Physiol_ 30, 1489–1498 (2010). Article CAS PubMed Google Scholar * Wang., X. Z. & Curtis, P. S. Gender-specific response of to atmospheric CO2 enrichment. _New Phytol_ 150,
675–684 (2001). Article CAS Google Scholar * Zhang, S., Jiang, H., Zhao, H., Korpelainen, H. & Li, C. Sexually different physiological responses of _Populus cathayana_ to nitrogen and
phosphorus deficiencies. _Tree Physiol_ 34, 343–354 (2014). Article CAS PubMed Google Scholar * Han, Y., Wang, L., Zhang, X., Korpelainen, H. & Li, C. Sexual differences in
photosynthetic activity, ultrastructure and phytoremediation potential of _Populus cathayana_ exposed to lead and drought. _Tree Physiol_ 33, 1043–1060 (2013). Article CAS PubMed Google
Scholar * Chen., L. _et al_. Sex-related adaptive responses to interaction of drought and salinity in _Populus yunnanensis_. _Plant, Cell Environ_ 33, 1767–1778 (2010). Article CAS Google
Scholar * Retuerto, R., Fernández-Lema, B., Rodríguez-Roiloa, S. & Obeso, J. R. Gender, light and water effects in carbon isotope discrimination, and growth rates in the dioecious tree
_Ilex aquifolium_. _Funct Ecol_ 14, 529–537 (2000). Article Google Scholar * Álvarez-Cansino, L., Díaz-Barradas, M. C., Zunzunegui, M., Esquivias, M. P. & Dawson, T. E.
Gender-specific variation in physiology in the dioecious shrub _Corema album_ throughout its distributional range. _Funct Plant Biol_ 39, 968–978 (2012). Article Google Scholar * Oñate,
M., García, M. B. & Munné-Bosch, S. Age and sex-related changes in cytokinins, auxins and abscisic acid in a centenarian relict herbaceous perennial. _Planta_ 235, 349–358 (2012).
Article PubMed Google Scholar * Gao, J. Q. _et al_. Effects of waterlogging on carbon assimilate partitioning in the Zoige alpine wetlands revealed by 13CO2 pulse labeling. _Sci Rep_ 5,
9411 (2015). Article CAS PubMed PubMed Central Google Scholar * Yin, D., Chen, S., Chen, F., Guan, Z. & Fang, W. Morphological and physiological responses of two chrysanthemum
cultivars differing in their tolerance to waterlogging. _Environ Exp Bot_ 67, 87–93 (2009). Article CAS Google Scholar * Yang, F., Han, C., Li, Z., Guo, Y. & Chan, Z. Dissecting
tissue- and species-specific responses of two _Plantago_ species to waterlogging stress at physiological level. _Environ Exp Bot_ 109, 177–185 (2015). Article Google Scholar *
Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. _Method Enzymol_ 148, 350–382 (1987). Article CAS Google Scholar * Han, C., Chan, Z. &
Yang, F. Comparative analyses of universal extraction buffers for assay of stress related biochemical and physiological parameters. _Prep Biochem Biotech_ 45, 684–695 (2015). Article CAS
Google Scholar * Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. _Anal Biochem_ 72,
248–254 (1976). Article CAS PubMed Google Scholar * Bates, C. J., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. _Plant Soil_ 39, 205–207
(1973). Article CAS Google Scholar * Sairam, R. K., Dharmar, K., Chinnusamy, V. & Meena, R. C. Waterlogging induced increase in sugar mobilization, fermentation, and related gene
expression in the roots of mung bean (_Vigna radiata_). _J Plant Physiol_ 166, 602–616 (2009). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was
sponsored by National Natural Science Foundation of China (No. 31660165; 31270449) and Scientific Research Starting Foundation of Hainan University to Fan Yang (kyqd1573). AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Institute of Tropical Agriculture and Forestry, Hainan University, Haikou, Hainan, 570228, P. R. China Ling-Feng Miao, Fan Yang, Chun-Yu Han, Yu-Jin Pu, Yang Ding
& Li-Jia Zhang Authors * Ling-Feng Miao View author publications You can also search for this author inPubMed Google Scholar * Fan Yang View author publications You can also search for
this author inPubMed Google Scholar * Chun-Yu Han View author publications You can also search for this author inPubMed Google Scholar * Yu-Jin Pu View author publications You can also
search for this author inPubMed Google Scholar * Yang Ding View author publications You can also search for this author inPubMed Google Scholar * Li-Jia Zhang View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS Miao L. performed the experiments and wrote the manuscript; Yang F. designed the experiments, analyzed the data, and
revised the manuscript; Han C. performed the experiments; Pu Y., Ding Y., and Zhang L. analyzed the data. CORRESPONDING AUTHOR Correspondence to Fan Yang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY TABLE 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Miao, LF., Yang, F., Han, CY. _et al._ Sex-specific responses to winter flooding,
spring waterlogging and post-flooding recovery in _Populus deltoides_ . _Sci Rep_ 7, 2534 (2017). https://doi.org/10.1038/s41598-017-02765-2 Download citation * Received: 16 February 2017 *
Accepted: 18 April 2017 * Published: 31 May 2017 * DOI: https://doi.org/10.1038/s41598-017-02765-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative