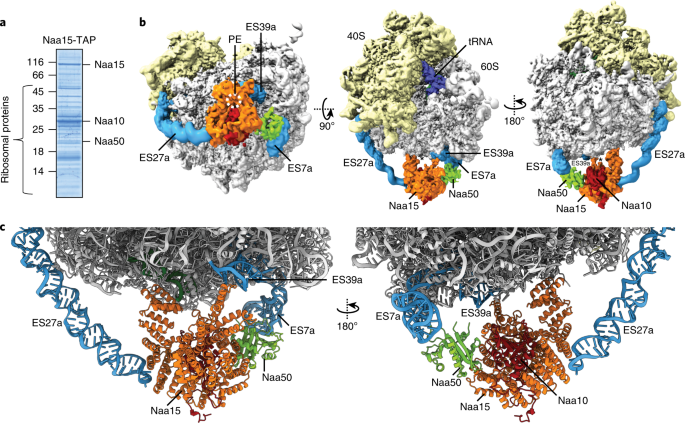
Ribosome–nata architecture reveals that rrna expansion segments coordinate n-terminal acetylation
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The majority of eukaryotic proteins are N-terminally α-acetylated by N-terminal acetyltransferases (NATs). Acetylation usually occurs co-translationally and defects have severe
consequences. Nevertheless, it is unclear how these enzymes act in concert with the translating ribosome. Here, we report the structure of a native ribosome–NatA complex from _Saccharomyces
cerevisiae_. NatA (comprising Naa10, Naa15 and Naa50) displays a unique mode of ribosome interaction by contacting eukaryotic-specific ribosomal RNA expansion segments in three out of four
binding patches. Thereby, NatA is dynamically positioned directly underneath the ribosomal exit tunnel to facilitate modification of the emerging nascent peptide chain. Methionine amino
peptidases, but not chaperones or signal recognition particle, would be able to bind concomitantly. This work assigns a function to the hitherto enigmatic ribosomal RNA expansion segments
and provides mechanistic insights into co-translational protein maturation by N-terminal acetylation. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS MULTI-PROTEIN ASSEMBLIES ORCHESTRATE CO-TRANSLATIONAL ENZYMATIC
PROCESSING ON THE HUMAN RIBOSOME Article Open access 03 September 2024 NAC GUIDES A RIBOSOMAL MULTIENZYME COMPLEX FOR NASCENT PROTEIN PROCESSING Article 21 August 2024 DIVERGENT ARCHITECTURE
OF THE HETEROTRIMERIC NATC COMPLEX EXPLAINS N-TERMINAL ACETYLATION OF COGNATE SUBSTRATES Article Open access 02 November 2020 DATA AVAILABILITY The cryo-EM map for the ribosome–NatA complex
and the respective atomic coordinates have been deposited in the EMDataBank and PDB with accession codes EMD-0201 and 6HD5 for local refinement of NatA, respectively, and with accession
codes EMD-0202 and 6HD7 for regular refinement. The map for RNaseI-treated ribosomes was deposited with accession code EMD-0203. Source data for Fig. 3c are available with the paper online.
Primary data are available upon reasonable request from the corresponding authors. REFERENCES * Gautschi, M. et al. The yeast N(alpha)-acetyltransferase NatA is quantitatively anchored to
the ribosome and interacts with nascent polypeptides. _Mol. Cell. Biol._ 23, 7403–7414 (2003). Article CAS Google Scholar * Polevoda, B., Brown, S., Cardillo, T. S., Rigby, S. &
Sherman, F. Yeast N(alpha)-terminal acetyltransferases are associated with ribosomes. _J. Cell. Biochem._ 103, 492–508 (2008). Article CAS Google Scholar * Magin, R. S., Deng, S., Zhang,
H., Cooperman, B. & Marmorstein, R. Probing the interaction between NatA and the ribosome for co-translational protein acetylation. _PLoS ONE_ 12, e0186278 (2017). Article Google
Scholar * Driessen, H. P., de Jong, W. W., Tesser, G. I. & Bloemendal, H. The mechanism of N-terminal acetylation of proteins. _CRC Crit. Rev. Biochem._ 18, 281–325 (1985). Article CAS
Google Scholar * Arnesen, T. et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. _Proc. Natl Acad. Sci.
USA_ 106, 8157–8162 (2009). Article CAS Google Scholar * Lee, K. E., Heo, J. E., Kim, J. M. & Hwang, C. S. N-terminal acetylation-targeted N-end rule proteolytic system: the Ac/N-end
rule pathway. _Mol. Cells_ 39, 169–178 (2016). Article CAS Google Scholar * Aksnes, H., Drazic, A., Marie, M. & Arnesen, T. First things first: vital protein marks by N-terminal
acetyltransferases. _Trends. Biochem. Sci._ 41, 746–760 (2016). Article CAS Google Scholar * Drazic, A. et al. NAA80 is actin’s N-terminal acetyltransferase and regulates cytoskeleton
assembly and cell motility. _Proc. Natl Acad. Sci. USA_ 115, 4399–4404 (2018). Article CAS Google Scholar * Polevoda, B., Norbeck, J., Takakura, H., Blomberg, A. & Sherman, F.
Identification and specificities of N-terminal acetyltransferases from _Saccharomyces cerevisiae_. _EMBO J._ 18, 6155–6168 (1999). Article CAS Google Scholar * Liszczak, G. et al.
Molecular basis for N-terminal acetylation by the heterodimeric NatA complex. _Nat. Struct. Mol. Biol._ 20, 1098–1105 (2013). Article CAS Google Scholar * Weyer, F. A. et al. Structural
basis of HypK regulating N-terminal acetylation by the NatA complex. _Nat. Commun._ 8, 15726 (2017). Article CAS Google Scholar * Gottlieb, L. & Marmorstein, R. Structure of human
NatA and its regulation by the Huntingtin interacting protein HYPK. _Structure_, https://doi.org/10.1016/j.str.2018.04.003 (2018). * Varland, S. & Arnesen, T. Investigating the
functionality of a ribosome-binding mutant of NAA15 using _Saccharomyces cerevisiae_. _BMC Res. Notes_ 11, 404 (2018). Article Google Scholar * Schmidt, C. et al. The cryo-EM structure of
a ribosome-Ski2-Ski3-Ski8 helicase complex. _Science_ 354, 1431–1433 (2016). Article CAS Google Scholar * Schmidt, C. et al. Structure of the hypusinylated eukaryotic translation factor
eIF-5A bound to the ribosome. _Nucleic Acids Res._ 44, 1944–1951 (2016). Article Google Scholar * Beckmann, R. et al. Architecture of the protein-conducting channel associated with the
translating 80S ribosome. _Cell_ 107, 361–372 (2001). Article CAS Google Scholar * Gerbi, S. A. Expansion segment: regions of variable size that interrupt the universal core secondary
structure of ribosomal RNA in _Ribosomal RNA: Structure, Evolution, Processing, and Function in Protein Synthesis_ (eds. Zimmermann, R. A. & Dahlberg, A. E.) 71–87 (CRC Press,
Boca-Raton, FL, USA, 1996). * Hashem, Y. et al. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. _Cell_ 153, 1108–1119 (2013). Article CAS
Google Scholar * Yamamoto, H. et al. Molecular architecture of the ribosome-bound Hepatitis C Virus internal ribosomal entry site RNA. _EMBO J._ 34, 3042–3058 (2015). Article CAS Google
Scholar * Bradatsch, B. et al. Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel. _Nat. Struct. Mol. Biol._ 19, 1234–1241 (2012). Article
CAS Google Scholar * Greber, B. J., Boehringer, D., Montellese, C. & Ban, N. Cryo-EM structures of Arx1 and maturation factors Rei1 and Jjj1 bound to the 60S ribosomal subunit. _Nat.
Struct. Mol. Biol._ 19, 1228–1233 (2012). Article CAS Google Scholar * Ramesh, M. & Woolford, J. L. Jr. Eukaryote-specific rRNA expansion segments function in ribosome biogenesis.
_RNA_ 22, 1153–1162 (2016). Article CAS Google Scholar * Kater, L. et al. Visualizing the assembly pathway of nucleolar pre-60S ribosomes. _Cell_ 171, 1599–1610, e1514 (2017). Article
CAS Google Scholar * Sweeney, R., Chen, L. & Yao, M. C. An rRNA variable region has an evolutionarily conserved essential role despite sequence divergence. _Mol. Cell. Biol._ 14,
4203–4215 (1994). Article CAS Google Scholar * Armache, J. P. et al. Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5-A resolution. _Proc. Natl Acad. Sci.
USA_ 107, 19748–19753 (2010). Article CAS Google Scholar * Gomez Ramos, L. M. et al. Yeast rRNA expansion segments: folding and function. _J. Mol. Biol._ 428, 4048–4059 (2016). Article
CAS Google Scholar * Vetro, J. A. & Chang, Y. H. Yeast methionine aminopeptidase type 1 is ribosome-associated and requires its N-terminal zinc finger domain for normal function in
vivo. _J. Cell. Biochem._ 85, 678–688 (2002). Article CAS Google Scholar * Sandikci, A. et al. Dynamic enzyme docking to the ribosome coordinates N-terminal processing with polypeptide
folding. _Nat. Struct. Mol. Biol._ 20, 843–850 (2013). Article CAS Google Scholar * Van Damme, P., Hole, K., Gevaert, K. & Arnesen, T. N-terminal acetylome analysis reveals the
specificity of Naa50 (Nat5) and suggests a kinetic competition between N-terminal acetyltransferases and methionine aminopeptidases. _Proteomics_ 15, 2436–2446 (2015). Article Google
Scholar * Neuwald, A. F. & Landsman, D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. _Trends Biochem. Sci._ 22,
154–155 (1997). Article CAS Google Scholar * Hou, F., Chu, C. W., Kong, X., Yokomori, K. & Zou, H. The acetyltransferase activity of San stabilizes the mitotic cohesin at the
centromeres in a shugoshin-independent manner. _J. Cell. Biol._ 177, 587–597 (2007). Article CAS Google Scholar * Evjenth, R. et al. Human Naa50p (Nat5/San) displays both protein N alpha-
and N epsilon-acetyltransferase activity. _J. Biol. Chem._ 284, 31122–31129 (2009). Article CAS Google Scholar * Arnesen, T. et al. Cloning and characterization of hNAT5/hSAN: an
evolutionarily conserved component of the NatA protein N-alpha-acetyltransferase complex. _Gene_ 371, 291–295 (2006). Article CAS Google Scholar * Forte, G. M., Pool, M. R. &
Stirling, C. J. N-terminal acetylation inhibits protein targeting to the endoplasmic reticulum. _PLoS Biol._ 9, e1001073 (2011). Article CAS Google Scholar * Defenouillere, Q. et al.
Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products. _Proc. Natl Acad. Sci. USA_ 110, 5046–5051 (2013). Article CAS Google
Scholar * Bhushan, S. et al. Structural basis for translational stalling by human cytomegalovirus and fungal arginine attenuator peptide. _Mol. Cell_ 40, 138–146 (2010). Article CAS
Google Scholar * Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. _Nat. Methods_ 14, 331–332 (2017). Article CAS Google
Scholar * Zhang, K. Gctf: real-time CTF determination and correction. _J. Struct. Biol._ 193, 1–12 (2016). Article CAS Google Scholar * Scheres, S. H. RELION: implementation of a
Bayesian approach to cryo-EM structure determination. _J. Struct. Biol._ 180, 519–530 (2012). Article CAS Google Scholar * Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E.
Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. _eLife_ 5, https://doi.org/10.7554/eLife.18722 (2016). * Emsley, P., Lohkamp, B., Scott, W. G. &
Cowtan, K. Features and development of Coot. _Acta Crystallogr. D. Biol. Crystallogr._ 66, 486–501 (2010). Article CAS Google Scholar * Jucker, F. M. & Pardi, A. Solution structure of
the CUUG hairpin loop: a novel RNA tetraloop motif. _Biochemistry_ 34, 14416–14427 (1995). Article CAS Google Scholar * Pettersen, E. F. et al. UCSF Chimera–a visualization system for
exploratory research and analysis. _J. Comput. Chem._ 25, 1605–1612 (2004). Article CAS Google Scholar * Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization
and analysis. _Protein Sci._ 27, 14–25 (2018). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank S. Rieder, J. Musial and H. Sieber for technical assistance. This
work was supported by the German Research Council (GRK1721 and FOR1805 to R.B.) and we acknowledge support by the Center for Integrated Protein Science Munich (CiPS-M). A.G.K. is supported
by a DFG fellowship through the Graduate School of Quantitative Biosciences Munich. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Gene Center and Center for Integrated Protein Science
Munich, Department of Biochemistry, University of Munich, Munich, Germany Alexandra G. Knorr, Christian Schmidt, Petr Tesina, Otto Berninghausen, Thomas Becker, Birgitta Beatrix & Roland
Beckmann Authors * Alexandra G. Knorr View author publications You can also search for this author inPubMed Google Scholar * Christian Schmidt View author publications You can also search
for this author inPubMed Google Scholar * Petr Tesina View author publications You can also search for this author inPubMed Google Scholar * Otto Berninghausen View author publications You
can also search for this author inPubMed Google Scholar * Thomas Becker View author publications You can also search for this author inPubMed Google Scholar * Birgitta Beatrix View author
publications You can also search for this author inPubMed Google Scholar * Roland Beckmann View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
B.B., T.B., A.G.K. and R.B. designed the study and wrote the manuscript. A.G.K. purified and biochemically characterized ribosome–NatA complexes and prepared samples for cryo-EM, processed
the data, analyzed the structures and, together with T.B., built the molecular model. O.B. collected cryo-EM data. A.G.K. and C.S. processed the cryo-EM data for RNaseI-treated uL4-RNCs.
B.B. cloned and established the purification of recombinant NatA. P.T. processed the cryo-EM data for the untreated RNCs. CORRESPONDING AUTHORS Correspondence to Thomas Becker, Birgitta
Beatrix or Roland Beckmann. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. INTEGRATED SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 PURIFICATION OF THE NATIVE RIBOSOME–NATA
COMPLEX. a, Schematic representation of the construct for TAP-tagged Naa15. CaMBD, calmodulin-binding domain; TEV, tobacco etch virus protease cleavage site; ProtA, protein A IgG binding
domains. b, Amido black–stained PVDF membrane after transfer of purification samples separated on a 4–12% NuPAGE gel. c, Western blot of purification samples probing with an antibody
directed against the CaMBD (anti-CAB, Thermo Fisher) and against uL29 (polyclonal chicken antiserum, Davids Biotechnolgie). L, lysate; FT, flow through; R, resuspension; W, wash; E, elution;
B, boiled beads. Naa15-CaMBD corresponds to TEV-cleaved product carrying a calmodulin-binding domain, which is recognized by the anti-CAB antibody. 0.1 A260 of E was loaded, for L and FT
1/40,000 and for wash fractions 1/7 of the volume was loaded on the gel. SUPPLEMENTARY FIGURE 2 3D CLASSIFICATION SCHEME FOR CRYO-EM RIBOSOME–NATA COMPLEX. Particles were picked
automatically with Gautomatch, and 2D classification was used to discard non-ribosomal particles. Remaining particles were subjected to 3D refinement and classification. The highest
populated classes with ES27a in the exit position were joined and classified according to their tRNA states. Particles containing tRNAs in the A- and P-site were merged and used for further
classification with a mask on the ribosomal tunnel exit region. One of the three classes showed well-defined additional density at the exit region for NatA and was refined to an overall
resolution of 3.4 Å. SUPPLEMENTARY FIGURE 3 LOCAL RESOLUTION AND FSC CURVE FOR THE RIBOSOME–NATA COMPLEX. The ribosome–NatA map (see Supplementary Fig. 2) was further refined focusing on the
tunnel exit region to improve the resolution of the bound NatA. a,b, Maps before (a) and after (b) focused refinement are shown colored according to their local resolution. Whereas the
overall resolution for the ribosome is below 4 Å, NatA displays a higher degree of flexibility indicated by a gradient of local resolution ranging from 7 to >12 Å. Focused refinement on
NatA improved the resolution for the ligand to below 10 Å and an average resolution of 8 Å. c,d, FSC curves for the maps shown in a and b, respectively. SUPPLEMENTARY FIGURE 4 DOCKING OF
NATA SUBUNITS INTO CRYO-EM MAP. a, Isolated densities for the NatA subunits extracted from the refined map focused on NatA are shown in transparent together with a ribbon representation of
the respective molecular models. Naa50 and Naa10 were docked essentially as rigid bodies (derived from PDB 4XNH); Naa15 required some adjustments, especially for the protruding basic helix
(see also Supplementary Fig. 7b). b, Zoom views focusing on the ribosome–NatA contacts using the focused refined map as in a. Views were chosen similar to Fig. 2 (see also Supplementary Fig.
3b). c, The upper panel shows the map after global refinement at 3.4 Å with the models for H24, H47 and the N terminus of Naa15. The middle panel shows the same map, but low-pass filtered
to 8 Å. Note that density for NatA becomes visible after low-pass filtering. The lower panel shows the map after local refinement low-pass filtered to 8 Å. The density for NatA becomes more
defined and distinct interaction sites of NatA with the ribosomal helices become evident. SUPPLEMENTARY FIGURE 5 SECONDARY STRUCTURE OF THE 28S RRNA OF _S. CEREVISIAE_. Expansion segments
ES7a, ES27a and ES39a are labeled in blue. Adopted form http://apollo.chemistry.gatech.edu/RibosomeGallery/ SUPPLEMENTARY FIGURE 6 OVERVIEW OF THE RIBOSOME-BOUND NATA STRUCTURE. Naa15 is
shown in beige, except for the 13 TPR motifs displayed alternating in yellow and blue and numbered according to Liscszak et al. (_Nat. Struct. Mol. Biol_. 20, 1098–1105, 2013) (PDB 4KVM).
TPR11 is not a canonical TPR; instead, it shows extended α-helices and is therefore labeled with 11-like and 11 ext for extended. Naa10 is shown in red and Naa50 is shown in green.
SUPPLEMENTARY FIGURE 7 SEQUENCE ALIGNMENT AND REARRANGEMENT OF BASIC HELIX Α33 OF _S. CEREVISIAE_ AND _S. POMBE_. a, Sequence alignment of the basic helix (α 33) of _S. pombe_ and _S.
cerevisiae_ Naa15 with Clustal O (1.2.4). Conserved lysines involved in binding ES39a are boxed. b, Comparison of the free structure of Naa15 of _S. pombe_ (PDB 4KVM) in green with Naa15 of
_S. cerevisiae_ in the ribosome-bound conformation in orange. In _S. cerevisiae_, this basic helix repositions towards ES39a when bound to the ribosome. SUPPLEMENTARY FIGURE 8 RIBOSOME
BINDING OF NATA. a, Western blot analysis comparing non-treated (RNC) and RNase I–treated (rtRNC) uL4-RNCs. Left, amido black–stained PVDF membrane after transfer of the RNC samples
separated on a 12% NuPAGE gel; right, membrane probed with an anti-HA antibody showing the peptidyl-tRNA, indicating that RNase I treatment does not affect the peptidyl-transferase center.
Shown is an overlay of the left panel with the antibody signal. b, 4–12% Nu-PAGE gel after an in vitro binding assay showing the supernatant (SN) and pellet (P) fractions of either empty 80S
ribosomes or NatA or 80S ribosomes incubated with recombinant NatA. This indicates that NatA is binding to empty ribosomes independently of an emerging nascent chain. c, 15% SDS–PAGE
supernatant and pellet fractions of a representative binding assay using either uL4-RNCs (RNCs) or RNase I–treated uL4-RNCs (rtRNCs). Note that significantly less NatA binds to rtRNCs. A
representative ribosomal band used for normalization is marked with an asterisk (see also Fig. 3c). SUPPLEMENTARY FIGURE 9 CRYO-EM STRUCTURES OF INTACT AND RNASE I–TREATED RIBOSOMES. Cryo-EM
maps of untreated (RNC; orange) and RNase I–treated (rtRNC; gray) ribosomes. The upper panel displays the front view, the middle panel the bottom view and the lower panel a side view. The
right column shows a difference map between RNC and rtRNC (red) superimposed on the map of the rtRNCs indicating statistically significant differences between the two maps. Note that mainly
density of protruding rRNA is missing in the rtRNCs, such as expansion segments ES7a, ES27a and ES39a or the L1 stalk. Stalk, L7/L12 stalk. All maps were at a resolution of 6.7 Å, low-pass
filtered to 8 Å and are shown at the same contour level (3.7_σ_). SUPPLEMENTARY INFORMATION SUPPLEMENTARY TEXT AND FIGURES Supplementary Figures 1–9 and Supplementary Note REPORTING SUMMARY
SOURCE DATA SOURCE DATA, FIGURE 3 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Knorr, A.G., Schmidt, C., Tesina, P. _et al._ Ribosome–NatA
architecture reveals that rRNA expansion segments coordinate N-terminal acetylation. _Nat Struct Mol Biol_ 26, 35–39 (2019). https://doi.org/10.1038/s41594-018-0165-y Download citation *
Received: 25 July 2018 * Accepted: 09 November 2018 * Published: 17 December 2018 * Issue Date: January 2019 * DOI: https://doi.org/10.1038/s41594-018-0165-y SHARE THIS ARTICLE Anyone you
share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative