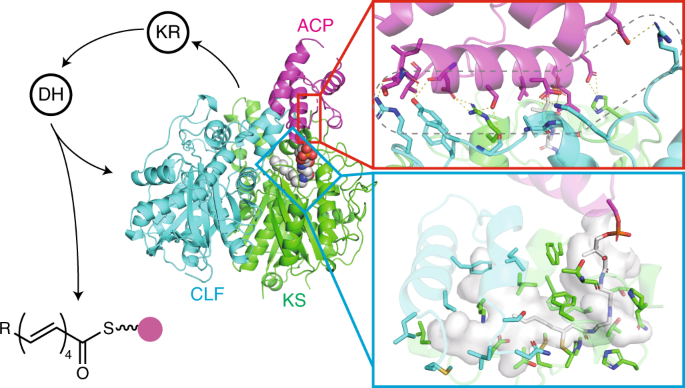
Structural basis for selectivity in a highly reducing type ii polyketide synthase
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In type II polyketide synthases (PKSs), the ketosynthase–chain length factor (KS–CLF) complex catalyzes polyketide chain elongation with the acyl carrier protein (ACP). Highly
reducing type II PKSs, represented by IgaPKS, produce polyene structures instead of the well-known aromatic skeletons. Here, we report the crystal structures of the Iga11–Iga12 (KS–CLF)
heterodimer and the covalently cross-linked Iga10=Iga11–Iga12 (ACP=KS–CLF) tripartite complex. The latter structure revealed the molecular basis of the interaction between Iga10 and
Iga11–Iga12, which differs from that between the ACP and KS of _Escherichia coli_ fatty acid synthase. Furthermore, the reaction pocket structure and site-directed mutagenesis revealed that
the negative charge of Asp 113 of Iga11 prevents further condensation using a β-ketoacyl product as a substrate, which distinguishes IgaPKS from typical type II PKSs. This work will
facilitate the future rational design of PKSs. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS C–N BOND FORMATION BY A POLYKETIDE SYNTHASE Article Open access 10 March 2023 STRUCTURE AND MECHANISM OF A DEHYDRATASE/DECARBOXYLASE ENZYME
COUPLE INVOLVED IN POLYKETIDE Β-METHYL BRANCH INCORPORATION Article Open access 18 September 2020 A POLYKETOACYL-COA THIOLASE-DEPENDENT PATHWAY FOR THE SYNTHESIS OF POLYKETIDE BACKBONES
Article 22 June 2020 DATA AVAILABILITY Structures have been deposited at the Protein Data Bank under accession codes 6KXD (Iga11–Iga12), 6KXE (C6=Iga11–Iga12) and 6KXF
(C8Cl-Iga10=Iga11–Iga12). REFERENCES * Klaus, M. & Grininger, M. Engineering strategies for rational polyketide synthase design. _Nat. Prod. Rep._ 35, 1070–1081 (2018). Article CAS
Google Scholar * Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. _Nat. Prod. Rep._ 18, 380–416 (2001). Article CAS Google Scholar * Hertweck, C. The
biosynthetic logic of polyketide diversity. _Angew. Chem. Int. Ed. Engl._ 48, 4688–4716 (2009). Article CAS Google Scholar * Hertweck, C., Luzhetskyy, A., Rebets, Y. & Bechthold, A.
Type II polyketide synthases: gaining a deeper insight into enzymatic teamwork. _Nat. Prod. Rep._ 24, 162–190 (2007). Article CAS Google Scholar * Das, A. & Khosla, C. Biosynthesis of
aromatic polyketides in bacteria. _Acc. Chem. Res._ 42, 631–639 (2009). Article CAS Google Scholar * Tsai, S.-C. The structural enzymology of iterative aromatic polyketide synthases: a
critical comparison with fatty acid synthases. _Annu. Rev. Biochem._ 87, 503–531 (2018). Article CAS Google Scholar * Dreier, J. & Khosla, C. Mechanistic analysis of a type II
polyketide synthase. Role of conserved residues in the β-ketoacyl synthase-chain length factor heterodimer. _Biochemistry_ 39, 2088–2095 (2000). Article CAS Google Scholar * Du, D. et al.
Production of a novel amide-containing polyene by activating a cryptic biosynthetic gene cluster in _Streptomyces_ sp. MSC090213JE08. _ChemBioChem_ 17, 1464–1471 (2016). Article CAS
Google Scholar * Du, D., Katsuyama, Y., Shin-Ya, K. & Ohnishi, Y. Reconstitution of a type II polyketide synthase that catalyzes polyene formation. _Angew. Chem. Int. Ed. Engl._ 57,
1954–1957 (2018). Article CAS Google Scholar * Grammbitter, G. L. C. et al. An uncommon type II PKS catalyzes biosynthesis of aryl polyene pigments. _J. Am. Chem. Soc._ 141, 16615–16623
(2019). Article CAS Google Scholar * Keatinge-Clay, A. T., Maltby, D. A., Medzihradszky, K. F., Khosla, C. & Stroud, R. M. An antibiotic factory caught in action. _Nat. Struct. Mol.
Biol._ 11, 888–893 (2004). Article CAS Google Scholar * Shen, H. C. et al. Synthesis and biological evaluation of platensimycin analogs. _Bioorg. Med. Chem. Lett._ 19, 1623–1627 (2009).
Article CAS Google Scholar * Trajtenberg, F. et al. Structural insights into bacterial resistance to cerulenin. _FEBS J._ 281, 2324–2338 (2014). Article CAS Google Scholar * Wang, J.
et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. _Nature_ 441, 358–361 (2006). Article CAS Google Scholar * Nanson, J. D., Himiari, Z., Swarbrick, C.
M. D. & Forwood, J. K. Structural characterisation of the beta-ketoacyl-acyl carrier protein synthases, FabF and FabH, of _Yersinia pestis_. _Sci. Rep._ 5, 14797 (2015). Article CAS
Google Scholar * Zhang, Y.-M., Hurlbert, J., White, S. W. & Rock, C. O. Roles of the active site water, histidine 303, and phenylalanine 396 in the catalytic mechanism of the elongation
condensing enzyme of _Streptococcus pneumoniae_. _J. Biol. Chem._ 281, 17390–17399 (2006). Article CAS Google Scholar * Moche, M., Schneider, G., Edwards, P., Dehesh, K. & Lindqvist,
Y. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. _J. Biol. Chem._ 274, 6031–6034 (1999). Article CAS Google Scholar
* Finzel, K., Lee, D. J. & Burkart, M. D. Using modern tools to probe the structure-function relationship of fatty acid synthases. _Chembiochem_ 16, 528–547 (2015). Article CAS Google
Scholar * Crosby, J. & Crump, M. P. The structural role of the carrier protein–active controller or passive carrier. _Nat. Prod. Rep._ 29, 1111–1137 (2012). Article CAS Google Scholar
* Shakya, G. et al. Modeling linear and cyclic PKS intermediates through atom replacement. _J. Am. Chem. Soc._ 136, 16792–16799 (2014). Article CAS Google Scholar * Chen, A., Re, R. N.
& Burkart, M. D. Type II fatty acid and polyketide synthases: deciphering protein-protein and protein-substrate interactions. _Nat. Prod. Rep._ 35, 1029–1045 (2018). Article CAS Google
Scholar * Miyanaga, A., Iwasawa, S., Shinohara, Y., Kudo, F. & Eguchi, T. Structure-based analysis of the molecular interactions between acyltransferase and acyl carrier protein in
vicenistatin biosynthesis. _Proc. Natl Acad. Sci. USA_ 113, 1802–1807 (2016). Article CAS Google Scholar * Miyanaga, A. et al. Structural basis of protein-protein interactions between a
trans-acting acyltransferase and acyl carrier protein in polyketide disorazole biosynthesis. _J. Am. Chem. Soc._ 140, 7970–7978 (2018). Article CAS Google Scholar * Nguyen, C. et al.
Trapping the dynamic acyl carrier protein in fatty acid biosynthesis. _Nature_ 505, 427–431 (2014). Article CAS Google Scholar * Milligan, J. C. et al. Molecular basis for interactions
between an acyl carrier protein and a ketosynthase. _Nat. Chem. Biol._ 15, 669–671 (2019). Article CAS Google Scholar * Dodge, G. J. et al. Structural and dynamical rationale for fatty
acid unsaturation in _Escherichia coli_. _Proc. Natl Acad. Sci. USA_ 116, 6775–6783 (2019). Article CAS Google Scholar * Herbst, D. A. et al. The structural organization of substrate
loading in iterative polyketide synthases. _Nat. Chem. Biol._ 14, 474–479 (2018). Article CAS Google Scholar * Worthington, A. S., Rivera, H., Torpey, J. W., Alexander, M. D. &
Burkart, M. D. Mechanism-based protein cross-linking probes to investigate carrier protein-mediated biosynthesis. _ACS Chem. Biol._ 1, 687–691 (2006). Article CAS Google Scholar *
Worthington, A. S. & Burkart, M. D. One-pot chemo-enzymatic synthesis of reporter-modified proteins. _Org. Biomol. Chem._ 4, 44–46 (2006). Article CAS Google Scholar * Clarke, K. M.,
Mercer, A. C., La Clair, J. J. & Burkart, M. D. In vivo reporter labeling of proteins via metabolic delivery of coenzyme A analogues. _J. Am. Chem. Soc._ 127, 11234–11235 (2005). Article
CAS Google Scholar * Haushalter, R. W., Worthington, A. S., Hur, G. H. & Burkart, M. D. An orthogonal purification strategy for isolating crosslinked domains of modular synthases.
_Bioorg. Med. Chem. Lett._ 18, 3039–3042 (2008). Article CAS Google Scholar * Worthington, A. S. et al. Probing the compatibility of type II ketosynthase-carrier protein partners.
_Chembiochem_ 9, 2096–2103 (2008). Article CAS Google Scholar * Kabsch, W. XDS. _Acta Crystallogr. D Biol. Crystallogr._ 66, 125–132 (2010). Article CAS Google Scholar * Evans, P. R.
& Murshudov, G. N. How good are my data and what is the resolution? _Acta Crystallogr. D Biol. Crystallogr._ 69, 1204–1214 (2013). Article CAS Google Scholar * Vagin, A. &
Teplyakov, A. Molecular replacement with MOLREP. _Acta Crystallogr. D Biol. Crystallogr._ 66, 22–25 (2010). Article CAS Google Scholar * Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M.
N. & Sternberg, M. J. E. The Phyre2 web portal for protein modeling, prediction and analysis. _Nat. Protoc._ 10, 845–858 (2015). Article CAS Google Scholar * Emsley, P., Lohkamp, B.,
Scott, W. G. & Cowtan, K. Features and development of Coot. _Acta Crystallogr. D Biol. Crystallogr._ 66, 486–501 (2010). Article CAS Google Scholar * Vagin, A. A. et al. REFMAC5
dictionary: organization of prior chemical knowledge and guidelines for its use. _Acta Crystallogr. D Biol. Crystallogr._ 60, 2184–2195 (2004). Article Google Scholar * Adams, P. D. et al.
PHENIX: a comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D Biol. Crystallogr._ 66, 213–221 (2010). Article CAS Google Scholar * Collaborative
Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. _Acta Crystallogr. D Biol. Crystallogr._ 50, 760–763 (1994). Article Google Scholar Download
references ACKNOWLEDGEMENTS We thank A. Harada and M. Senda, and the staff of the Photon Factory and Swiss Light Source (proposal number 2017G165) for the X-ray data collection. The research
is supported by Grants-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (JP18H02144 and JP19H04645 to
Y.K.), a Research Fellow Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS; JP17J09439, to D.D.), JSPS A3 Foresight Program grant (to Y.O.), NIH R01 grant GM095970 to
M.D.B. and NIH K12 GM068524 grant, to T.D.D. (San Diego IRACDA Postdoctoral Fellow). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biotechnology, Graduate School of
Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan Danyao Du, Yohei Katsuyama, Masanobu Horiuchi, Shinya Fushinobu & Yasuo Ohnishi * Collaborative Research Institute
for Innovative Microbiology, The University of Tokyo, Tokyo, Japan Yohei Katsuyama, Shinya Fushinobu & Yasuo Ohnishi * Department of Chemistry and Biochemistry, University of California,
San Diego, San Diego, CA, USA Aochiu Chen, Tony D. Davis & Michael D. Burkart Authors * Danyao Du View author publications You can also search for this author inPubMed Google Scholar *
Yohei Katsuyama View author publications You can also search for this author inPubMed Google Scholar * Masanobu Horiuchi View author publications You can also search for this author inPubMed
Google Scholar * Shinya Fushinobu View author publications You can also search for this author inPubMed Google Scholar * Aochiu Chen View author publications You can also search for this
author inPubMed Google Scholar * Tony D. Davis View author publications You can also search for this author inPubMed Google Scholar * Michael D. Burkart View author publications You can also
search for this author inPubMed Google Scholar * Yasuo Ohnishi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.K., M.D.B. and D.D.
designed the research. T.D.D. synthesized the chemical probes. The crystallization of the proteins was done by D.D. The X-ray diffraction experiment was carried out by Y.K. and D.D. The
experimental phasing was done by Y.K. Refinement and validation of the structure was done by D.D., Y.K. and S.F. Site-directed mutagenesis and analysis of mutants were done by D.D. and M.H.
The cross-linked complex was prepared by D.D. and A.C. D.D. and Y.K. wrote the draft manuscript. A.C., T.D.D., M.D.B., S.F. and Y.O. commented on the draft. Y.K. and Y.O. finalized the
manuscript and all authors approved it. Y.K., M.D.B. and Y.O. directed the research. CORRESPONDING AUTHORS Correspondence to Yohei Katsuyama or Michael D. Burkart. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL INFORMATION Supplementary Figs. 1–14 and Tables 1–4. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Du, D., Katsuyama, Y., Horiuchi, M. _et al._ Structural basis for selectivity in a highly reducing type II polyketide synthase. _Nat Chem Biol_ 16,
776–782 (2020). https://doi.org/10.1038/s41589-020-0530-0 Download citation * Received: 02 October 2019 * Accepted: 27 March 2020 * Published: 04 May 2020 * Issue Date: July 2020 * DOI:
https://doi.org/10.1038/s41589-020-0530-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative