Mechanism of receptor assembly via the pleiotropic adipokine leptin
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However,
the structure and mechanism of Leptin-mediated LEP-R assemblies has remained unclear. Intriguingly, the signaling-competent isoform of LEP-R is only lowly abundant amid several inactive
short LEP-R isoforms contributing to a mechanistic conundrum. Here we show by X-ray crystallography and cryo-EM that, in contrast to long-standing paradigms, Leptin induces type I cytokine
receptor assemblies featuring 3:3 stoichiometry and demonstrate such Leptin-induced trimerization of LEP-R on living cells via single-molecule microscopy. In mediating these assemblies,
Leptin undergoes drastic restructuring that activates its site III for binding to the Ig domain of an adjacent LEP-R. These interactions are abolished by mutations linked to obesity.
Collectively, our study provides the structural and mechanistic framework for how evolutionarily conserved Leptin:LEP-R assemblies with 3:3 stoichiometry can engage distinct LEP-R isoforms
to achieve signaling. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS STRUCTURAL INSIGHTS INTO THE MECHANISM OF LEPTIN RECEPTOR ACTIVATION Article Open access 31 March 2023 CRYSTAL STRUCTURE OF ISTHMIN-1 AND REASSESSMENT OF ITS
FUNCTIONAL ROLE IN PRE-ADIPOCYTE SIGNALING Article Open access 15 April 2025 STRUCTURAL BASIS OF CYTOKINE-MEDIATED ACTIVATION OF ALK FAMILY RECEPTORS Article 13 October 2021 DATA
AVAILABILITY Crystallographic coordinates and structure factors have been deposited in the PDB, and cryo-EM maps and models have been deposited in the Electron Microscopy Data Bank (EMDB)
and PDB data banks with accession codes listed in Tables 1 and 2. Briefly, the crystal structure of mLeptin:mLEP-RCRH2 is deposited under the accession code PDB 7z3p, hLeptin:hLEP-RCRH2
under PDB 7z3q, mLeptin:mLEP-RIgCRH2 under PDB 7z3r, and mLEP-RFNIII:VHH-4.80 under PDB 8av2. The cryo-EM map of the open 1:2 mLeptin:mLEP-RECD complex has been deposited under the accession
codes EMD-15677 and PDB 8avb. The cryo-EM map of the closed 3:3 mLeptin:mLEP-RECD-tGCN4 complex has been deposited under the accession codes EMD-15678 and PDB 8avc, after local refinement
under accession codes EMD-15679 and PDB 8avd, and after local refinement and symmetry expansion under accession codes EMD-15899 and PDB 8b7q. The cryo-EM map of the open 2:2
hLeptin:hLEP-RECD-tGCN4 complex has been deposited under the accession codes EMD-15680 and PDB 8ave, the closed 3:3 hLeptin:hLEP-RECD-tGCN4 complex under EMD-15681 and PDB 8avf, and the open
3:3 hLeptin:hLEP-RECD-tGCN4 complex under EMD-15683 and PDB 8avo. Search models for molecular replacement were prepared using PDB 1ax8 (hLeptin), PDB 3v6o (hLEP-RCRH2 fragment), PDB 1OHQ
(VHH model) and AF-P48356-F1-model_v1 (for mLEP-RFnIII). Coordinates for the IL6 (PDB 1p9m) and GCSF (PDB 2d9q) receptor complexes were used for comparative analysis. Structural predictions
via Alphafold were used to generate starting models for real-space cryo-EM refinement: mLEP-R (AF-P48356-F1-model_v1), hLeptin (AF-P41159-F1-model_v2) and hLEP-R (AF-P48357-F1-model_v2).
TIRF microscopy data from localization and co-tracking analysis have been uploaded into the publicly available repository Zenodo together with representative raw imaging data and are
available at https://doi.org/10.5281/zenodo.7419603. AUC data are publicly available at https://doi.org/10.5281/zenodo.7566572. All remaining data generated or analyzed during this study are
included in this published article (and its supplementary information files). Source data are provided with this paper. REFERENCES * Zhang, F. et al. Crystal structure of the obese protein
leptin-E100. _Nature_ 387, 206–209 (1997). Article CAS PubMed Google Scholar * Wauman, J., Zabeau, L. & Tavernier, J. The leptin receptor complex: heavier than expected? _Front.
Endocrinol._ 8, 30 (2017). Article Google Scholar * de Candia, P. et al. The pleiotropic roles of leptin in metabolism, immunity, and cancer. _J. Exp. Med._ 218, e20191593 (2021). Article
PubMed PubMed Central Google Scholar * Friedman, J. M. Leptin and the endocrine control of energy balance. _Nat. Metab._ 1, 754–764 (2019). Article CAS PubMed Google Scholar *
Salum, K. C. R. et al. When leptin is not there: a review of what nonsyndromic monogenic obesity cases tell us and the benefits of exogenous leptin. _Front. Endocrinol._ 12, 722441 (2021).
Article Google Scholar * Farooqi, I. S. et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. _N. Engl. J. Med._ 356, 237–247 (2007). Article CAS
PubMed PubMed Central Google Scholar * Gorska, E. et al. Leptin receptors. _Eur. J. Med. Res._ 15, 50–54 (2010). Article PubMed PubMed Central Google Scholar * Fei, H. et al.
Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. _Proc. Natl Acad. Sci. USA_ 94, 7001–7005 (1997). Article CAS PubMed PubMed
Central Google Scholar * Haniu, M. et al. Human leptin receptor. Determination of disulfide structure and N-glycosylation sites of the extracellular domain. _J. Biol. Chem._ 273,
28691–28699 (1998). Article CAS PubMed Google Scholar * Zabeau, L. et al. Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of
transcription complementation assay. _Mol. Endocrinol._ 18, 150–161 (2004). Article CAS PubMed Google Scholar * Moharana, K. et al. Structural and mechanistic paradigm of leptin receptor
activation revealed by complexes with wild-type and antagonist leptins. _Structure_ 22, 866–877 (2014). Article CAS PubMed Google Scholar * Peelman, F. et al. Mapping of binding site
III in the leptin receptor and modeling of a hexameric leptin.leptin receptor complex. _J. Biol. Chem._ 281, 15496–15504 (2006). Article CAS PubMed Google Scholar * Zabeau, L. et al.
Leptin receptor activation depends on critical cysteine residues in its fibronectin type III subdomains. _J. Biol. Chem._ 280, 22632–22640 (2005). Article CAS PubMed Google Scholar * Tu,
H., Hsuchou, H., Kastin, A. J., Wu, X. & Pan, W. Unique leptin trafficking by a tailless receptor. _FASEB J._ 24, 2281–2291 (2010). Article CAS PubMed PubMed Central Google Scholar
* Ge, H., Huang, L., Pourbahrami, T. & Li, C. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. _J. Biol. Chem._ 277,
45898–45903 (2002). Article CAS PubMed Google Scholar * Ferrao, R. D., Wallweber, H. J. & Lupardus, P. J. Receptor-mediated dimerization of JAK2 FERM domains is required for JAK2
activation. _eLife_ 7, e38089 (2018). Article PubMed PubMed Central Google Scholar * Glassman, C. R. et al. Structure of a Janus kinase cytokine receptor complex reveals the basis for
dimeric activation. _Science_ 376, 163–169 (2022). Article CAS PubMed PubMed Central Google Scholar * Mancour, L. V. et al. Ligand-induced architecture of the leptin receptor signaling
complex. _Mol. Cell_ 48, 655–661 (2012). Article CAS PubMed PubMed Central Google Scholar * White, D. W., Kuropatwinski, K. K., Devos, R., Baumann, H. & Tartaglia, L. A. Leptin
receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. _J. Biol. Chem._ 272, 4065–4071 (1997). Article CAS PubMed Google Scholar
* Bacart, J. et al. Evidence for leptin receptor isoforms heteromerization at the cell surface. _FEBS Lett._ 584, 2213–2217 (2010). Article CAS PubMed Google Scholar * Iserentant, H.
et al. Mapping of the interface between leptin and the leptin receptor CRH2 domain. _J. Cell Sci._ 118, 2519–2527 (2005). Article CAS PubMed Google Scholar * Peelman, F. et al. Mapping
of the leptin binding sites and design of a leptin antagonist. _J. Biol. Chem._ 279, 41038–41046 (2004). Article CAS PubMed Google Scholar * Danielsson, J. et al. The pierced lasso
topology leptin has a bolt on dynamic domain composed by the disordered loops I and III. _J. Mol. Biol._ 432, 3050–3063 (2020). Article CAS PubMed Google Scholar * Niv-Spector, L. et al.
Mapping leptin-interacting sites in recombinant leptin-binding domain (LBD) subcloned from chicken leptin receptor. _Biochem. J._ 390, 475–484 (2005). Article CAS PubMed PubMed Central
Google Scholar * Verploegen, S. A., Plaetinck, G., Devos, R., Van der Heyden, J. & Guisez, Y. A human leptin mutant induces weight gain in normal mice. _FEBS Lett._ 405, 237–240 (1997).
Article CAS PubMed Google Scholar * Carpenter, B. et al. Structure of the human obesity receptor leptin-binding domain reveals the mechanism of leptin antagonism by a monoclonal
antibody. _Structure_ 20, 487–497 (2012). Article CAS PubMed Google Scholar * Spangler, J. B., Moraga, I., Mendoza, J. L. & Garcia, K. C. Insights into cytokine–receptor interactions
from cytokine engineering. _Annu. Rev. Immunol._ 33, 139–167 (2015). Article CAS PubMed Google Scholar * Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the
structural coverage of protein-sequence space with high-accuracy models. _Nucleic Acids Res._ 50, D439–D444 (2022). Article CAS PubMed Google Scholar * Niv-Spector, L. et al.
Identification of the hydrophobic strand in the A–B loop of leptin as major binding site III: implications for large-scale preparation of potent recombinant human and ovine leptin
antagonists. _Biochem. J._ 391, 221–230 (2005). Article CAS PubMed PubMed Central Google Scholar * Gromada, J. S. P., Altarejos, J., & Murphy, A. J. A leptin receptor agonist
antibody for use in treating a metabolic dysfunction or hypoleptinemia. Patent No. WO2019195796A1 (2019). * Harbury, P. B., Kim, P. S. & Alber, T. Crystal structure of an
isoleucine-zipper trimer. _Nature_ 371, 80–83 (1994). Article CAS PubMed Google Scholar * Walls, A. C. et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein
trimer. _Nature_ 531, 114–117 (2016). Article CAS PubMed PubMed Central Google Scholar * Xu, Y. et al. Crystal structure of the entire ectodomain of gp130: insights into the molecular
assembly of the tall cytokine receptor complexes. _J. Biol. Chem._ 285, 21214–21218 (2010). Article CAS PubMed PubMed Central Google Scholar * Mirdita, M. et al. ColabFold: making
protein folding accessible to all. _Nat. Methods_ 19, 679–682 (2022). Article CAS PubMed PubMed Central Google Scholar * Evans, R. et al. Protein complex prediction with
AlphaFold-Multimer. Preprint at _bioRxiv_ https://doi.org/10.1101/2021.10.04.463034 (2022). * Liongue, C., Sertori, R. & Ward, A. C. Evolution of cytokine receptor signaling. _J.
Immunol._ 197, 11–18 (2016). Article CAS PubMed Google Scholar * Sotolongo Bellon, J. et al. Four-color single-molecule imaging with engineered tags resolves the molecular architecture
of signaling complexes in the plasma membrane. _Cell Rep. Methods_ 2, 100165 (2022). Article CAS PubMed PubMed Central Google Scholar * Morris, R., Kershaw, N. J. & Babon, J. J. The
molecular details of cytokine signaling via the JAK/STAT pathway. _Protein Sci._ 27, 1984–2009 (2018). Article CAS PubMed PubMed Central Google Scholar * Richter, D. et al.
Ligand-induced type II interleukin-4 receptor dimers are sustained by rapid re-association within plasma membrane microcompartments. _Nat. Commun._ 8, 15976 (2017). Article CAS PubMed
PubMed Central Google Scholar * Wilmes, S. et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. _Science_ 367, 643–652 (2020). Article
CAS PubMed PubMed Central Google Scholar * Martinez-Fabregas, J. et al. Kinetics of cytokine receptor trafficking determine signaling and functional selectivity. _eLife_ 8, e49314
(2019). Article PubMed PubMed Central Google Scholar * Wilmes, S. et al. Receptor dimerization dynamics as a regulatory valve for plasticity of type I interferon signaling. _J. Cell
Biol._ 209, 579–593 (2015). Article CAS PubMed PubMed Central Google Scholar * Gorby, C. et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses.
_Sci. Signal._ 13, eabc0653 (2020). Article CAS PubMed PubMed Central Google Scholar * Mazen, I., El-Gammal, M., Abdel-Hamid, M. & Amr, K. A novel homozygous missense mutation of
the leptin gene (N103K) in an obese Egyptian patient. _Mol. Genet. Metab._ 97, 305–308 (2009). Article CAS PubMed Google Scholar * Wabitsch, M. et al. Biologically inactive leptin and
early-onset extreme obesity. _N. Engl. J. Med._ 372, 48–54 (2015). Article PubMed Google Scholar * Wabitsch, M. et al. Measurement of immunofunctional leptin to detect and monitor
patients with functional leptin deficiency. _Eur. J. Endocrinol._ 176, 315–322 (2017). Article CAS PubMed Google Scholar * Zastrow, O. et al. The soluble leptin receptor is crucial for
leptin action: evidence from clinical and experimental data. _Int. J. Obes. Relat. Metab. Disord._ 27, 1472–1478 (2003). Article CAS PubMed Google Scholar * Djogo, T. et al. Adult
NG2-glia are required for median eminence-mediated leptin sensing and body weight control. _Cell Metab._ 23, 797–810 (2016). Article CAS PubMed Google Scholar * Butiaeva, L. I. et al.
Leptin receptor-expressing pericytes mediate access of hypothalamic feeding centers to circulating leptin. _Cell Metab._ 33, 1433–1448.e5 (2021). Article CAS PubMed Google Scholar *
Langendonk, J. G. et al. Circadian rhythm of plasma leptin levels in upper and lower body obese women: influence of body fat distribution and weight loss. _J. Clin. Endocrinol. Metab._ 83,
1706–1712 (1998). Article CAS PubMed Google Scholar * Li, Z., Ceccarini, G., Eisenstein, M., Tan, K. & Friedman, J. M. Phenotypic effects of an induced mutation of the ObRa isoform
of the leptin receptor. _Mol. Metab._ 2, 364–375 (2013). Article CAS PubMed PubMed Central Google Scholar * Bjorbaek, C. et al. Expression of leptin receptor isoforms in rat brain
microvessels. _Endocrinology_ 139, 3485–3491 (1998). Article CAS PubMed Google Scholar * Bjorbaek, C., Uotani, S., da Silva, B. & Flier, J. S. Divergent signaling capacities of the
long and short isoforms of the leptin receptor. _J. Biol. Chem._ 272, 32686–32695 (1997). Article CAS PubMed Google Scholar * Bahrenberg, G. et al. Identification of the critical
sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. _Mol.
Endocrinol._ 16, 859–872 (2002). Article CAS PubMed Google Scholar * Vernooy, J. H. et al. Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in
mice. _J. Immunol._ 184, 7169–7177 (2010). Article CAS PubMed Google Scholar * Aricescu, A. R., Lu, W. & Jones, E. Y. A time- and cost-efficient system for high-level protein
production in mammalian cells. _Acta Crystallogr. D_ 62, 1243–1250 (2006). Article PubMed Google Scholar * Zabeau, L. et al. A novel leptin receptor antagonist uncouples leptin’s
metabolic and immune functions. _Cell. Mol. Life Sci._ 76, 1201–1214 (2019). Article CAS PubMed Google Scholar * Reeves, P. J., Callewaert, N., Contreras, R. & Khorana, H. G.
Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative
HEK293S stable mammalian cell line. _Proc. Natl Acad. Sci. USA_ 99, 13419–13424 (2002). Article CAS PubMed PubMed Central Google Scholar * Kabsch, W. XDS. _Acta Crystallogr. D_ 66,
125–132 (2010). Article CAS PubMed PubMed Central Google Scholar * Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? _Acta Crystallogr. D_ 69,
1204–1214 (2013). Article CAS PubMed PubMed Central Google Scholar * Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent
developments in Phenix. _Acta Crystallogr. D_ 75, 861–877 (2019). Article CAS Google Scholar * McCoy, A. J. et al. Phaser crystallographic software. _J. Appl. Crystallogr._ 40, 658–674
(2007). Article CAS PubMed PubMed Central Google Scholar * Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. _Acta Crystallogr. D_ 60, 2126–2132 (2004).
Article PubMed Google Scholar * Bricogne, G. B. E. et al. BUSTER version 2.10.4. (Global Phasing Ltd., 2017); https://www.globalphasing.com/buster/ * Eisenberg, D., Schwarz, E., Komaromy,
M. & Wall, R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. _J. Mol. Biol._ 179, 125–142 (1984). Article CAS PubMed Google Scholar * Schuck,
P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. _Biophys. J._ 78, 1606–1619 (2000). Article CAS PubMed PubMed
Central Google Scholar * Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. _Anal. Biochem._ 320, 104–124 (2003). Article CAS
PubMed Google Scholar * Brautigam, C. A. Calculations and publication-quality illustrations for analytical ultracentrifugation data. _Methods Enzymol._ 562, 109–133 (2015). Article CAS
PubMed Google Scholar * DuBridge, R. B. et al. Analysis of mutation in human cells by using an Epstein–Barr virus shuttle system. _Mol. Cell. Biol._ 7, 379–387 (1987). CAS PubMed
PubMed Central Google Scholar * Eyckerman, S. et al. Analysis of Tyr to Phe and fa/fa leptin receptor mutations in the PC12 cell line. _Eur. Cytokine Netw._ 10, 549–556 (1999). CAS PubMed
Google Scholar * Götzke, H. et al. The ALFA-tag is a highly versatile tool for nanobody-based bioscience applications. _Nat. Commun._ 10, 4403 (2019). Article PubMed PubMed Central
Google Scholar * You, C., Richter, C. P., Löchte, S., Wilmes, S. & Piehler, J. Dynamic submicroscopic signaling zones revealed by pair correlation tracking and localization microscopy.
_Anal. Chem._ 86, 8593–8602 (2014). Article CAS PubMed Google Scholar * Serge, A., Bertaux, N., Rigneault, H. & Marguet, D. Dynamic multiple-target tracing to probe spatiotemporal
cartography of cell membranes. _Nat. Methods_ 5, 687–694 (2008). Article CAS PubMed Google Scholar * Niewidok, B. et al. Single-molecule imaging reveals dynamic biphasic partition of
RNA-binding proteins in stress granules. _J. Cell Biol._ 217, 1303–1318 (2018). Article CAS PubMed PubMed Central Google Scholar * Jaqaman, K. et al. Robust single-particle tracking in
live-cell time-lapse sequences. _Nat. Methods_ 5, 695–702 (2008). Article CAS PubMed PubMed Central Google Scholar * Blanchet, C. E. et al. Versatile sample environments and automation
for biological solution X-ray scattering experiments at the P12 beamline (PETRA III, DESY). _J. Appl. Crystallogr._ 48, 431–443 (2015). Article CAS PubMed PubMed Central Google Scholar
* Manalastas-Cantos, K. et al. ATSAS 3.0: expanded functionality and new tools for small-angle scattering data analysis. _J. Appl. Crystallogr._ 54, 343–355 (2021). Article CAS PubMed
PubMed Central Google Scholar * Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. _Nat. Methods_ 14, 331–332 (2017).
Article CAS PubMed PubMed Central Google Scholar * Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure
determination. _Nat. Methods_ 14, 290–296 (2017). Article CAS PubMed Google Scholar * Bepler, T. et al. Positive-unlabeled convolutional neural networks for particle picking in
cryo-electron micrographs. _Nat. Methods_ 16, 1153–1160 (2019). Article CAS PubMed PubMed Central Google Scholar * Jumper, J. et al. Highly accurate protein structure prediction with
AlphaFold. _Nature_ 596, 583–589 (2021). Article CAS PubMed PubMed Central Google Scholar * Cianfrocco MA, W. M., Youn, C., Wagner, R. & Leschziner, A. E. COSMIC2: a science gateway
for cryo-electron microscopy structure determination. In _Proc. Practice and Experience in Advanced Research Computing 2017 on Sustainability, Success and Impact_ (eds. Hart, D.) 22 (ACM,
2017). * Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. _Commun. Biol._ 4, 874 (2021). Article PubMed PubMed Central Google Scholar
* Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. _J. Mol. Biol._ 333,
721–745 (2003). Article CAS PubMed Google Scholar * Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by
single particle electron cryomicroscopy. _Ultramicroscopy_ 135, 24–35 (2013). Article CAS PubMed PubMed Central Google Scholar * Cardone, G., Heymann, J. B. & Steven, A. C. One
number does not fit all: mapping local variations in resolution in cryo-EM reconstructions. _J. Struct. Biol._ 184, 226–236 (2013). Article PubMed Google Scholar * Pettersen, E. F. et al.
UCSF ChimeraX: structure visualization for researchers, educators, and developers. _Protein Sci._ 30, 70–82 (2021). Article CAS PubMed Google Scholar * The PyMOL Molecular Graphics
System, Version 2.3.3 (Schrödinger, 2019). * Peelman, F., Zabeau, L., Moharana, K., Savvides, S. N. & Tavernier, J. 20 years of leptin: insights into signaling assemblies of the leptin
receptor. _J. Endocrinol._ 223, T9–T23 (2014). Article CAS PubMed Google Scholar * Boulanger, M. J., Chow, D. C., Brevnova, E. E. & Garcia, K. C. Hexameric structure and assembly of
the interleukin-6/IL-6 alpha-receptor/gp130 complex. _Science_ 300, 2101–2104 (2003). Article CAS PubMed Google Scholar * Tamada, T. et al. Homodimeric cross-over structure of the human
granulocyte colony-stimulating factor (GCSF) receptor signaling complex. _Proc. Natl Acad. Sci. USA_ 103, 3135–3140 (2006). Article CAS PubMed PubMed Central Google Scholar * Amemiya,
C. T. et al. The African coelacanth genome provides insights into tetrapod evolution. _Nature_ 496, 311–316 (2013). Article CAS PubMed PubMed Central Google Scholar * Londraville, R.
L., Prokop, J. W., Duff, R. J., Liu, Q. & Tuttle, M. On the molecular evolution of leptin, leptin receptor, and endospanin. _Front. Endocrinol._ 8, 58 (2017). Article Google Scholar
Download references ACKNOWLEDGEMENTS We thank the staff of beamlines P12, P13 and P14 (Petra III, Deutsches Elektronen-Synchrotron), and Proxima 2A (SOLEIL) for technical support and
beamtime allocation. We are grateful to the staff of the VIB-VUB facility for Bio Electron Cryogenic Microscopy (BECM, Brussels, Belgium), the staff of the Laboratory of Cell Biology &
Histology (CBH) and the Antwerp Centre for Advanced Microscopy (ACAM) (UAntwerpen, Antwerp, Belgium), and the electron Bio-Imaging Centre (eBIC) at the Diamond Light Source (Didcot, UK) for
technical support and infrastructural access, and H. Kenneweg for production of labeled NBs. The pGL3-rPAPluc plasmid containing the luciferase gene was a kind gift of F. Peelman (VIB-UGent,
Ghent, Belgium). This work was supported by grants from the Research Foundation-Flanders (grant G0G0619N to K.V.) and the VIB (to S.N.S.). This work benefited from access to the Integrated
Structural Biology platform of the Strasbourg Instruct-ERIC center IGBMC-CBI. Financial support was provided by FRISBI (ANR-10-INBS-0005 to C.B.), Instruct-ERIC (PID 15107 to K.V.),
iNext-Discovery (project number 17947 to K.V. and funded by the Horizon 2020 program of the European Commission) and the DFG (SFB 944, projects P8 and Z, to J.P.). AUTHOR INFORMATION Author
notes * Dominiek Catteeuw Present address: Department of Biochemistry and Microbiology, Ghent University, Ghent, Belgium * Jan Tavernier Present address: Orionis Biosciences, Ghent, Belgium
AUTHORS AND AFFILIATIONS * Unit for Structural Biology, Department of Biochemistry and Microbiology, Ghent University, Ghent, Belgium Alexandra Tsirigotaki, Ann Dansercoer, Koen H. G.
Verschueren, Iva Marković, Jan Felix, Savvas N. Savvides & Kenneth Verstraete * Unit for Structural Biology, VIB-UGent Center for Inflammation Research, Ghent, Belgium Alexandra
Tsirigotaki, Ann Dansercoer, Koen H. G. Verschueren, Iva Marković, Jan Felix, J. Fernando Bazan, Savvas N. Savvides & Kenneth Verstraete * Department of Biology/Chemistry and Center for
Cellular Nanoanalytics, Osnabrück University, Osnabrück, Germany Christoph Pollmann, Maximillian Hafer & Jacob Piehler * Integrated Structural Biology Platform, Centre for Integrative
Biology (CBI), Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), CNRS UMR 7104, INSERM U1258, University of Strasbourg, Illkirch, France Catherine Birck * PUXANO BV,
Ghent, Belgium Wouter Van Putte * VIB-UGent Center for Medical Biotechnology, Ghent, Belgium Dominiek Catteeuw & Jan Tavernier * Department of Biomolecular Medicine, Ghent University,
Ghent, Belgium Dominiek Catteeuw & Jan Tavernier * ħ Bioconsulting llc, Stillwater, MN, USA J. Fernando Bazan Authors * Alexandra Tsirigotaki View author publications You can also search
for this author inPubMed Google Scholar * Ann Dansercoer View author publications You can also search for this author inPubMed Google Scholar * Koen H. G. Verschueren View author
publications You can also search for this author inPubMed Google Scholar * Iva Marković View author publications You can also search for this author inPubMed Google Scholar * Christoph
Pollmann View author publications You can also search for this author inPubMed Google Scholar * Maximillian Hafer View author publications You can also search for this author inPubMed Google
Scholar * Jan Felix View author publications You can also search for this author inPubMed Google Scholar * Catherine Birck View author publications You can also search for this author
inPubMed Google Scholar * Wouter Van Putte View author publications You can also search for this author inPubMed Google Scholar * Dominiek Catteeuw View author publications You can also
search for this author inPubMed Google Scholar * Jan Tavernier View author publications You can also search for this author inPubMed Google Scholar * J. Fernando Bazan View author
publications You can also search for this author inPubMed Google Scholar * Jacob Piehler View author publications You can also search for this author inPubMed Google Scholar * Savvas N.
Savvides View author publications You can also search for this author inPubMed Google Scholar * Kenneth Verstraete View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS A.T. prepared constructs, and performed protein expression and purification with contributions from A.D. and K.V. A.T., K.H.G.V. and K.V. determined and analyzed
crystallographic structures with contributions from S.N.S. K.V. collected and analyzed cryo-EM data, with contributions from J.F., W.V.P. and S.N.S. A.T. performed biolayer interferometry,
and SEC–MALLS experiments. SAXS data were analyzed by K.V. and A.T. I.M. performed cellular assays. M.H. and C.P. performed smTIRFM experiments and data analysis. J.F.B. carried out
evolutionary structural analyses, and J.P. supervised the smTIRFM experiments. C.B. performed SV–AUC experiments and analyzed data. D.C. and J.T. contributed critical reagents. A.T., K.V.
and S.N.S. wrote the paper with contributions and approval from all authors. K.V. and S.N.S. conceived and supervised the project and procured funding. CORRESPONDING AUTHORS Correspondence
to Savvas N. Savvides or Kenneth Verstraete. ETHICS DECLARATIONS COMPETING INTERESTS W.V.P. is the founder and chief executive officer of PUXANO. All other authors declare no competing
interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Structural & Molecular Biology_ thanks Eunhee Choi, Ellinor Haglund and the other, anonymous, reviewer(s) for their contribution to
the peer review of this work. Primary Handling Editors: Florian Ullrich and Katarzyna Ciazynska, in collaboration with the _Nature Structural & Molecular Biology_ team. Peer reviewer
reports are available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED
DATA EXTENDED DATA FIG. 1 STRUCTURAL AND BIOPHYSICAL CHARACTERIZATION OF THE LEPTIN:LEP-R ASSEMBLY. A-C, Characterization of the minimal Leptin:LEP-R recognition complex. A, Structural
superposition of the glycan-trimmed hLeptin:hLEP-RCRH2 complex crystal structure (LEP-RCRH2 fragment 428-635 N516Q/C604S in blue; Leptin in yellow) with the mouse complex crystal structure
(colored in gray). The structure of the individual components and the interaction interface thereof is highly similar between the two homologues, consistent with their high sequence identity
(83.2% for Leptin, 86.9% for LEP-RCRH2, Supplementary Fig. 1). (r.m.s.d.: root mean square deviation of alignment, 161 Cα atoms) B, Molar mass determination of the glycan-trimmed
mLeptin:mLEP-RCRH2 complex using Size Exclusion Chromatography coupled with Multi-Angle Laser Light Scattering (SEC-MALLS). The complex (50 μM) was resolved in a Superdex 200 increase column
pre-equilibrated in HBS buffer (20 mM HEPES pH 7.4, 150 mM NaCl). The measured mass (39.3 kDa) approximates the theoretical of 40.3 kDa for 1:1 stoichiometry. _(n_ = _1)_ C, Biolayer
interferometry (BLI) sensograms of the mouse Leptin:LEP-R interaction. Left: mLeptin was titrated, as indicated on the side, against the _in vitro_ biotinylated mLEP-RCRH2 fragment
immobilized on Streptavidin sensors _(n_ = _4)_. Right: mLEP-RECD was titrated against _in vitro_ biotinylated mLeptin _(n_ = _3)_. Comparison of the kinetic rates and derived affinities
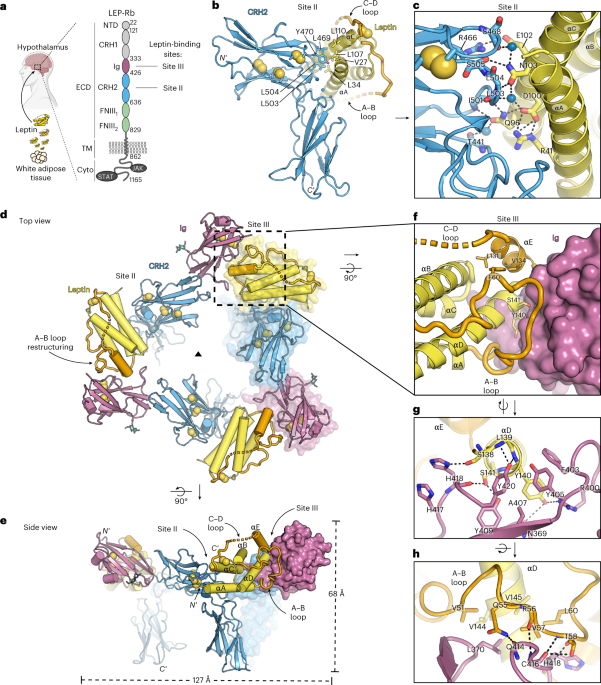
suggest that the high-affinity Leptin:LEP-R interaction is attributed to the Leptin:LEP-RCRH2 interface, in agreement with previous studies89. D–G, Characterization of the
mLeptin:LEP-RIgCRH2 oligomeric assembly. D, The asymmetric unit of the glycan-trimmed mLeptin:mLEP-RIgCRH2 crystal structure resolved at 2.9 Å (coloured as in Fig. 1a) superimposed to the
mLeptin:mLEP-RCRH2 structure (grey) (r.m.s.d. of alignment =0.4 Å, 257 Cα atoms). Interacting residues at the Ig-CRH2 interface are highlighted in the insert. A glycan chain (green sticks)
was resolved at position Asn393 of the LEP-RIg domain. E, The oligomeric assembly of mLeptin:mLEP-RIgCRH2 (left) derived from the 3- and 2-fold crystallographic symmetry operations as
detailed on the right (space group H 3 2). The assembly comprised of a 3:3 mLeptin:mLEP-RIgCRH2 stoichiometric complex through site II and site III contacts, and an artifactual head-to-head
dimerization thereof, resulting in a 6:6 complex. The head-to-head dimer associates purely by mirroring Ig-Ig’ contacts at an exposed hydrophobic patch shown in the insert. The LEP-R
N-terminal domain is expected to disrupt this interface (N-termini indicated). F, In solution molar mass determination of LEP-RIgCRH2 and complexes thereof with Leptin and the Leptin
antagonist a1 (140ST141/AA)22,29 using SEC-MALLS (Superdex 200 increase column). Protein molar masses are plotted after protein conjugate analysis of the glycosylated complexes.
Experimentally determined and theoretical masses are given on the right. h: human; m: mouse complexes. _n_ = _1_. G, SDS-PAGE (17%) of isolated 6:6 and 1:1 Leptin:LEP-RIgCRH2 complexes under
different sample preparation conditions. Multimeric artifacts are observed only with the combination of reduction and boiling, raising awareness for sample preparation for determining
stoichiometry from SDS-PAGE. Leptin migrates slower under reducing conditions. (β-merc: β-mercaptoethanol). Source data EXTENDED DATA FIG. 2 EVOLUTIONARY AND CO-EVOLUTIONARY FEATURES OF
LEP-RIGCRH2 AND ITS INTERACTION INTERFACES WITH LEPTIN. A, Structural superposition (top) and sequence alignment (bottom) of the LEP-RIgCRH2 domains with those of human gp130 (IL6Rβ)90 and
GCSFR91 in their cytokine-bound state (pdb: 1p9m, 2d9q respectively). After alignment at the first subdomain of the CRH2 module (r.m.s.d. indicated; 75 and 60 Cα atoms respectively), the
shift distance was determined between the highly conserved cysteine residues of the Ig motif, as indicated. Structure-based sequence alignment was performed using UCSF Chimera and shows a
shorter linker between the Ig and CRH2 domains for LEP-R relatively to its structural homologues. B, Structural superposition of the site III interface in evolutionary relatives. For
visualization purposes only the α-helix D is shown for mLeptin, hIL6 and hGCSF, featuring the aromatic blueprint of site III in sticks. IL6Rβ:IL6 and GCSFR:GCSF were aligned to the Ig domain
of mLeptin:LEP-RIgCRH2 (r.m.s.d. indicated; 41 and 59 Cα atoms respectively). C-F, Structural superposition of the crystallographically-distilled mLeptin:mLEP-RIgCRH2 assembly with
AlphaFold models, as indicated, accompanied with predicted Aligned Error plots (PAE) of each prediction. AlphaFold2-ptm was used for the prediction of mLEP-RIgCRH2 (panels C-D) and
AlphaFold-Multimer (version 2.2) for the prediction of the mLeptin:mLEP-RIgCRH2 interaction interfaces (panels E-F). G, Evolutionary conservation of the Leptin:LEP-RIgCRH2 hexameric assembly
across the animal phylogenetic spectrum, tested by AlphaFold-Multimer (version 2.2, with associated ipTM scores)34,35 with orthologous 3:3 pairings of Leptin and LEP-R sequences harvested
from UniProt. Drawing the branching of jawed vertebrates from the coelacanth phylogenetic tree92, birds are represented by the chicken _G. gallus_ Leptin:LEP-R complex (respectively UniProt
codes O42164 and Q9I8V6), reptiles by the alligator _A. sinensis_ (A0A1U8DFZ0 and A0A3Q0H8E2), mammals by human _H. sapiens_ (P41159 and P48357), amphibians by _X. tropicalis_ (A0A803KEF7
and F6RVW6), lobe-finned fishes by the living fossil _Coelacanth L. chalumnae_ (H3AP27 and H3AG22), cartilaginous fishes by the Australian ghostshark _C. milii_ (A0A4W3H871 and V9KNM1), and
ray-finned fishes by the eel _A. anguilla_ (A0A0C7AV37 and A0A0C7AV33) and tilapia _O. niloticus_ (I3KCE8 and A0A067Z8Z1). Sequence evolutionary relationships were based on LEP_R orthologues
and were adapted from Londraville et al., 201793. Complexes were visualized and superposed to the X-ray structure of mLeptin:mLEP-RIgCRH2 by PyMOL 2.3.4 (www.pymol.org) with listed r.m.s.d
fit to the 3:3 complex. EXTENDED DATA FIG. 3 BIOPHYSICAL AND FUNCTIONAL CHARACTERIZATION OF LEP-R COMPLEXES. A-E, Biophysical analysis of mouse Leptin:LEP-RECD in solution. A, Sedimentation
coefficient distributions _c(s)_ for different concentrations of glycan-trimmed mLeptin:mLEP-RECD. B, SEC-MALLS analysis (Superdex 200 increase column) of glycan-trimmed wild type mLEP-RECD
and mLEP-RECD-A407 mutant and their complexes with mLeptin. Similar results were obtained from at least 3 independent repetitions. C AND E, SEC-MALLS analysis (Superdex 200 increase column)
of glycan-trimmed (panel C) or glycosylated (panel E) mLEP-RECD-ΔFNIII and their complexes with mLeptin. Similar results were obtained from _n_ = _2_ at selected similar concentrations for
panel c, _n_ = _3_ for panel e. D, SEC-MALLS analysis (Superose 6 increase column) of glycosylated mLEP-RECD and its complexes with mLeptin, as a function of complex concentration. Similar
results were obtained from at least _n_ = _2_ independent experiments at a range of concentrations. F, J, K, Characterization of human LEP-R complexes. F, SEC-MALLS analysis (Superdex 200
increase column) of glycosylated hLEP-RECD and its complexes with hLeptin. Similar results were obtained from _n_ = _2_ independent experiments at selected concentrations. J, SEC-MALLS
analysis (Superdex 200 increase column) of glycosylated hLEP-RECD and its complexes with the agonistic antibody mibavademab30. _n_ = _1_. K, Activation of hLEP-Rb by Leptin and variants, as
well as by the agonistic antibody mibavademab, probed by a STAT3-responsive luciferase reporter in HEK293T cells. _n_ = _3_ independent experiments with 3 technical replicates each. Here,
mean values and standard deviations from a representative independent experiment are shown. G, H, I, Interspecies cross-reactivity probed with SEC-MALLS (Superdex 200 increase column). _n_ =
_1_. G, Complexes of hLEP-RECD with mouse and human Leptin. H, Complexes of glycan-trimmed mLEP-RECD with mouse and human Leptin. I, Complexes of glycan-trimmed mLEP-RECD with ‘murinized’
human Leptin. Specifically, residues close to the Site III interface that differ between mouse and human Leptin (Supplementary Fig. 1a) were mutated on hLeptin to the corresponding of the
mouse homologue (that is CD-loop residues 118-129; G139L; I85V/M89L/G132D/G139L). Source data EXTENDED DATA FIG. 4 CRYO-EM ANALYSIS OF MLEP:MLEP-RECD COMPLEXES. A. The COSMIC2 webserver was
used to align projections of the trimeric mLeptin:mLEP-RIgCRH2 crystal structure, the 1:2 mLeptin:LEP-RIgCRH2FnIII cryo-EM model, and projections of a 1:1 mLeptin:mLEP-RECD model (based on
the Alphafold2-model for mLEP-RECD and the mLeptin:mLEP-RCRH2 crystal structure) with high-resolution 2D-classes obtained for the mLeptin:mLEP-RECD complex sample. In the gallery 2D cryo-EM
classes matching projections are indicated with a colored dot. B, Cryo-EM workflow towards the reconstruction of the 1:2 mLeptin:LEP-RECD complex. We note that 3D reconstruction of the
remaining two observed states was hindered due to preferred orientation. NU, non-uniform refinement; GSFSC: gold-standard Fourier shell correlation. EXTENDED DATA FIG. 5 CRYO-EM DATA
ANALYSIS WORKFLOW FOR THE MLEPTIN:MLEP-RTGCN4 COMPLEX. A, SEC-MALLS analysis of the glycosylated mLeptin:mLEP-RECD-tGCN4 complex. The SEC-elution profile is plotted as the ultraviolet
absorbance at 280 nm (left Y-axis) in function of elution volume. The total, protein and glycan molecular mass (right Y-axis) as determined by MALLS are reported as the average molecular
mass (and s.d.) across the elution peak. Number of samples for mLeptin:mLEP-RECD-tGCN4 analyzed: _n_ = _1_. B, SAXS scattering curves of EndoH-treated samples for mLEP-RECD and
mLeptin:mLEP-RECD complexes plotted as the scattered intensity in function of scattering vector _s_ = 4_π_sin_θ_/_λ. (i)_ mLEP-RECD (grey), (_ii_) non-stabilized mLeptin:mLEP-RECD complex
(black), (_iii_) stabilized mLeptin:mLEP-RECD-tGCN4 complex (yellow), _(iv)_ scaled and overlayed scattering profiles for mLeptin:mLEP-RECD and mLeptin:mLEP-RECD-tGCN4. Similar results were
obtained from _n_ = _2_ independent experiments for mLeptin:mLEP-RECD. For the rest _n_ = _1_. C. Cryo-EM data processing workflow in cryoSPARC for the glycosylated
mLeptin:mLEP-RECD-ECD-tGCN4 complex. GSFSC: gold-standard Fourier shell correlation. D. Fourier shell correlation plots, local resolution map and sharpened map following symmetry expansion
of the particle data set and local refinement around one LEP-RCRH2:Leptin:LEP-RIg' subcomplex. Source data EXTENDED DATA FIG. 6 COMPARATIVE ANALYSIS BETWEEN MOUSE AND HUMAN LEPTIN:LEP-R
COMPLEX STRUCTURES AS DERIVED FROM CRYO-EM AND X-RAY CRYSTALLOGRAPHIC ANALYSIS. A, Real-space refined atomic model for the trimeric mLeptin:mLEP-RIgCRH2 core region overlayed with the
sharpened cryo-EM map in C1 symmetry for the mLeptin:mLEP-RECD-tGCN4 complex following local map refinement. The cryo-EM map is contoured at 0.313 V. In the cryo-EM map strong additional
density is observed at all three LEP-RCRH2:Leptin interface regions (site II) that likely corresponds to a trapped Ni-ion coordinated by histidine residues in the LEP-RCRH2 module and the
N-terminal His-tag present on mLeptin. B, Structural superposition of the crystallographically-distilled 3:3 mLeptin:mLEP-RIgCRH2 assembly (black) with the real-space refined
mLeptin:mLEP-RIgCRH2 assembly via cryo-EM. C–D, Cryo-EM maps and fitted atomic models for the mouse and human Leptin:LEP-RECD-tGCN4 complexes. The sharpened cryo-EM maps are colored per zone
with Leptin in yellow, the CRH1 module in grey, the Ig domain in magenta, the CRH2 module in blue and the FnIII module in green. The fitted atomic model models are shown as a cartoon
overlayed with the cryo-EM maps as a transparent volume. The mouse and human cryo-EM maps are contoured at 0.178 V and 0.550 V, respectively. E, Structural superposition of the real-space
refined atomic models for the mouse and human Leptin:LEP-RECD-tGCN4 complexes. F, Structural superposition of the real-space refined model for the hLeptin:hLEP-RIgCRH2 core with the
structural prediction for hLEP-RECD via AlphaFold2 overlayed with the sharpened cryo-EM map for the hLeptin:hLEP-RECD-tGCN4 complex. EXTENDED DATA FIG. 7 STRUCTURAL INSIGHTS INTO THE
MLEP-RFNIII CONFORMATION AND MAP OF NATURALLY OCCURRING MUTATIONS LIKELY RELATED TO OBESITY ON THE LEPTIN:RECEPTOR ASSEMBLY. A, Cartoon representation of the determined crystal structure of
mLEP-RFnIII:VHH-4.80 complex. Evolutionary conserved Trp across the interdomain interface and crystallographically observed N-linked glycosylation sites are shown as sticks. B, Mass
spectrometric confirmation of the disulfide bridge between residues Cys602-Cys672 of the CRH2 and FNIII domains respectively in mLEP-RECD. The MS (top) and MS/MS spectra (bottom) are shown
for the disulfide-linked peptides that are indicated on the left. C–F, Map of naturally occurring mutations likely related to obesity on the Leptin:receptor assembly. C, Domain distribution
of missense variants identified in the human LEP-R gene of obese individuals (Supplementary Table 1), that have no currently known effect on protein secretion and stability. Functionally
validated pathogenic mutations are highlighted in bold. D-E, Localization of mutations in the LEP and LEP-R genes (Supplementary Table 1) in the assembly. Mutated residues are shown as
spheres. Functionally validated pathogenic mutations are highlighted in bold. F, Structural superposition of the crystallographically-resolved hLEP-RCRH2 domain (gray) with the AlphaFold
model of hLEP-R (r.m.s.d.=0.669 Å, 171 Cα atoms). Residues found in variants likely related to obesity are annotated. A disulfide bridge is predicted to be formed between the CRH2 and FNIII
domains (Cys604-Cys674), as shown in the insert. Both residues are functionally important1113. The disulfide bridge was confirmed by peptide mapping for mLEP-RECD, as shown in panel b.
Cysteine 602 (604 in human) was mutated to serine in FNIII-deletion permutations of LEP-R in this study to prevent artificial disulfide-linked clusters. EXTENDED DATA FIG. 8 CRYO-EM DATA
ANALYSIS WORKFLOW FOR THE HLEPTIN:HLEP-RTGCN4 COMPLEX. A, SEC-MALLS analysis of the glycosylated hLeptin:hLEP-RECD-tGCN4 complex. The SEC-elution profile is plotted as the ultraviolet
absorbance at 280 nm (left Y-axis) in function of elution volume. The total, protein and glycan molecular mass (right Y-axis) as determined by MALLS are reported as the average molecular
mass (and s.d.) across the elution peak. Number of samples for hLeptin:hLEP-RECD-tGCN4 analyzed: _n_ = _1_. B, Cryo-EM data processing workflow in cryoSPARC for the glycosylated
hLeptin:hLEP-RECD-tGCN4 complex. NU, non-uniform refinement; GSFSC: gold-standard Fourier shell correlation. Source data EXTENDED DATA FIG. 9 RECEPTOR CO-LOCALIZATION AT THE CELL SURFACE
PROBED BY SMTIRFM. A, Schematic overview of single molecule tracking and data evaluation. B, Dual-color (2 C) co-tracking. C, Triple-color (3 C) co-tracking. D, Diffusion properties; arrows
indicate percentage of diffusional decrease E, fraction of immobilized emitters. For B-E, the number of cells (N) examined over n = 2 independent experiments for all LepR experiments, and n
= 1 control experiment for TpoR, is indicated in the top row. Statistical differences were calculated with two-tailed two-sample Kolmogorov-Smirnov tests (**P ≤ 0.01, ***P ≤ 0.001; ns: not
significant). Exact P-values for each comparison are given in the source data file of Fig. 6. Box and whisker plots show the five number summaries of the data: minimum, first quartile,
median, third quartile, and maximum values. Outliers are indicated with an asterisk. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–5, Tables 1 and 2, and
References. REPORTING SUMMARY PEER REVIEW FILE mLep:mLEP-Rb assembly in living cells. Co-localization of mLEP-Rb in the absence (left) and presence of ligand (middle: two-color
co-localization; right: three-color co-localization, 10 nM mLeptin, 10 min). Green, red and blue signals correspond to receptors labeled with Cy3BNB, Atto643NB and Dy752NB, respectively.
Out-of-focus immobile signals observed in the presence of mLeptin can be explained by membrane-proximal endosomes. Acquisition frame rate: 33 Hz. Playback: real time. mLep:mLEP-RΔ866-tFoldon
assembly in living cells. Co-localization of mLEP-RΔ866-tFoldon in presence of ligand (left: two-color co-localization; right: three-color co-localization, 10 nM mLeptin, 10 min). Green,
red and blue signals correspond to receptors labeled with Cy3BNB, Atto643NB and Dy752NB, respectively. Acquisition frame rate: 33 Hz. Playback: real time. SOURCE DATA SOURCE DATA FIG. 3
SEC–MALLS and signaling source data. SOURCE DATA FIG. 6 Statistical source data. SOURCE DATA EXTENDED DATA FIG. 1 SEC–MALLS source data, biolayer interferometry source data and statistical
analysis. SOURCE DATA EXTENDED DATA FIG. 1 Uncropped SDS–PAGE gel. SOURCE DATA EXTENDED DATA FIG. 3 SEC–MALLS and signaling source data. SOURCE DATA EXTENDED DATA FIG. 5 SEC–MALLS and SAXS
source data. SOURCE DATA EXTENDED DATA FIG. 8 SEC–MALLS source data. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this
article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of
such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tsirigotaki, A., Dansercoer, A., Verschueren, K.H.G. _et al._ Mechanism of
receptor assembly via the pleiotropic adipokine Leptin. _Nat Struct Mol Biol_ 30, 551–563 (2023). https://doi.org/10.1038/s41594-023-00941-9 Download citation * Received: 20 October 2022 *
Accepted: 06 February 2023 * Published: 23 March 2023 * Issue Date: April 2023 * DOI: https://doi.org/10.1038/s41594-023-00941-9 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative