Extranodal extension of lymph node metastasis influences recurrence in prostate cancer: a systematic review and meta-analysis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The extranodal extension (ENE) of nodal metastasis involves the extension of neoplastic cells through the lymph node capsule into the perinodal adipose tissue. This morphological
feature has recently been indicated as an important prognostic factor in various cancer types, but its role in prostate cancer is still unclear. We aimed to clarify it, performing the first
meta-analysis on this issue, comparing prognostic parameters in surgically treated, node-positive prostate cancer patients with (ENE+) vs. without (ENE−) ENE. Data were summarized using risk
ratios (RRs) for number of deaths/recurrences and hazard ratios (HRs), with 95% confidence intervals (CI), for the time-dependent risk related to ENE positivity. Six studies followed-up
1,113 patients with N1 prostate cancer (658 ENE+ vs. 455 ENE−) for a median of 83 months. The presence of ENE was associated with a significantly higher risk of biochemical recurrence (RR =
1.15; 95%CI: 1.03–1.28; I2 = 0%; HR = 1.40, 95%CI: 1.12–1.74; I2 = 0%) and “global” (biochemical recurrence and distant metastasis) recurrence (RR = 1.15; 95%CI: 1.04–1.28; I2 = 0%; HR =
1.41, 95%CI: 1.14–1.74; I2 = 0%). ENE emerged as a potential prognostic moderator, earmarking a subgroup of patients at higher risk of recurrence. It may be considered for the prognostic
stratification of metastatic patients. New possible therapeutic approaches may explore more in depth this prognostic parameter. SIMILAR CONTENT BEING VIEWED BY OTHERS ANALYSIS OF THE
SURGICAL APPROACH IN PROSTATE CANCER STAGING: RESULTS FROM THE SURVEILLANCE, EPIDEMIOLOGY AND END RESULTS PROGRAM Article Open access 19 June 2023 REEVALUATING THE THERAPEUTIC ROLE OF
EXTENDED LYMPH NODE DISSECTION IN THE ERA OF ROBOT-ASSISTED RADICAL PROSTATECTOMY Article Open access 21 May 2025 LENGTH OF POSITIVE SURGICAL MARGINS AFTER RADICAL PROSTATECTOMY: DOES SIZE
MATTER? – A SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 01 March 2023 INTRODUCTION Prostate cancer (PCa) is one of the most common cancers in men worldwide1,2,3,4, and deaths
from prostate cancer are second only to those due to lung cancer3. The incidence and prevalence of prostate cancer are different in each area of the world, the highest being in North America
and the lowest in Southern Asia2, but the worldwide incidence of PCa has grown substantially in recent years1,2,3,4. The prognosis of PCa patients depends mainly on the presence or absence
of distant metastases5, and lymph node metastases are a particularly crucial prognostic factor6. Several researchers have analyzed the different morphological features of lymph node
metastases in an effort to identify their most prognostically significant characteristics7,8,9,10,11,12,13,14,15. Some features (e.g. the involvement of multiple versus single lymph nodes,
or nodal cancer volume) have revealed a strong prognostic value. Conventional staging for PCa does not differentiate between subgroups of node-positive disease; stratification is only based
on the absence or presence of nodal metastases (N0 versus N1)16. There are also certain morphological aspects of lymph node metastases that have no clearly-established prognostic role yet,
as different studies have generated different conclusions. One of the morphological features to consider is the presence of extranodal extension (ENE) of nodal metastases, which is indicated
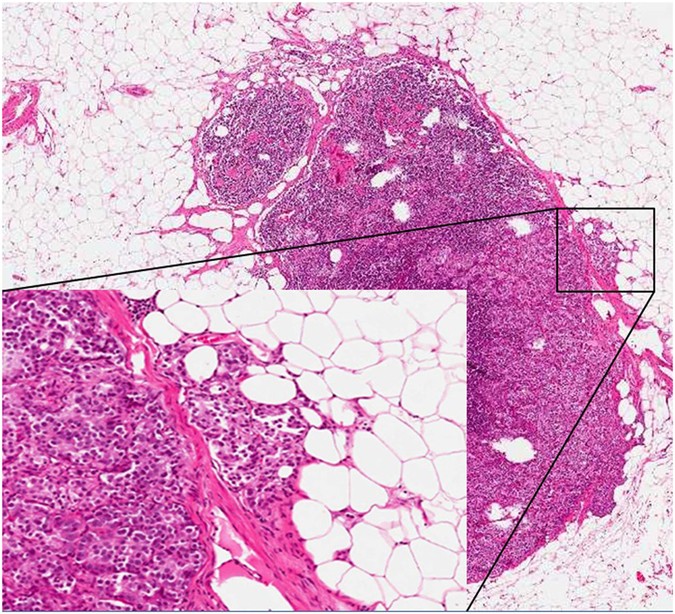
as the extension of metastatic cells beyond the nodal capsule into the perinodal soft tissue (Fig. 1). ENE has been recently indicated as a significant prognostic factor in many cancer
types17,18,19,20,21,22,23,24,25, but its role in PCa is still unclear. For these reasons, and also because identifying generally-acceptable prognostic indicators is still a very important
challenge in these times of personalized medicine, the aim of the present study was to establish the weight and determining the role of ENE on the prognosis of patients with N1 (nodal
metastasis/metastases) PCa by performing the first meta-analysis on this argument. RESULTS SEARCH RESULTS Altogether, 1051 non-duplicated articles were identified by our literature search.
We have excluded 1024 articles after title/abstract review; the remaining 27 articles were retrieved for full text review. At last, after a complete analysis based on the criteria of
eligibility, 6 studies resulted suitable for this meta-analysis (Supplementary Figure 1). STUDY AND PATIENT CHARACTERISTICS The 6 meta-analyzed studies followed up 1,113 cases (658 ENE+ vs.
455 ENE−) over a median period of 83 months (range: 16–92) (Supplementary Table 1)10,11,12,13,14,15. The quality of the studies seemed to be good, and none of them showed a potentially high
risk of bias (median NOS score = 8, range: 7–9; Supplementary Tables 1 and 2). The mean age of patients was 65 ± 10 years; we do not find significant differences between ENE+ and ENE−
patients in terms of mean age (Student’s t-test for independent samples, p = 0.88) or Gleason score (chi-square test, p = 0.21) (Supplementary Table 1). ALL-CAUSE MORTALITY (ACM),
CANCER-SPECIFIC MORTALITY (CSM) AND RISK OF RECURRENCE (ROR) One study reported that 20/71 patients (=28.2%) with ENE+ vs. 5/31 (=16.1%) with ENE− died, meaning an increased risk of ACM in
the former that was not statistically significant (RR = 1.75; 95%CI: 0.72–4.23; p = 0.22) (Table 1)12. Similarly, four studies reported that ENE + was not significantly associated with a
higher risk of CSM (RR = 1.21; 95%CI: 0.98–1.50; p = 0.08; I2 = 0%)10,11,12,13. With regard to ROR, ENE+ status was associated with a higher risk of BCR (RR = 1.15; 95%CI: 1.03–1.28; p =
0.01; I2 = 0%) in two studies12, 15, while this association was not significant (RR = 1.91; 95%CI: 0.72–5.11; p = 0.20) in one study11 that considered metastasis as an indicator of
recurrence (Fig. 2). Pooling data of all the three studies about ROR11, 12, 15, the global association with ENE was also significant (RR = 1.15; 95%CI: 1.04–1.28; p = 0.008; I2 = 0%, Table 1
and Fig. 2). The p for the interaction between BCR and solid metastasis was = 0.46, however, suggesting that the type of outcome was not a significant moderator of these findings. No
publication bias emerged for any of the outcomes considered (Table 1). ADJUSTED HRS ON ACM, CSM AND ROR In secondary analyses, we analyzed whether using HRs (hazard ratios were adjusted for
the maximum number of covariates available in each study) instead of RRs could affect our results. In the survival analyses, the median number of adjustments used was 1 (range: 0–8)
(Supplementary Table 1). Table 2 shows the adjusted HRs by ENE status. ENE+ status was not associated with a significantly worse prognosis than ENE− status when ACM (1 study; HR = 1.50,
95%CI: 0.59–3.82; p = 0.85)12 (Table 2), and CSM (3 studies; HR = 1.42, 95%CI: 0.98–2.05; p = 0.07; I2 = 0%)10,11,12 (Table 2) were considered as outcomes. In the adjusted estimates, the
risk of recurrence was more significant for the outcome BCR (3 studies; HR = 1.42; 95%CI: 0.98–2.05; p = 0.07; I2 = 0%) than for distant metastasis (1 study; HR = 1.50; 95%CI: 0.59–3.82; p =
0.85), as shown in Fig. 3 (p for interaction = 0.74). Pooling together the indexes of recurrence, ENE+ status remained associated with a significantly higher risk of recurrence in four
studies (HR = 1.41, 95%CI: 1.14–1.74; p = 0.001; I2 = 0%, Fig. 3)11, 12, 14, 15, which was unaffected by publication bias (adjusted estimate: HR = 1.32, 95%CI: 1.11–1.62, with two studies
trimmed to the left of the mean). None of the outcomes analyzed indicated a high heterogeneity (as indicated by I2 ≥ 50%), so meta-regression and sensitivity analyses were not performed.
DISCUSSION For this study we analyzed 6 observational studies involving 1,113 patients with N1 PCa, 658 of them ENE+, and 455 ENE−. Our results indicate that ENE is associated with a higher
risk of recurrence (ROR) in PCa, and this association is particularly strong for the risk of BCR. Notably, the association was not just maintained, but even reinforced when HRs adjusted for
potential confounders were considered. The robustness of our findings is confirmed by the fact that we did not find any significant heterogeneity or publication bias. At the same time, ENE
was not associated with ACM or CSM in our meta-analysis. Judging from our results, ENE appears to be another factor capable of influencing the clinical history, and consequently also the
quality of life of patients with PCa, but it does not seem to significantly affect their survival. So far, too few morphological features of nodal metastases have been identified as
significant prognostic moderators, and that is why our results seem important. The prognostic predictor generally thought to be the most significant is nodal cancer volume, which has been
described as the strongest indicator of systemic progression and CSM7, 8. The factors judged important for stratifying patient survival include the total metastatic tumor volume and the
diameter of the largest metastasis7, 8, 12, 26. Metastatic tumor volume has been strongly associated with other important prognostic factors like the Gleason score and DNA ploidy, confirming
this parameter’s close link with the biological behavior of metastasizing PCa27. Notably, ENE was not strictly correlated with metastatic tumor volume, and this may be one of the reasons
for its limited influence on the global survival of PCa patients. At the same time, ENE is a classic example of local aggressiveness (with the tumor invading perinodal adipose tissue), and
this can explain its association with ROR. Another parameter that pathologists can document with ease and that correlates with prognosis in PCa patients is the number of positive lymph
nodes8. A recent expert report suggested sub-stratifying N1 patients as: N1 if there is a single positive lymph node, and N2 if there are two or more. N1 could also be further divided
according to the size of the tumor metastasis27. On the basis of our study, N1 patients could be subdivided by ENE status too, with ENE+ cases indicating a subgroup of N1 patients at greater
ROR. Surprisingly, both the previous and the currently used staging system (the new AJCC cancer staging manual, recently released) only distinguishes between PCa patients with and without
nodal involvement in a dichotomous way (N0 versus N1)16, 28. Future staging systems may consider the above-mentioned morphological features of lymph node metastases to better stratify
patients and facilitate the identification of higher-risk patients who need to be followed up more closely, also on the basis of possible findings of future researches. Another relevant
implication emerging from this meta-analysis is about the sphere of surgical pathology, and gross sampling in particular. Standard sampling usually involves examining manually isolated,
palpable lymph nodes29. Using this approach, a single lymph node may be oversampled and also counted more than once, since several different pieces can be obtained by the pathologist,
especially in the case of large lymph nodes, while small metastatic lymph nodes may be overlooked. To avoid this problem, a recent report suggested using a technique that involves submitting
all nodal and perinodal tissue, and examining palpable lymph nodes and the remaining tissue separately30. Consistently with this view, in the light of our finding that ENE has a certain
prognostic importance in PCa, and the fact that it may be a very focal aspect in a metastatic lymph node, we would emphasize the importance of examining the whole of each lymph node, however
large. Montironi _et al_. recently analyzed the potential clinical significance of the so-called large-format histology coupled with the total submission of nodal and perinodal tissue31,
finding that this method improves the number of lymph nodes recovered and the detection of metastases. The approach has other advantages too, including a shorter time spent on sampling and
fewer blocks needing to be cut and fewer slides to be examined31. Despite criticism relating to its higher costs, the superiority of such method is demonstrated by the fact that it enables
nearly three times more lymph nodes to be identified than the standard sampling technique31. The use of fat-cleaning agents could be of help in this line, reducing the amount of tissue which
has to be examined. On the grounds of these considerations, also regarding the increased opportunities in ENE detection, this technique may be taken into account by pathologists. In our
study the prognostic parameter most associated with the presence of ENE was BCR. Fleischmann _et al_. classified BCR as a PSA level >0.2 ng/ml. Hofer _et al_. indicated BCR as a
postoperative increase in serum PSA to more than 0.4 ng/ml on two consecutive measurements. Passoni _et al_. defined BCR as two consecutive PSA readings >0.2 ng/ml. Irrespective of such
small differences in the threshold considered, BCR seems to be significantly influenced by the presence of ENE, in terms of both risk ratios (RRs, Fig. 2), and hazard ratios (HRs, Fig. 3).
Since BCR is considered an important prognostic parameter (also influencing PCa patients’ quality of life), we would emphasize the prognostic importance of ENE. ENE retains its prognostic
significance for all types of recurrence (distant metastasis, BCR) (Figs 2 and 3), and it may be that, with a longer follow-up, the power of ENE to predict ACM and CSM may increase, and
become statistically significant. Its impact on ROR is already important, however. Another point of interest concerns adjuvant therapy. In a recent study on pancreatic cancer, patients with
ENE seemed to have a better prognosis with the use of adjuvant chemoradiation, but not from chemotherapy alone32. This matter has to be further investigated because, if confirmed in PCa,
such results could orient towards particular therapeutic options. The standard definition of ENE is another important issue that warrants attention. Five out of the six studies considered
here assessed ENE using a classical definition, such as the extension of tumor cells into the perinodal soft tissue10, 12,13,14,15. On the other hand, Cheng _et al_. considered metastatic
deposits within adipose tissue as ENE as well, introducing a possible bias. It would be best to arrive at a standardized definition of ENE because of its possible importance in
histopathological diagnostics. This parameter must be documented correctly in future studies, also because it may be an important prognostic moderator. Whilst the results of this
investigation appear as reliable, we must also consider some limitations. The first is represented by the small number of studies involved (which came as a surprise, considering the large
body of literature on PCa). The sizable number of patients considered in each study (mean 186 patients/study), the studies’ high NOS scores (median = 8), and the limited heterogeneity of the
results provide some guarantee of reliability, however. Further studies on ENE in PCa are nonetheless needed to clarify its prognostic potential. In addition, data on other comorbidities
were not indicated in the studies analyzed, though they are known to play a significant part in the prognosis of patients with PCa. In conclusion, our meta-analysis indicates that ENE is
associated with the risk of BCR and of disease recurrence in general, even after adjusting for potential confounders. Notably, ENE is identified in a remarkable proportion of N1 PCa
patients. However, we have to recognize that, at this moment, there are no different treatment options for patients with or without ENE, at the time that the initial pathology report is
generated. Also on the basis of our work, this parameter may be taken into account by future studies about advanced PCa with nodal metastases. At the other hand, it is also true that now the
follow-up will be the same for either type (ENE+ and ENE−) of patient. Concluding this part, if ENE becomes part of a standard CAP synoptic report, at this moment it should be only one of
the “optional parameters”. Further studies are needed, preferably with a longer follow-up, to shed more light on the potential role of this parameter in influencing PCa patients’ survival.
Our results may become more relevant with the passage of time and the accumulation of additional data, and the development of new therapeutic treatments options for advanced prostate cancer.
A final consideration regards the recent development of molecular techniques. Indeed, it has been indicated that pathology reports will be integrated with a specific molecular profile of a
patient’s cancer33,34,35,36,37, but, before taking up such a proposal, all the prognostic value of all morphological aspects, such as ENE, have to be clarified. METHODS This systematic
review has been performed following the Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) guidelines38 and the Preferred Reporting Items for Systematic reviews and Meta-Analyses
(PRISMA) statement39 (Supplementary Fugure 1). DATA SOURCES AND LITERATURE SEARCH STRATEGY Two investigators (C.L., A.N.) independently conducted a literature search in PubMed and SCOPUS
with no language restrictions, from the inception of the databases up until 30 June 2016, seeking prospective studies that compared any prognostic parameters (all-cause mortality,
cancer-specific mortality and recurrent disease) in patients with a diagnosis of nodal positive PCa with or without extranodal disease (ENE+ vs. ENE−). In PubMed, controlled vocabulary terms
and the following keywords were used: (“extracapsular” OR “pericapsular” OR “extranodal” OR “perilymphatic” OR “perinodal” OR “extra capsular” OR “peri capsular” OR “extra nodal” OR “peri
lymphatic” OR “peri nodal” OR “extra-capsular” OR “peri-capsular” OR “extra-nodal” OR “peri-lymphatic” OR “peri-nodal”) AND (“prostat*”) AND (“mortality” OR “mortalities” OR “fatality” OR
“fatalities” OR “death*” OR “survival” OR “prognosis” OR “hazard ratio” OR “HR” OR “relative risk” OR “RR” OR “progression” OR “recurrence”). The same search was conducted also in SCOPUS.
Conference abstracts were also evaluated, and so were the reference lists of included articles, and those identified as relevant to the topic were hand-searched to identify any additional,
potentially relevant articles. Any inconsistencies were solved by consensus. STUDY SELECTION For this meta-analysis, as inclusion criteria we considered: (1) prospective, observational
cohort studies; (2) comparison of prognostic factors between ENE− vs. ENE+ cases; (3) a clear diagnosis of nodal-positive acinar prostate adenocarcinoma; (4) data concerning mortality or
recurrent disease, considering not only local recurrences or distant metastases, but also biochemical recurrence (BCR). As exclusion criteria we considered: (1) no ongoing cancer; (2)
absence of data on prognostic indicator in the title/abstract; (3) comparisons between ENE + vs. N0 (no lymph node metastases); (4) a diagnosis of cancer histotypes other than acinar
prostate adenocarcinoma; and (5) animal or _in vitro_ studies. DATA EXTRACTION One author (M.S.) was involved in data extraction from the articles included in the analysis and a second
author (C.L.) checked independently the resulting data. The information extracted regarded the authors, year and country of publication, exclusion criteria, number of patients with
metastatic lymph nodes, patients’ age, type and mode of the recurrence of disease (if any), number of adjustments in survival analyses, and duration of follow-up. If we did not find some
information on ENE or outcomes, we contacted the first and/or corresponding authors of the specific original articles to obtain unpublished data. In case two articles referred to the same
cohort, the most recent study was considered. OUTCOMES We considered as primary outcomes these parameters as follows: the number of deaths irrespective of their cause (all-cause mortality);
the number of deaths due to cancer; and the number of recurrences in ENE+ vs. ENE− node positive PCa patients during the follow-up. We considered as secondary outcomes these parameters as
follows: hazard ratios (HRs), adjusted for the maximum number of confounders available, for the above-mentioned parameters, taking ENE− patients for reference. ASSESSMENT OF STUDY QUALITY
The Newcastle-Ottawa Scale (NOS) was used to judge the quality of the studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm), using a score of ≤5 (out of 9) as a indicator
for a high risk of bias40. DATA SYNTHESIS AND STATISTICAL ANALYSIS We performed the analyses using Comprehensive Meta-Analysis (CMA) 3 (http://www.meta-analysis.com). To test for normality
in the case of continuous variables, the Shapiro-Wilk test was used. If normality was satisfied, we presented the variables as means ± standard deviations; if not, we considered medians and
ranges. In primary analyses, pooled risk ratios (RRs) and 95%CIs for all-cause mortality, cancer-specific mortality, and disease recurrence were calculated between ENE+ and ENE− cases
applying DerSimonian-Laird random-effects models41. In secondary analyses, pooled HRs with 95%CIs adjusted for the maximum number of covariates available in the papers were also calculated
to clarify whether potential confounders may affect the relationship between ENE status and outcomes. We assessed heterogeneity across studies with the Cochrane I2 metric and chi square
statistics. In case of significant heterogeneity (p < 0.05)42, a series of meta-regression analyses by ENE status and each of the prognostic parameters considered will be conducted.
Publication bias was assessed by visual inspection of funnel plots and also using the Begg-Mazumdar Kendall tau43, and the Egger bias tests44. The trim-and-fill method was also applied for a
final check about publication bias44, 45. REFERENCES * Center, M. M. _et al_. International variation in prostate cancer incidence and mortality rates. _Eur. Urol._ 61, 1079–1092 (2012).
Article PubMed Google Scholar * Bashir, M. N. Epidemiology of prostate cancer. _Asian Pac. J. Cancer Prev._ 16, 5137–5141 (2015). Article PubMed Google Scholar * Schröder, F. H. _et
al_. Prostate-cancer mortality at 11 years of follow-up. _N. Engl. J. Med._ 366, 981–990 (2012). Article PubMed Google Scholar * Torre, L. A. _et al_. Global cancer statistics, 2012. CA.
_Cancer J. Clin._ 65, 87–108 (2015). Article Google Scholar * Santoni, M. _et al_. The origin of prostate metastases: emerging insights. _Cancer Metastasis Rev._ 34, 765–773 (2015).
Article CAS PubMed Google Scholar * Adams, J. & Cheng, L. Lymph node-positive prostate cancer: current issues, emerging technology and impact on clinical outcome. _Expert Rev.
Anticancer Ther._ 11, 1457–69 (2011). Article PubMed Google Scholar * Cheng, L. _et al_. Cancer volume of lymph node metastasis predicts progression in prostate cancer. _Am. J. Surg.
Pathol._ 22, 1491–500 (1998). Article CAS PubMed Google Scholar * Cheng, L. _et al_. Risk of prostate carcinoma death in patients with lymph node metastasis. _Cancer._ 91, 66–73 (2001).
Article CAS PubMed Google Scholar * Daneshmand, S. _et al_. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. _J. Urol._
172(6 Pt 1), 2252–2255 (2004). Article PubMed Google Scholar * Boormans, J. L., Wildhagen, M. F., Bangma, C. H., Verhagen, P. C. & van Leenders, G. J. Histopathological
characteristics of lymph node metastases predict cancer-specific survival in node-positive prostate cancer. _BJU Int_ 102, 1589–1593 (2008). Article PubMed Google Scholar * Cheng, L. _et
al_. Extranodal extension in lymph node-positive prostate cancer. _Mod. Pathol._ 13, 113–118 (2000). Article CAS PubMed Google Scholar * Fleischmann, A. _et al_. Prognostic factors in
lymph node metastases of prostatic cancer patients: the size of the metastases but not extranodal extension independently predicts survival. _Histopathology._ 53, 468–475 (2008). Article
CAS PubMed Google Scholar * Griebling, T. L., Ozkutlu, D., See, W. A. & Cohen, M. B. Prognostic implications of extracapsular extension of lymph node metastases in prostate cancer.
_Mod. Pathol._ 10, 804–809 (1997). CAS PubMed Google Scholar * Hofer, M. D. _et al_. Prognostic factors in lymph node-positive prostate cancer. _Urology._ 67, 1016–1021 (2006). Article
PubMed Google Scholar * Passoni, N. M. _et al_. Prognosis of patients with pelvic lymph node (LN) metastasis after radical prostatectomy: value of extranodal extension and size of the
largest LN metastasis. _BJU Int._ 114, 503–510 (2014). Article PubMed Google Scholar * Edge, S. B., Byrd, D. R., Compton, C. C., Fritz, A. G. & Trotti A. _AJCC Cancer Staging Manual_.
7th ed. (Springer-Verlag 2010). * Luchini, C. _et al_. Extra-nodal extension in N1-adenocarcinoma of pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic
significance. _Eur. J. Gastroenterol. Hepatol._ 28, 205–209 (2016). PubMed Google Scholar * Veronese, N. _et al_. Prognostic impact and implications of extra-capsular lymph node
involvement in colorectal cancer: a systematic review with meta-analysis. _Ann. Oncol._ 27, 42–48 (2016). Article CAS PubMed Google Scholar * Luchini, C. _et al_. Extra-nodal extension
is an important prognostic parameter for both colonic and rectal cancer. _Ann. Oncol._ 27, 955–956 (2016). CAS PubMed Google Scholar * Wind, J. _et al_. A systematic review on the
significance of extracapsular lymph node involvement in gastrointestinal malignancies. _Eur. J. Surg. Oncol._ 33, 401–408 (2007). Article CAS PubMed Google Scholar * Veronese, N. _et
al_. Prognostic impact of extra-nodal extension in thyroid cancer: a meta-analysis. _J. Surg. Oncol._ 112, 828–833 (2015). Article PubMed Google Scholar * Luchini, C. _et al_. Prognostic
implications of extra-nodal extension in node-positive squamous cell carcinoma of the vulva: a systematic review and meta-analysis. _Surg. Oncol._ 25, 60–65 (2016). Article PubMed Google
Scholar * Nottegar, A. _et al_. Extra-nodal extension of sentinel lymph node metastasis is a marker of poor prognosis in breast cancer patients: A systematic review and an exploratory
meta-analysis. _Eur. J Surg. Oncol._ 42, 919–925 (2016). Article CAS PubMed Google Scholar * Veronese, N. _et al_. Extranodal extension of nodal metastases is a poor prognostic indicator
in gastric cancer: a systematic review and meta-analysis. _J. Gastrointest. Surg._ 20, 1692–1698 (2016). Article PubMed Google Scholar * Luchini, C. _et al_. Extranodal extension of
lymph node metastasis is a marker of poor prognosis in oesophageal cancer: a systematic review with meta-analysis. _J. Clin. Pathol_. [Epub ahead of print] doi: 10.1136/jclinpath-2016-203830
(2016). * Fleischmann, A., Schobinger, S., Schumacher, M., Thalmann, G. N. & Studer, U. E. Survival in surgically treated, nodal positive prostate cancer patients is predicted by
histopathological characteristics of the primary tumor and its lymph node metastases. _Prostate._ 69, 352–362 (2009). Article PubMed Google Scholar * Cheng, L., Montironi, R., Bostwick,
D. G., Lopez-Beltran, A. & Berney, D. M. Staging of prostate cancer. _Histopathology._ 60, 87–117 (2012). Article PubMed Google Scholar * Amin, M. B _et al_. AJCC Cancer Staging
Manual, _8th ed_. (Springer-Verlag 2017). * Conti, A. _et al_. Update on histopathological evaluation of lymphadenectomy specimens from prostate cancer patients. _World J. Urol_. [Epub ahead
of print] (2015). * Perry-Keene, J., Ferguson, P., Samaratunga, H., Nacey, J. N. & Delahunt, B. Total submission of pelvic lymphadenectomy tissues removed during radical prostatectomy
for prostate cancer increases lymph node yield and detection of micrometastases. _Histopathology._ 64, 399–404 (2014). Article PubMed Google Scholar * Montironi, R. _et al_. Total
submission of lymphadenectomy tissues removed during radical prostatectomy for prostate cancer: possible clinical significance of large-format histology. _Hum. Pathol._ 45, 2059–2062 (2014).
Article PubMed Google Scholar * Sergeant, G., Ectors, N., Fieuws, S., Aerts, R. & Topal, B. Prognostic relevance of extracapsular lymph node involvement in pancreatic ductal
adenocarcinoma. _Ann. Surg. Oncol._ 16, 3070–3079 (2009). Article PubMed Google Scholar * Luchini, C. _et al_. Next generation histopathologic diagnosis: a lesson from a hepatic
carcinosarcoma. _J. Clin. Oncol._ 32, e63–6 (2014). Article PubMed Google Scholar * Luchini, C. _et al_. Prognostic role and implications of mutation status of tumor suppressor gene
ARID1A in cancer: a systematic review and meta-analysis. _Oncotarget_ 6, 39088–39097 (2015). PubMed PubMed Central Google Scholar * Luchini, C. _et al_. Different prognostic roles of
tumor suppressor gene BAP1 in cancer: A systematic review with meta-analysis. _Genes Chromosomes Cancer_ 55, 741–749 (2016). Article CAS PubMed Google Scholar * Yachida, S. _et al_.
Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. _Cancer Cell._ 29, 229–240 (2016). Article CAS PubMed Google Scholar * Mafficini, A. _et al_. BRCA somatic and
germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing. _Oncotarget_ 7, 1076–1083 (2016). PubMed PubMed Central Google Scholar * Stroup, D. F. _et
al_. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. _JAMA._ 283, 2008–2012 (2000).
Article CAS PubMed Google Scholar * Liberati, A. _et al_. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions:
explanation and elaboration. _BMJ._ 339, b2700 (2009). Article PubMed PubMed Central Google Scholar * Wells, G. A. _et al_. The Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Date of access: 22/07/2016). * DerSimonian, R. & Laird, N. Meta-analysis in clinical
trials. _Control Clin. Trials._ 7, 177–188 (1986). Article CAS PubMed Google Scholar * Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. _Stat. Med._
21, 1539–1558 (2002). Article PubMed Google Scholar * Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. _Biometrics._ 50, 1088–1101
(1994). Article CAS PubMed MATH Google Scholar * Egger, M., Davey Smith, G. & Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. _BMJ._ 315,
629–634 (1997). Article CAS PubMed PubMed Central Google Scholar * Duval, S. & Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication
bias in meta-analysis. _Biometrics._ 56, 455–463 (2000). Article CAS PubMed MATH Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Diagnostics and Public Health, University and Hospital Trust of Verona, Verona, Italy Claudio Luchini, Alessia Nottegar, Matteo Brunelli & Aldo Scarpa * ARC-NET Research Center,
University and Hospital Trust of Verona, Verona, Italy Claudio Luchini & Aldo Scarpa * Department of Pathology, Santa Chiara Hospital, Trento, Italy Claudio Luchini * Institute of
Pathology, University of Bern, CH-3010, Bern, Switzerland Achim Fleischmann * Department of Urology, Erasmus MC - Cancer Institute, Rotterdam, The Netherlands Joost L. Boormans * Department
of Medicine, DIMED, University of Padua, Padua, Italy Matteo Fassan & Paola Lucato * Health Service and Population Research Department, King’s College London, De Crespigny Park, London,
SE5 8AF, United Kingdom Brendon Stubbs * Department of Neuroscience, University of Padua, Padua, Italy Marco Solmi * Urologic Clinic, University and Hospital trust of Verona, Verona, Italy
Antonio Porcaro * National Research Council, Neuroscience Institute, Aging Branch, Padova, Italy Nicola Veronese * Institute for clinical Research and Education in Medicine (IREM), Padova,
Italy Nicola Veronese * Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN, USA Liang Cheng Authors * Claudio Luchini View author
publications You can also search for this author inPubMed Google Scholar * Achim Fleischmann View author publications You can also search for this author inPubMed Google Scholar * Joost L.
Boormans View author publications You can also search for this author inPubMed Google Scholar * Matteo Fassan View author publications You can also search for this author inPubMed Google
Scholar * Alessia Nottegar View author publications You can also search for this author inPubMed Google Scholar * Paola Lucato View author publications You can also search for this author
inPubMed Google Scholar * Brendon Stubbs View author publications You can also search for this author inPubMed Google Scholar * Marco Solmi View author publications You can also search for
this author inPubMed Google Scholar * Antonio Porcaro View author publications You can also search for this author inPubMed Google Scholar * Nicola Veronese View author publications You can
also search for this author inPubMed Google Scholar * Matteo Brunelli View author publications You can also search for this author inPubMed Google Scholar * Aldo Scarpa View author
publications You can also search for this author inPubMed Google Scholar * Liang Cheng View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
Study concepts: C.L., L.C.; Study design: C.L., L.C.; Manuscript writing: C.L., L.C.; Manuscript editing: C.L., A.F., J.L.B., M.F., A.N., P.L., B.S., M.S., A.P., N.V., M.B., A.S., L.C.; Data
extraction: C.L., A.F., J.L.B., A.N., M.S.,; Data elaboration and interpretation: C.L., A.F., J.L.B., M.F., A.N., P.L., B.S., M.S., A.P., N.V., M.B., A.S., L.C.; Statistical analysis: C.L.,
Manuscript revision and approval of submission in its present form: C.L., A.F., J.L.B., M.F., A.N., P.L., B.S., M.S., A.P., N.V., M.B., A.S., L.C., Important intellectual content: N.V.,
A.S., L.C. CORRESPONDING AUTHORS Correspondence to Claudio Luchini or Liang Cheng. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY
MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Luchini, C., Fleischmann, A., Boormans, J.L. _et al._ Extranodal extension of lymph node metastasis influences recurrence in prostate cancer: a systematic
review and meta-analysis. _Sci Rep_ 7, 2374 (2017). https://doi.org/10.1038/s41598-017-02577-4 Download citation * Received: 07 November 2016 * Accepted: 12 April 2017 * Published: 24 May
2017 * DOI: https://doi.org/10.1038/s41598-017-02577-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative