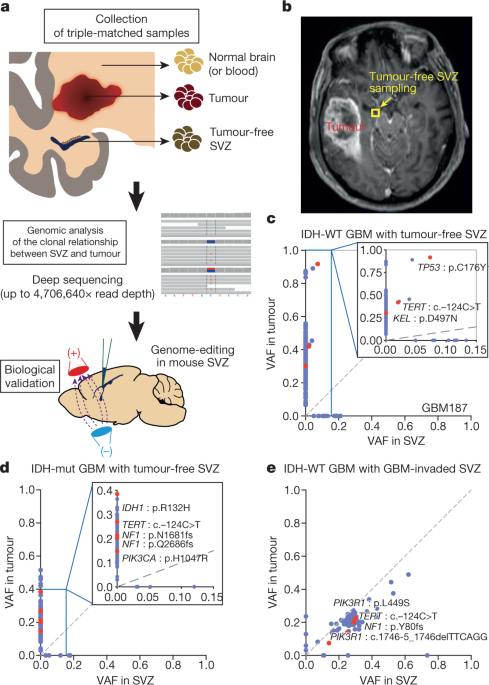
Human glioblastoma arises from subventricular zone cells with low-level driver mutations
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Glioblastoma (GBM) is a devastating and incurable brain tumour, with a median overall survival of fifteen months1,2. Identifying the cell of origin that harbours mutations that
drive GBM could provide a fundamental basis for understanding disease progression and developing new treatments. Given that the accumulation of somatic mutations has been implicated in
gliomagenesis, studies have suggested that neural stem cells (NSCs), with their self-renewal and proliferative capacities, in the subventricular zone (SVZ) of the adult human brain may be
the cells from which GBM originates3,4,5. However, there is a lack of direct genetic evidence from human patients with GBM4,6,7,8,9,10. Here we describe direct molecular genetic evidence
from patient brain tissue and genome-edited mouse models that show astrocyte-like NSCs in the SVZ to be the cell of origin that contains the driver mutations of human GBM. First, we
performed deep sequencing of triple-matched tissues, consisting of (i) normal SVZ tissue away from the tumour mass, (ii) tumour tissue, and (iii) normal cortical tissue (or blood), from 28
patients with isocitrate dehydrogenase (IDH) wild-type GBM or other types of brain tumour. We found that normal SVZ tissue away from the tumour in 56.3% of patients with wild-type IDH GBM
contained low-level GBM driver mutations (down to approximately 1% of the mutational burden) that were observed at high levels in their matching tumours. Moreover, by single-cell sequencing
and laser microdissection analysis of patient brain tissue and genome editing of a mouse model, we found that astrocyte-like NSCs that carry driver mutations migrate from the SVZ and lead to
the development of high-grade malignant gliomas in distant brain regions. Together, our results show that NSCs in human SVZ tissue are the cells of origin that contain the driver mutations
of GBM. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature
and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues
and online access $199.00 per year only $3.90 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local
taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING
VIEWED BY OTHERS HUMAN FETAL CEREBELLAR CELL ATLAS INFORMS MEDULLOBLASTOMA ORIGIN AND ONCOGENESIS Article 30 November 2022 SEQUENTIAL FATE-SWITCHES IN STEM-LIKE CELLS DRIVE THE TUMORIGENIC
TRAJECTORY FROM HUMAN NEURAL STEM CELLS TO MALIGNANT GLIOMA Article Open access 04 January 2021 EVOLVING CELL STATES AND ONCOGENIC DRIVERS DURING THE PROGRESSION OF IDH-MUTANT GLIOMAS
Article 21 November 2024 REFERENCES * Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a
randomised phase III study: 5-year analysis of the EORTC-NCIC trial. _Lancet Oncol_. 10, 459–466 (2009). Article PubMed CAS Google Scholar * Wen, P. Y. & Kesari, S. Malignant gliomas
in adults. _N. Engl. J. Med_. 359, 492–507 (2008). Article PubMed CAS Google Scholar * Sanai, N. et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks
chain migration. _Nature_ 427, 740–744 (2004). Article ADS PubMed CAS Google Scholar * Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells
in a somatic tumor suppressor mouse model. _Cancer Cell_ 15, 45–56 (2009). Article PubMed PubMed Central CAS Google Scholar * Tomasetti, C., Li, L. & Vogelstein, B. Stem cell
divisions, somatic mutations, cancer etiology, and cancer prevention. _Science_ 355, 1330–1334 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Chow, L. M. et al.
Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. _Cancer Cell_ 19, 305–316 (2011). Article PubMed PubMed Central CAS Google
Scholar * Zong, H., Parada, L. F. & Baker, S. J. Cell of origin for malignant gliomas and its implication in therapeutic development. _Cold Spring Harb. Perspect. Biol_. 7, a020610
(2015). Article PubMed PubMed Central CAS Google Scholar * Jacques, T. S. et al. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour
phenotypes. _EMBO J_. 29, 222–235 (2010). Article PubMed CAS Google Scholar * Friedmann-Morvinski, D. et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas
in mice. _Science_ 338, 1080–1084 (2012). Article ADS PubMed PubMed Central CAS Google Scholar * Alcantara Llaguno, S. R. et al. Adult lineage-restricted CNS progenitors specify
distinct glioblastoma subtypes. _Cancer Cell_ 28, 429–440 (2015). Article PubMed PubMed Central CAS Google Scholar * Li, Y. M., Suki, D., Hess, K. & Sawaya, R. The influence of
maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? _J. Neurosurg_. 124, 977–988 (2016). Article PubMed Google Scholar *
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. _Cell_ 155, 462–477 (2013). Article PubMed PubMed Central CAS Google Scholar * Suzuki, H. et al. Mutational
landscape and clonal architecture in grade II and III gliomas. _Nat. Genet_. 47, 458–468 (2015). Article PubMed CAS Google Scholar * Quiñones-Hinojosa, A. et al. Cellular composition and
cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. _J. Comp. Neurol_. 494, 415–434 (2006). Article PubMed Google Scholar * Eriksson, P. S. et al.
Neurogenesis in the adult human hippocampus. _Nat. Med_. 4, 1313–1317 (1998). Article PubMed CAS Google Scholar * Spalding, K. L. et al. Dynamics of hippocampal neurogenesis in adult
humans. _Cell_ 153, 1219–1227 (2013). Article PubMed PubMed Central CAS Google Scholar * Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. _Nature_ 500,
415–421 (2013). Article PubMed PubMed Central CAS Google Scholar * Alexandrov, L. B. et al. Clock-like mutational processes in human somatic cells. _Nat. Genet_. 47, 1402–1407 (2015).
Article PubMed PubMed Central CAS Google Scholar * Ju, Y. S. et al. Somatic mutations reveal asymmetric cellular dynamics in the early human embryo. _Nature_ 543, 714–718 (2017).
Article ADS PubMed CAS PubMed Central Google Scholar * Calzolari, F. et al. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. _Nat.
Neurosci_. 18, 490–492 (2015). Article PubMed CAS Google Scholar * Liu, C. et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. _Cell_ 146, 209–221 (2011).
Article PubMed PubMed Central CAS Google Scholar * Lee, J. H. Somatic mutations in disorders with disrupted brain connectivity. _Exp. Mol. Med_. 48, e239 (2016). Article PubMed PubMed
Central CAS Google Scholar * Chen, L. et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. _Int. J. Radiat.
Oncol. Biol. Phys_. 86, 616–622 (2013). Article PubMed PubMed Central Google Scholar * Khalifa, J. et al. Subventricular zones: new key targets for glioblastoma treatment. _Radiat.
Oncol_. 12, 67 (2017). Article PubMed PubMed Central CAS Google Scholar * Bell, R. J. et al. Understanding TERT promoter mutations: a common path to immortality. _MCR_ 14, 315–323
(2016). Article PubMed CAS Google Scholar * Lim, J. S. et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. _Nat. Med_. 21,
395–400 (2015). Article PubMed CAS Google Scholar * McConnell, M. J. et al. Intersection of diverse neuronal genomes and neuropsychiatric disease: The Brain Somatic Mosaicism Network.
_Science_ 356, eaal1641 (2017). Article PubMed PubMed Central CAS Google Scholar * Moon, J. H. et al. Histopathological implications of ventricle wall 5-aminolevulinic acid-induced
fluorescence in the absence of tumor involvement on magnetic resonance images. _Oncol. Rep_. 36, 837–844 (2016). Article PubMed CAS Google Scholar * Roh, T. H. et al. Long-term outcomes
of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: A single-center analysis. _Medicine (Baltimore)_ 96, e7422 (2017). Article CAS Google Scholar
* Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. _Acta Neuropathol_. 131, 803–820 (2016). Article PubMed Google
Scholar * Verhaak, R. G. W. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in _PDGFRA_, _IDH1_, _EGFR_, and
_NF1_. _Cancer Cell_ 17, 98–110 (2010). Article PubMed PubMed Central CAS Google Scholar * Barbie, D. A. _et al_. Systematic RNA interference reveals that oncogenic KRAS-driven cancers
require TBK1. _Nature_ 462, 108–112 (2009). Article ADS PubMed PubMed Central CAS Google Scholar * Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced
tumor-normal sample pairs. _Bioinformatics_ 28, 1811–1817 (2012). Article PubMed CAS Google Scholar * Ramos, A. H. et al. Oncotator: cancer variant annotation tool. _Hum. Mutat_. 36,
E2423–E2429 (2015). Article PubMed Google Scholar * Kuilman, T. et al. CopywriteR: DNA copy number detection from off-target sequence data. _Genome Biol_. 16, 49 (2015). Article PubMed
PubMed Central Google Scholar * Eckel-Passow, J. E. et al. Glioma groups based on 1p/19q, _IDH_, and _TERT_ promoter mutations in tumors. _N. Engl. J. Med_. 372, 2499–2508 (2015). Article
PubMed PubMed Central CAS Google Scholar * Untergasser, A. et al. Primer3Plus, an enhanced web interface to Primer3. _Nucleic Acids Res_. 35, W71-4 (2007). Article PubMed Google
Scholar * Johnson, B. E. et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. _Science_ 343, 189–193 (2014). Article ADS PubMed CAS Google
Scholar * Kong, B. H. et al. Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens. _Childs Nerv. Syst_. 29, 217–229 (2013). Article PubMed Google
Scholar * Kwak, J. et al. Isolation and characterization of tumorspheres from a recurrent pineoblastoma patient: feasibility of a patient-derived xenograft. _Int. J. Oncol_. 49, 569–578
(2016). Article PubMed CAS Google Scholar * Bae, T. et al. Different mutational rates and mechanisms in human cells at pregastrulation and neurogenesis. _Science_ (2017). * Baslan, T. et
al. Genome-wide copy number analysis of single cells. _Nat. Protoc._ 7, 1024–1041 (2012). Article PubMed CAS Google Scholar * Rosenthal, R., McGranahan, N., Herrero, J., Taylor, B. S.
& Swanton, C. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. _Genome Biol_. 17, 31 (2016).
Article PubMed PubMed Central CAS Google Scholar * Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian
cells. _Nat. Biotechnol_. 33, 543–548 (2015). Article PubMed CAS Google Scholar * Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. _Nat. Protoc._ 8, 2281–2308 (2013).
Article PubMed CAS Google Scholar * Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. _Cell_ 159, 440–455 (2014). Article PubMed PubMed Central CAS
Google Scholar * Zhu, H. et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. _Proc. Natl Acad. Sci. USA_ 106, 2712–2716 (2009).
Article ADS PubMed Google Scholar * Guo, P. et al. A simplified purification method for AAV variant by polyethylene glycol aqueous two-phase partitioning. _Bioengineered_ 4, 103–106
(2013). Article PubMed PubMed Central Google Scholar * Park, J., Lim, K., Kim, J.-S. & Bae, S. Cas-analyzer: an online tool for assessing genome editing results using NGS data.
_Bioinformatics_ 33, 286–288 (2017). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank J. K. Kim for discussing single-cell sequencing. This work was
supported by grants from Suh Kyungbae Foundation and IBS-R002-D1 to J.H.L. (last author), the Korean Health Technology R&D Project, Ministry of Health & Welfare, South Korea
(H15C3143 and H16C0415 to J.H.L. (last author), HI14C1324 and HI17C2586 to S.G.K.), the Global PhD Fellowship Program through the National Research Foundation (NRF) of Korea funded by the
Ministry of Education, Republic of Korea (NRF-2014H1A2A1021321 to J.H.L. (first author)), the Basic Science Research Program through the NRF of Korea (NRF-2016R1D1A1A09916521 to S.G.K.)
funded by the Ministry of Education, the NRF of Korea grant (NRF-2017M2A2A7A01071036 to S.G.K.) funded by the Korean Ministry of Science, ICT and Future Planning, the Basic Science Research
Program through the NRF of Korea (NRF-2017R1A2B2006526 to S.P.) funded by the Ministry of Science, ICT and Future Planning, and Korea Health Technology R&D Project through the Korea
Health Industry Development Institute (HI16C2387 to Y.S.J.) funded by the Ministry of Health and Welfare. Non-cancer brain tissues were provided by the Netherlands Brain Bank (project number
Lee-835) to J.H.L. (last author). REVIEWER INFORMATION _Nature_ thanks M. Taylor and the other anonymous reviewer(s) for their contribution to the peer review of this work. AUTHOR
INFORMATION Author notes * These authors contributed equally: Joo Ho Lee, Jeong Eun Lee * These authors jointly supervised this work: Seok-Gu Kang, Jeong Ho Lee AUTHORS AND AFFILIATIONS *
Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, South Korea Joo Ho Lee, Jeong Eun Lee, Jee Ye Kahng, Jun Sung Park,
Woo Kyeong Kim, June-Koo Lee, Young Seok Ju, Sung-Hong Park & Jeong Ho Lee * Department of Internal Medicine, College of Medicine, Chungnam National University, Daejeon, South Korea
Jeong Eun Lee * Department of Biological Sciences, KAIST, Daejeon, South Korea Jee Ye Kahng, Joon-Hyuk Lee & Won-Suk Chung * Department of Pathology, Brain Korea 21 Project for Medical
Science, Yonsei University College of Medicine, Seoul, South Korea Se Hoon Kim * Department of Neurosurgery, Brain Tumor Center, Severance Hospital, Yonsei University College of Medicine,
Seoul, South Korea Seon Jin Yoon, Junseong Park, Eui Hyun Kim, Ji-Hyun Lee, Jong Hee Chang & Seok-Gu Kang * Department of Bio and Brain Engineering, KAIST, Daejeon, South Korea Ji-Yong
Um & Sung-Hong Park * Center for Synaptic Brain Dysfunctions, Institute for Basic Science, Daejeon, South Korea Jeong Ho Lee Authors * Joo Ho Lee View author publications You can also
search for this author inPubMed Google Scholar * Jeong Eun Lee View author publications You can also search for this author inPubMed Google Scholar * Jee Ye Kahng View author publications
You can also search for this author inPubMed Google Scholar * Se Hoon Kim View author publications You can also search for this author inPubMed Google Scholar * Jun Sung Park View author
publications You can also search for this author inPubMed Google Scholar * Seon Jin Yoon View author publications You can also search for this author inPubMed Google Scholar * Ji-Yong Um
View author publications You can also search for this author inPubMed Google Scholar * Woo Kyeong Kim View author publications You can also search for this author inPubMed Google Scholar *
June-Koo Lee View author publications You can also search for this author inPubMed Google Scholar * Junseong Park View author publications You can also search for this author inPubMed Google
Scholar * Eui Hyun Kim View author publications You can also search for this author inPubMed Google Scholar * Ji-Hyun Lee View author publications You can also search for this author
inPubMed Google Scholar * Joon-Hyuk Lee View author publications You can also search for this author inPubMed Google Scholar * Won-Suk Chung View author publications You can also search for
this author inPubMed Google Scholar * Young Seok Ju View author publications You can also search for this author inPubMed Google Scholar * Sung-Hong Park View author publications You can
also search for this author inPubMed Google Scholar * Jong Hee Chang View author publications You can also search for this author inPubMed Google Scholar * Seok-Gu Kang View author
publications You can also search for this author inPubMed Google Scholar * Jeong Ho Lee View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
S.-G.K. and Jeong Ho Lee conceived the idea of the project. Joo Ho Lee, S.-G.K. and Jeong Ho Lee organised the project. Joo Ho Lee and J.E.L. performed genetic studies. Joo Ho Lee, J.-K.L.
and Y.S.J. performed the analysis of the mutational signature. S.H.K. performed pathological studies in human tissues. Joo Ho Lee and J.Y.K. performed the generation and analysis of mice.
Joo Ho Lee and J.Y.K. performed single-cell sequencing and laser microdissection. J.-Y.U. and S.-H.P. performed the analysis of mouse brain MRIs. J.H.C., E.H.K. and S.-G.K. performed
surgeries, collected patient samples and managed tissue information with S.J.Y., Ji-Hyun Lee, Joo Ho Lee and W.K.K. J.P. performed subtype classification. J.S.P. performed genetic studies of
non-cancer brain tissues. Joon-Hyuk Lee and W.-S.C. produced viral vectors. Joo Ho Lee and Jeong Ho Lee wrote the manuscript. S.-G.K. and Jeong Ho Lee led the project. CORRESPONDING AUTHORS
Correspondence to Seok-Gu Kang or Jeong Ho Lee. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 RADIOLOGICAL AND HISTOLOGICAL
CONFIRMATION OF SAMPLING AND TISSUES FROM PATIENTS WITH IDH-WILD-TYPE GBM. A, The distance between the tumour-margin and the sampling site of SVZ tissue was measured using 3D-reconstruced
MRI images. B, H&E staining of the GBM tumour shows pseudopalisading necrosis. Scale bar, 200 µm. C, H&E staining of the SVZ tissue away from the tumour shows tumour-free status with
intact architecture. Scale bar, 200 µm. Radiological and histological confirmation was performed in all 30 patients. D, Pre-operation brain MRI of a patient with IDH-wild-type GBM shows the
locations of sampling sites of tumour and tumour-free SVZ tissue. Multiple samplings from tumour-free SVZs were performed in two patients (GBM187 and GBM499). EXTENDED DATA FIG. 2 VAF
SCATTERPLOTS OF SNV AND INDELS, AND PATTERNS OF CNVS OF MATCHED TUMOUR AND TUMOUR-FREE SVZ TISSUE. A–C, Scatterplots from seven patients with IDH-wild-type GBM with tumour-free SVZ (A),
meningioma with tumour-free SVZ (B), anaplastic oligodendroglioma, and IDH-mutant with tumour-free SVZ (C). Shared and private somatic mutations in paired SVZ and tumour (_x_ and _y_ axes,
respectively) tissue specimens are indicated as a function of the VAF. A single point represents an individual mutation. Red dots indicate cancer-driver mutations (see Methods). D,
Quantitative analysis of CNVs in _EGFR_ in patients with IDH-wild-type GBM who harboured driver mutations thereof in tumour-free SVZ. The bar graph shows relative fold changes in _EGFR_ copy
numbers in patients GBM26, GBM187, GBM245, GBM276, GBM499 and GBM520 (relative to the matched normal control) based on qPCR. E, Genome-wide CNV plots for patients with IDH-wild-type GBM and
matching tumour-free SVZs harbouring shared mutations in WES. Red and blue boxes indicate shared and tumour-private CNVs between tumour-free SVZ and tumour, respectively. F, Genome-wide CNV
plots for a patient with IDH-wild-type GBM and a GBM-invaded SVZ. G, _EGFR_ amplification was found at the cellular level through FISH of the tumour-free SVZ specimen from one patient
(GBM520). FISH was performed in patients GBM26, GBM276, GBM499 and GBM520. _EGFR_ and control probes were red and green, respectively. Scale bars, 10 µm. Source data EXTENDED DATA FIG. 3
VAFS OF NONSYNONYMOUS SOMATIC MUTATIONS IN GLIOMA-RELATED GENES FROM TRIPLE-MATCHED SAMPLES OF 23 PATIENTS WITH GBM AND OTHER TYPES OF BRAIN TUMOUR. A, VAFs of mutations in patients with
IDH-wild-type GBM and tumour-free SVZs. B, VAFs of mutations in other types of brain tumour with tumour-free SVZs. C, VAFs of mutations in patients with IDH-wild-type GBM and GBM-invaded
SVZs. The lengths of the bars are proportional to the VAF (the scale bar under the last column applies to all columns). EXTENDED DATA FIG. 4 SCHEMATIC OF THE EXPERIMENTAL PROCEDURES FOR
SINGLE-CELL SEQUENCING OF TUMOUR-PRIVATE MUTATIONS AND SHARED DRIVER MUTATIONS IN FROZEN TISSUE. Nuclei are isolated from homogenized tissue and stained with DAPI. Flow cytometry sorts
DAPI-stained nuclei as a single nucleus into each well of a 96-well plate, which is confirmed by fluorescent microscopy. Then, single-cell PCR followed by Sanger sequencing is performed.
EXTENDED DATA FIG. 5 IDENTIFICATION OF THE CHRONOLOGICAL ORDER AND CLONAL EVALUATION BETWEEN SVZ AND TUMOUR. A, B, Single-cell sequencing of tumours and tumour-free SVZ. Single-cell Sanger
sequencing of tumour-private and shared driver mutations (left) and a summary of the sequencing results (right) in the tumours (A) and in the tumour-free SVZ (B) from patients who had
somatic mutations shared between SVZ and tumour tissue. The numbers in the tables on the right indicate the number of sequenced clones. C, D, LCM of the three layers that make up the SVZ,
followed by site-specific amplicon sequencing. C, LCM in patient GBM187 captured approximately 30 nuclei from each defined structure. Scale bar, 100 µm. D, Site-specific amplicon sequencing
reveals increases in C228T mutant allele frequency from the bulk DNA to the micro-dissected astrocytic ribbon, whereas the mutation was not found in the other micro-dissected regions.
Control reflects a randomly micro-dissected region from the same tumour-free SVZ specimen. E, The frequency of patients harbouring _TERT_ promoter mutations. Numbers indicate the number
(percentage) of patients. Deep-target sequencing of _TERT_ promoter mutations (C228T and C250T) was performed. Non-cancer aged brain refers to autopsy samples of the hippocampus from
non-cancer aged control brain tissues with an average age of 84 years. *_P_ = 0.005, Fisher’s exact test. F, Mutation spectra incorporating the substitution type of mutations in the
GBM-invaded SVZ. The mutation types are displayed on the horizontal axis, and the vertical axis indicates the fractions of mutations attributed to a specific mutation type. G, The number of
mutations contributing to each mutation signature. Source data EXTENDED DATA FIG. 6 DEVELOPMENT OF HIGH-GRADE GLIOMA IN GENOME-EDITED MICE HARBOURING P53/PTEN/EGFR MUTATIONS IN THE SVZ. A,
The map of a single vector expressing Cas9 and Cre recombinase with the sgRNAs targeting _p53_/_Pten_. B, In vitro screen of sgRNAs targeted to _p53_ and _Pten_ in the Neuro-2a cell line by
transient transfection and T7E1 assay. C, Immunostaining image of markers for neural stem cells at 3 days after electroporation of the vector in P53/PTEN/EGFR-mutant mice; the image
highlights the localization of tdTomato-positive cells along the SVZ co-stained with GFAP or nestin. Scale bars, 50 μm. D, A scatter dot graph showing the percentage of tdTomato-positive
cells co-stained with nestin or GFAP (P53/PTEN/EGFR-mutant mouse: _n_ = 5; mean ± s.e.m.). E, A Kaplan–Meier survival graph of mice (_n_ = 10 mice in each group). _P_ = 0.000063, log-rank
test. F, Representative H&E-stained images reflect the classical features of high-grade glioma, such as necrosis (Fig. 3e), microvascular proliferation (M), and mitoses (arrow). Scale
bars, 100 μm. G, Representative MRI images of the bulk tumours formed in the 3 mice after 16 weeks. H, Immunostaining of various high-grade glioma-related markers, including nestin, GFAP,
OLIG2, S100β, MBP and Ki67, as well as the neuronal maker NeuN, in tumours (_n_ = 4 tumours). Scale bars, 50 μm. I, The bar graph shows the percentage of sequencing reads with indels in one
high-grade glioma from mutant mice, using site-specific amplicon sequencing of the CRISPR targeting region in _p53_ and _Pten_. J, Detection of EGFRviii (360 bp) in tumours from
P53/PTEN/EGFR-mutant mice using quantitative PCR with reverse transcription (qRT–PCR). _Actb_ was used as an internal control (_n_ = 3 mice with tumours). Source data EXTENDED DATA FIG. 7
THE FORMATION OF GLIOMA AS OBSERVED IN SERIAL SECTIONS OF MOUSE BRAIN. A, Images from the P53/PTEN/EGFR-mutant mice show that the tdTomato-positive cells initially locate in the rostral SVZ,
where mutations are edited. Over time, these cells migrate to distant cortical regions and proliferate to form the tumour. B, Images from wild-type mice at 16 weeks after the
electroporation of the plasmid containing sgRNA for _lacZ_ gene with the expression of Cas9/Cre as a control. In panels A and B, section points were the middle of the olfactory bulb at 0.7,
−1, −2.5 and −3.5 mm apart from bregma; time intervals were 2 days, 8 weeks, 13 weeks and 16 weeks after electroporation. Scale bars, 500 μm. C, Quantification of tdTomato signal intensities
corresponding to the average of cortical regions from four serial sections in the affected side. Section points were 0.7, −1, −2.5 and −3.5 mm apart from bregma. Control indicates the
wild-type mouse electroporated with the plasmid containing sgRNA for the _lacZ_ gene with the expression of Cas9/Cre. *_P_ = 0.007, **_P_ = 0.0002 (_n_ = 6 for control and mutant mice at
each time point), Student’s two-tailed _t_-test. Error bars represent mean ± s.e.m. Source data EXTENDED DATA FIG. 8 THE NORMAL CYTOARCHITECTURE OF THE SVZ AND OLFACTORY BULB CONTAINING
MUTATION-CARRYING CELLS AND VIRAL INJECTION TARGETING DRIVER MUTATIONS IN THE CORTEX. A, B, Immunostaining of S100β (A, ependymal cell marker), GFAP and nestin (B, stem-cell marker) in
mutation-arising SVZ tissue from the mice with high-grade glioma in the distant cortical region (_n_ = 6 mice with tumours). C, Targeted deep sequencing of TP53 and PTEN in tdTomato-positive
neurons laser-captured from the olfactory bulb. The tdTomato-positive cells in the olfactory bulb were labelled with yellow dots in LCM images (targeting). Labelled cells were
micro-dissected (LCM). D, Deep amplicon sequencing of CRISPR target sites showed that tdTomato-positive cells in the olfactory bulb contained P53/PTEN mutations. Scale bar, 50 μm. LCM and
sequencing were repeated in 3 mice. E, Experimental scheme showing the procedure for viral injection of AAV5 containing sgRNAs for _p53_ and _Pten_ genes, with the expression of Cas9 and Cre
recombinase into the SVZ in LSL-EGFRviii; LSL-Cas9-GFP mice. F, G, Representative images of the injection site in the cortex at 1 week and 6 weeks after viral injection. Virus-expressing
cells are GFP-positive. Scale bar, 500 μm. H, Quantification of the number of GFP-positive cells at the representative image. The section points were the sites where the highest number of
GFP-positive cells are found (mice at 1 week: _n_ = 3, mice at 4–6 weeks: _n_ = 5). Not significant (NS), Student’s two-tailed _t_-test. Error bars represent mean ± s.e.m. I, Images from
mouse at 6 weeks after viral injection to cortex. No signal was found in the distant brain as well as the SVZ. J, Images from mouse at 6 weeks after electroporation to the SVZ. For panels D
and E, section points were 0.7, −1, −2.5 and −3.5 mm apart from bregma (mice at 1 week: _n_ = 3, mice at 4–6 weeks: _n_ = 5). Scale bars, 500 μm. K, Representative immunohistochemical images
of GFAP-, OLIG2-, NeuN-, and GFP-positive cells at 1 week after viral injection) mice at 1 week: _n_ = 3, mice at 4–6 weeks: _n_ = 5). Scale bars, 100 μm. Source data EXTENDED DATA FIG. 9
ABERRANT GROWTH OF OPC LINEAGE IN THE DISTANT REGION. A, The scatter dot graph shows the percentage of cells positive for various NSC-derived cell lineage markers, such as NeuN for neurons,
MBP for oligodendrocytes, GFAP for astrocytes, and PDGFRα and OLIG2 for oligodendrocyte-progenitor cells (OPCs). The average of four representative cortical regions at the caudal cortex
(−3.5 mm apart from bregma) away from the mutation-arising SVZ were analysed (_n_ = 6 for control and mutant mice). Error bars represent mean ± s.e.m. B, Representative immunostaining images
of OLIG2-, PDGFRα-, GFAP- and tdTomato-positive cell regions at the caudal cortex (−3.5 mm apart from bregma). White arrows indicate tdTomato-positive cells co-stained with OLIG2 or PDGFRα.
Scale bars, 50 µm. _n_ = 6 mice C, Immunostaining of Ki67, a marker of proliferation, and cell-type markers PDGFRα, OLIG2 and GFAP in P53/PTEN/EGFR-mutant mice before the formation of a
visible glioma. White arrows indicate Ki67-positive cells. Scale bars, 50 μm. _n_ = 3 mice. D, Illustration of the progress of migration and tumour development via the aberrant growth of
OPCs. Source data SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE 1 Summary of clinical and sequencing information of patients. EGFR expression grade was evaluated by
immunohistochemistry in patient samples. Gene amplification of EGFR was evaluated by fluorescence in situ hybridization. N/A, not available; WES, whole-exome sequencing; MGMT,
_O_6-alkylguanine DNA alkyltransferase SUPPLEMENTARY TABLE 2 A list of glioma-related genes for targeted sequencing SUPPLEMENTARY TABLE 3 Sequencing primers for validation of mutations and
the states thereof SUPPLEMENTARY TABLE 4 Primers for quantitative PCR analysis of copy number variations in EGFR SUPPLEMENTARY TABLE 5 Primers for single-cell sequencing SUPPLEMENTARY TABLE
6 A list of oligonucleotides used for sgRNA construction SOURCE DATA SOURCE DATA FOR FIG. 2 SOURCE DATA FOR FIG. 3 SOURCE DATA FOR EXTENDED DATA FIG. 2 SOURCE DATA FOR EXTENDED DATA FIG. 5
SOURCE DATA FOR EXTENDED DATA FIG. 6 SOURCE DATA FOR EXTENDED DATA FIG. 7 SOURCE DATA FOR EXTENDED DATA FIG. 8 SOURCE DATA FOR EXTENDED DATA FIG. 9 RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, J.H., Lee, J.E., Kahng, J.Y. _et al._ Human glioblastoma arises from subventricular zone cells with low-level driver mutations. _Nature_
560, 243–247 (2018). https://doi.org/10.1038/s41586-018-0389-3 Download citation * Received: 25 May 2017 * Accepted: 11 June 2018 * Published: 01 August 2018 * Issue Date: 09 August 2018 *
DOI: https://doi.org/10.1038/s41586-018-0389-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative