The nlrp3 inflammasome: molecular activation and regulation to therapeutics
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
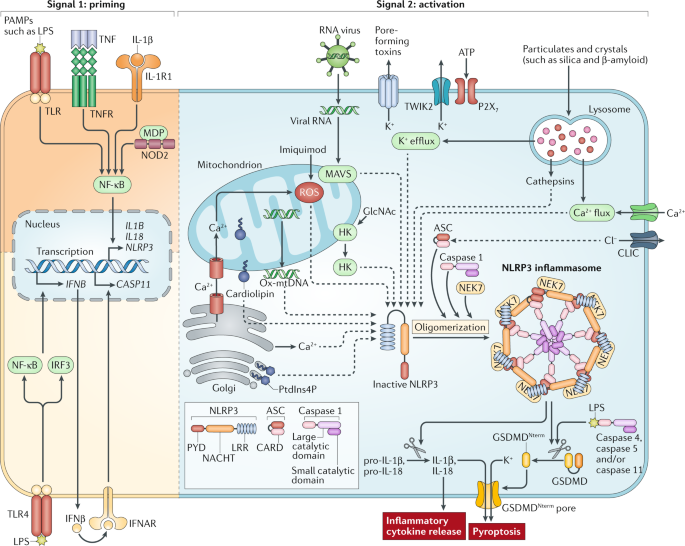
ABSTRACT NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) is an intracellular sensor that detects a broad range of microbial motifs, endogenous danger signals and environmental
irritants, resulting in the formation and activation of the NLRP3 inflammasome. Assembly of the NLRP3 inflammasome leads to caspase 1-dependent release of the pro-inflammatory cytokines
IL-1β and IL-18, as well as to gasdermin D-mediated pyroptotic cell death. Recent studies have revealed new regulators of the NLRP3 inflammasome, including new interacting or regulatory
proteins, metabolic pathways and a regulatory mitochondrial hub. In this Review, we present the molecular, cell biological and biochemical bases of NLRP3 activation and regulation and
describe how this mechanistic understanding is leading to potential therapeutics that target the NLRP3 inflammasome. Access through your institution Buy or subscribe This is a preview of
subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value
online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS HOW LOCATION AND CELLULAR SIGNALING COMBINE TO ACTIVATE
THE NLRP3 INFLAMMASOME Article Open access 20 September 2022 UPDATED INSIGHTS INTO THE MOLECULAR NETWORKS FOR NLRP3 INFLAMMASOME ACTIVATION Article Open access 30 April 2025 DRUGGING THE
NLRP3 INFLAMMASOME: FROM SIGNALLING MECHANISMS TO THERAPEUTIC TARGETS Article 29 November 2023 REFERENCES * Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R.
D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. _Nat. Genet._ 29, 301–305 (2001). CAS PubMed
PubMed Central Google Scholar * Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural
deafness, and AA amyloidosis. _Arthritis Rheum._ 46, 2445–2452 (2002). CAS PubMed Google Scholar * Aksentijevich, I. et al. De novo _CIAS1_ mutations, cytokine activation, and evidence
for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases.
_Arthritis Rheum._ 46, 3340–3348 (2002). REFERENCES 1–3 REPORT THAT NLRP3 GAIN-OF-FUNCTION MUTATIONS PROMOTE HUMAN AUTOINFLAMMATORY DISEASES. CAS PubMed PubMed Central Google Scholar *
Mangan, M. S. et al. Targeting the NLRP3 inflammasome in inflammatory diseases. _Nat. Rev. Drug Discov._ 17, 588 (2018). CAS PubMed Google Scholar * Guo, H., Callaway, J. B. & Ting,
J. P. Inflammasomes: mechanism of action, role in disease, and therapeutics. _Nat. Med._ 21, 677–687 (2015). PubMed PubMed Central Google Scholar * Martinon, F., Burns, K. & Tschopp,
J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. _Mol. Cell_ 10, 417–426 (2002). THIS IS THE FIRST REPORT DESCRIBING AN
INFLAMMASOME COMPLEX THAT MEDIATES CLEAVAGE OF IL-1Β. CAS PubMed Google Scholar * Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in
Muckle-Wells autoinflammatory disorder. _Immunity_ 20, 319–325 (2004). THIS IS THE FIRST REPORT SHOWING THAT NLRP3 ASSEMBLES AN INFLAMMASOME COMPLEX THAT MEDIATES CLEAVAGE OF IL-1Β BY
CASPASE 1. CAS PubMed Google Scholar * Duncan, J. A. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. _Proc. Natl Acad.
Sci. USA_ 104, 8041–8046 (2007). THIS STUDY SHOWS THAT ATP BINDING IS ESSENTIAL FOR NLRP3 FUNCTION, SUGGESTING A THERAPEUTIC TARGET FOR TREATING NLRP3-RELATED DISEASES. CAS PubMed PubMed
Central Google Scholar * Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. _Cell_ 156, 1207–1222 (2014). CAS
PubMed PubMed Central Google Scholar * Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. _Cell_ 156, 1193–1206 (2014). REFERENCES 9 AND 10
SHOW THAT THE PYDS FROM INFLAMMASOME SENSORS NUCLEATE THE POLYMERIZATION OF ASC. CAS PubMed PubMed Central Google Scholar * Schmidt, F. I. et al. A single domain antibody fragment that
recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. _J. Exp. Med._ 213, 771–790 (2016). CAS PubMed PubMed Central Google Scholar * Boucher, D. et al.
Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. _J. Exp. Med._ 215, 827–840 (2018). CAS PubMed PubMed Central Google Scholar * Schmid-Burgk, J. L.
et al. A genome-wide CRISPR screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. _J. Biol. Chem._ 291, 103–109 (2015). PubMed PubMed Central Google Scholar *
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. _Nature_ 530, 354–357 (2016). CAS PubMed PubMed
Central Google Scholar * Shi, H. et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. _Nat. Immunol._ 17, 250–258 (2016).
REFERENCES 13–15 IDENTIFY NEK7 AS AN INTEGRAL COMPONENT OF THE NLRP3 INFLAMMASOME. CAS PubMed Google Scholar * Bauernfeind, F. G. et al. Cutting edge: NF-kappaB activating pattern
recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. _J. Immunol._ 183, 787–791 (2009). CAS PubMed Google Scholar * Franchi, L.,
Eigenbrod, T. & Núñez, G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. _J. Immunol._ 183, 792–796
(2009). CAS PubMed Google Scholar * Xing, Y. et al. Cutting edge: TRAF6 mediates TLR/IL-1R signaling-induced nontranscriptional priming of the NLRP3 inflammasome. _J. Immunol._ 199,
1561–1566 (2017). CAS PubMed Google Scholar * Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. _Nature_ 496, 238–242 (2013). CAS PubMed
PubMed Central Google Scholar * Perregaux, D. & Gabel, C. A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by
these agents is a necessary and common feature of their activity. _J. Biol. Chem._ 269, 15195–15203 (1994). CAS PubMed Google Scholar * Walev, I., Reske, K., Palmer, M., Valeva, A. &
Bhakdi, S. Potassium-inhibited processing of IL-1 beta in human monocytes. _EMBO J._ 14, 1607–1614 (1995). CAS PubMed PubMed Central Google Scholar * Surprenant, A., Rassendren, F.,
Kawashima, E., North, R. A. & Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). _Science_ 272, 735–738 (1996). CAS PubMed Google Scholar
* Samways, D. S., Li, Z. & Egan, T. M. Principles and properties of ion flow in P2X receptors. _Front. Cell Neurosci._ 8, 6 (2014). PubMed PubMed Central Google Scholar * Di, A. et
al. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. _Immunity_ 49, 56–65 (2018). CAS PubMed PubMed Central Google Scholar *
Triantafilou, K., Hughes, T. R., Triantafilou, M. & Morgan, B. P. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. _J.
Cell Sci._ 126, 2903–2913 (2013). CAS PubMed Google Scholar * Laudisi, F. et al. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1β release. _J.
Immunol._ 191, 1006–1010 (2013). CAS PubMed PubMed Central Google Scholar * Asgari, E. et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent
NLRP3 inflammasome activation. _Blood_ 122, 3473–3481 (2013). CAS PubMed Google Scholar * Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by
bacterial toxins and particulate matter. _Immunity_ 38, 1142–1153 (2013). PubMed PubMed Central Google Scholar * Pétrilli, V. et al. Activation of the NALP3 inflammasome is triggered by
low intracellular potassium concentration. _Cell Death Differ._ 14, 1583–1589 (2007). PubMed Google Scholar * Gaidt, M. M. et al. Human monocytes engage an alternative inflammasome
pathway. _Immunity_ 44, 833–846 (2016). CAS PubMed Google Scholar * Groß, C. J. et al. K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria.
_Immunity_ 45, 761–773 (2016). PubMed Google Scholar * Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. _Cell_ 166, 624–636 (2016).
CAS PubMed PubMed Central Google Scholar * Murakami, T. et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. _Proc. Natl Acad. Sci. USA_ 109,
11282–11287 (2012). CAS PubMed PubMed Central Google Scholar * Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. _Nature_ 492,
123–127 (2012). CAS PubMed PubMed Central Google Scholar * Yaron, J. R. et al. K+ regulates Ca2+ to drive inflammasome signaling: dynamic visualization of ion flux in live cells. _Cell
Death Dis._ 6, e1954 (2015). CAS PubMed PubMed Central Google Scholar * Katsnelson, M. A., Rucker, L. G., Russo, H. M. & Dubyak, G. R. K+ efflux agonists induce NLRP3 inflammasome
activation independently of Ca2+ signaling. _J. Immunol._ 194, 3937–3952 (2015). CAS PubMed Google Scholar * Domingo-Fernández, R., Coll, R. C., Kearney, J., Breit, S. & O’Neill, L.
A. J. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1β transcription and activate the NLRP3 inflammasome. _J. Biol. Chem._ 292, 12077–12087 (2017). PubMed PubMed
Central Google Scholar * Tang, T. et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. _Nat. Commun._ 8, 202 (2017). PubMed
PubMed Central Google Scholar * Green, J. P. et al. Chloride regulates dynamic NLRP3-dependent ASC oligomerization and inflammasome priming. _Proc. Natl Acad. Sci. USA_ 115, E9371–E9380
(2018). CAS PubMed PubMed Central Google Scholar * Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. _Nat.
Immunol._ 9, 847–856 (2008). CAS PubMed PubMed Central Google Scholar * Orlowski, G. M. et al. Multiple cathepsins promote pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. _J.
Immunol._ 195, 1685–1697 (2015). CAS PubMed Google Scholar * Katsnelson, M. A., Lozada-Soto, K. M., Russo, H. M., Miller, B. A. & Dubyak, G. R. NLRP3 inflammasome signaling is
activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. _Am. J. Physiol. Cell Physiol._ 311, C83–C100 (2016). PubMed
PubMed Central Google Scholar * Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. _Nature_ 469, 221–225 (2011). CAS PubMed
Google Scholar * Cruz, C. M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. _J. Biol. Chem._
282, 2871–2879 (2007). CAS PubMed Google Scholar * Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. _Science_ 320, 674–677 (2008).
CAS PubMed PubMed Central Google Scholar * Courbet, A. et al. Imidazoquinoxaline anticancer derivatives and imiquimod interact with tubulin: Characterization of molecular microtubule
inhibiting mechanisms in correlation with cytotoxicity. _PLOS ONE_ 12, e0182022 (2017). PubMed PubMed Central Google Scholar * Nakahira, K. et al. Autophagy proteins regulate innate
immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. _Nat. Immunol._ 12, 222–230 (2011). CAS PubMed Google Scholar * Bauernfeind, F. et al.
Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. _J. Immunol._ 187, 613–617 (2011). CAS PubMed Google Scholar * Liu, X. et
al. Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. _Antioxid. Redox Signal._ 26, 28–43
(2017). CAS PubMed PubMed Central Google Scholar * Li, W. et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. _Biochem.
Pharmacol._ 76, 1485–1489 (2008). CAS PubMed PubMed Central Google Scholar * Sussan, T. E. et al. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated
atherosclerosis in mice. _PLOS ONE_ 3, e3791 (2008). PubMed PubMed Central Google Scholar * Freigang, S. et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation
and exacerbation of atherosclerosis. _Eur. J. Immunol._ 41, 2040–2051 (2011). CAS PubMed Google Scholar * Zhao, C., Gillette, D. D., Li, X., Zhang, Z. & Wen, H. Nuclear factor
E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. _J. Biol. Chem._ 289, 17020–17029 (2014). CAS PubMed PubMed Central Google Scholar * Sogawa, Y. et al.
Infiltration of M1, but not M2, macrophages is impaired after unilateral ureter obstruction in Nrf2-deficient mice. _Sci. Rep._ 7, 8801 (2017). PubMed PubMed Central Google Scholar *
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. _Nature_ 464, 104–107 (2010). CAS PubMed PubMed Central Google Scholar * Zhong, Z. et al. New
mitochondrial DNA synthesis enables NLRP3 inflammasome activation. _Nature_ 560, 198–203 (2018). CAS PubMed PubMed Central Google Scholar * Shimada, K. et al. Oxidized mitochondrial DNA
activates the NLRP3 inflammasome during apoptosis. _Immunity_ 36, 401–414 (2012). CAS PubMed PubMed Central Google Scholar * Lemasters, J. J., Theruvath, T. P., Zhong, Z. & Nieminen,
A. L. Mitochondrial calcium and the permeability transition in cell death. _Biochim. Biophys. Acta_ 1787, 1395–1401 (2009). CAS PubMed PubMed Central Google Scholar * Man, S. M. et al.
The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. _Nat. Immunol._ 16, 467–475 (2015). CAS PubMed PubMed
Central Google Scholar * Kuriakose, T., Zheng, M., Neale, G. & Kanneganti, T. D. IRF1 is a transcriptional regulator of ZBP1 promoting NLRP3 inflammasome activation and cell death
during influenza virus infection. _J. Immunol._ 200, 1489–1495 (2018). CAS PubMed Google Scholar * Allam, R. et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome
activation but non-apoptotic caspase-8 is required for inflammasome priming. _EMBO Rep._ 15, 982–990 (2014). CAS PubMed PubMed Central Google Scholar * Subramanian, N., Natarajan, K.,
Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. _Cell_ 153, 348–361 (2013). CAS PubMed PubMed
Central Google Scholar * Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. _Front. Cell Dev. Biol._ 5, 90 (2017). PubMed PubMed Central Google Scholar * Iyer, S. S. et
al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. _Immunity_ 39, 311–323 (2013). CAS PubMed PubMed Central Google Scholar * Elliott, E. I. et al. Cutting edge:
mitochondrial assembly of the NLRP3 inflammasome complex is initiated at priming. _J. Immunol._ 200, 3047–3052 (2018). CAS PubMed Google Scholar * Franchi, L. et al. Cytosolic
double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. _J. Immunol._ 193, 4214–4222 (2014). CAS PubMed Google Scholar * Park, S. et
al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. _J. Immunol._ 191, 4358–4366 (2013). CAS PubMed Google Scholar * Ichinohe, T.,
Yamazaki, T., Koshiba, T. & Yanagi, Y. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. _Proc. Natl Acad. Sci. USA_ 110,
17963–17968 (2013). CAS PubMed PubMed Central Google Scholar * Krawczyk, C. M. et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation.
_Blood_ 115, 4742–4749 (2010). CAS PubMed PubMed Central Google Scholar * Sanman, L. E. et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell
death. _eLife_ 5, e13663 (2016). PubMed PubMed Central Google Scholar * Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. _Nat.
Immunol._ 12, 408–415 (2011). CAS PubMed PubMed Central Google Scholar * Moon, J. S. et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. _J.
Clin. Invest._ 125, 665–680 (2015). PubMed PubMed Central Google Scholar * Moon, J. S. et al. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages.
_Nat. Med._ 22, 1002–1012 (2016). CAS PubMed PubMed Central Google Scholar * Li, X. N. et al. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular
reactive oxygen species by upregulating thioredoxin. _Diabetes_ 58, 2246–2257 (2009). CAS PubMed PubMed Central Google Scholar * Youm, Y. H. et al. The ketone metabolite
β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. _Nat. Med._ 21, 263–269 (2015). THIS STUDY SHOWS THAT AN ENDOGENOUS MOLECULE PRODUCED DURING FASTING POTENTLY
INHIBITS NLRP3 ACTIVATION. CAS PubMed PubMed Central Google Scholar * Truax, A. D. et al. The inhibitory innate immune sensor NLRP12 maintains a threshold against obesity by regulating
gut microbiota homeostasis. _Cell Host Microbe_ 24, 364–378 (2018). CAS PubMed PubMed Central Google Scholar * Hughes, M. M. & O’Neill, L. A. J. Metabolic regulation of NLRP3.
_Immunol. Rev._ 281, 88–98 (2018). CAS PubMed Google Scholar * Chen, J. & Chen, Z. J. PtdIns4P on dispersed _trans_-Golgi network mediates NLRP3 inflammasome activation. _Nature_ 564,
71–76 (2018). THIS STUDY SHOWS THAT DISASSEMBLY OF THE _TRANS_ -GOLGI NETWORK SERVES AS A SCAFFOLD FOR NLRP3 AGGREGATION AND ACTIVATION. CAS PubMed Google Scholar * Guo, C. et al.
Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 inflammasome activation and cholesterol biosynthetic signaling in macrophages. _Immunity_ 49, 842–856 (2018). CAS PubMed
Google Scholar * Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. _Nature_ 526, 660 (2015). CAS PubMed Google Scholar * He, W.-t et al.
Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. _Cell Res._ 25, 1285 (2015). CAS PubMed PubMed Central Google Scholar * Ding, J. et al. Pore-forming
activity and structural autoinhibition of the gasdermin family. _Nature_ 535, 111 (2016). CAS PubMed Google Scholar * Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis
by forming membrane pores. _Nature_ 535, 153 (2016). CAS PubMed PubMed Central Google Scholar * Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1
secretion from living macrophages. _Immunity_ 48, 35–44 (2018). CAS PubMed Google Scholar * Monteleone, M. et al. Interleukin-1β maturation triggers its relocation to the plasma membrane
for gasdermin-D-dependent and -independent secretion. _Cell Rep._ 24, 1425–1433 (2018). CAS PubMed Google Scholar * Groß, O. et al. Inflammasome activators induce interleukin-1α secretion
via distinct pathways with differential requirement for the protease function of caspase-1. _Immunity_ 36, 388–400 (2012). PubMed Google Scholar * Antonopoulos, C. et al. Caspase-8 as an
effector and regulator of NLRP3 inflammasome signaling. _J. Biol. Chem._ 290, 20167–20184 (2015). CAS PubMed PubMed Central Google Scholar * Antonopoulos, C., El Sanadi, C., Kaiser, W.
J., Mocarski, E. S. & Dubyak, G. R. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1β via caspase-8 in dendritic cells. _J. Immunol._ 191, 4789–4803
(2013). CAS PubMed Google Scholar * Bossaller, L. et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. _J.
Immunol._ 189, 5508–5512 (2012). CAS PubMed Google Scholar * Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. _Nature_ 479, 117–121 (2011). THIS STUDY
IDENTIFIES NON-CANONICAL NLRP3 ACTIVATION VIA LPS-MEDIATED CASPASE 11 ACTIVATION AND SHOWS IT IS AN IMPORTANT IMMUNE MEDIATOR DURING SEPSIS. CAS PubMed Google Scholar * Aachoui, Y. et al.
Caspase-11 protects against bacteria that escape the vacuole. _Science_ 339, 975–978 (2013). CAS PubMed PubMed Central Google Scholar * Kayagaki, N. et al. Noncanonical inflammasome
activation by intracellular LPS independent of TLR4. _Science_ 341, 1246–1249 (2013). CAS PubMed Google Scholar * Shi, J. et al. Inflammatory caspases are innate immune receptors for
intracellular LPS. _Nature_ 514, 187–192 (2014). THIS STUDY SHOWS THAT CASPASES 4, 5 AND 11 ARE INTRACELLULAR RECEPTORS FOR LPS, ACTIVATION OF WHICH INDUCES NON-CANONICAL INFLAMMASOME
ACTIVATION. CAS PubMed Google Scholar * Napier, B. A. et al. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. _J. Exp. Med._ 213,
2365–2382 (2016). CAS PubMed PubMed Central Google Scholar * Man, S. M. et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. _Cell_ 167,
382–396 (2016). CAS PubMed PubMed Central Google Scholar * Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. _Nature_ 509,
366 (2014). CAS PubMed Google Scholar * Lee, B. L. et al. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. _J. Exp. Med._ 215, 2279–2288 (2018). CAS
PubMed PubMed Central Google Scholar * Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. _Nature_ 526, 666–671 (2015). TOGETHER WITH REFERENCES
80 AND 81 , THIS PAPER SHOWS THAT CLEAVAGE OF GSDMD BY CASPASE 1, 4, 5 OR 11 DURING INFLAMMASOME ACTIVATION CAUSES PYROPTOSIS. CAS PubMed Google Scholar * Zanoni, I. et al. An endogenous
caspase-11 ligand elicits interleukin-1 release from living dendritic cells. _Science_ 352, 1232–1236 (2016). CAS PubMed PubMed Central Google Scholar * Kerur, N. et al. cGAS drives
noncanonical-inflammasome activation in age-related macular degeneration. _Nat. Med._ 24, 50–61 (2018). CAS PubMed Google Scholar * Chu, L. H. et al. The oxidized phospholipid oxPAPC
protects from septic shock by targeting the non-canonical inflammasome in macrophages. _Nat. Commun._ 9, 996 (2018). PubMed PubMed Central Google Scholar * Chen, K. W. et al. Noncanonical
inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. _Sci. Immunol._ 3, eaar6676 (2018). PubMed Google Scholar * Kahlenberg, J. M., Carmona-Rivera, C.,
Smith, C. K. & Kaplan, M. J. Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. _J. Immunol._ 190, 1217–1226 (2012).
PubMed Google Scholar * Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. _Blood_
113, 2324–2335 (2009). CAS PubMed PubMed Central Google Scholar * Piccini, A. et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta
and IL-18 secretion in an autocrine way. _Proc. Natl Acad. Sci. USA_ 105, 8067–8072 (2008). CAS PubMed PubMed Central Google Scholar * He, Y., Franchi, L. & Núñez, G. TLR agonists
stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. _J. Immunol._ 190, 334–339 (2013). CAS PubMed Google Scholar *
Lin, K.-M. et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. _Proc. Natl Acad. Sci. USA_ 111, 775–780 (2014). CAS PubMed Google Scholar *
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against _Salmonella_. _J. Exp. Med._ 207, 1745–1755 (2010). CAS PubMed PubMed Central Google
Scholar * Kalantari, P. et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. _Cell Rep._ 6, 196–210 (2014). CAS PubMed PubMed
Central Google Scholar * Karki, R. et al. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. _Cell Host Microbe_ 17,
357–368 (2015). CAS PubMed PubMed Central Google Scholar * Freeman, L. et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. _J. Exp.
Med._ 214, 1351–1370 (2017). CAS PubMed PubMed Central Google Scholar * Swanson, K. V. et al. A noncanonical function of cGAMP in inflammasome priming and activation. _J. Exp. Med._ 214,
3611–3626 (2017). PubMed PubMed Central Google Scholar * Man, S. M. et al. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. _Proc.
Natl Acad. Sci. USA_ 111, 7403–7408 (2014). CAS PubMed PubMed Central Google Scholar * Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic
bacteria and DNA viruses. _Nat. Immunol._ 11, 395–402 (2010). CAS PubMed PubMed Central Google Scholar * Gaidt, M. M. et al. The DNA inflammasome in human myeloid cells is initiated by a
STING-cell death program upstream of NLRP3. _Cell_ 171, 1110–1124 (2017). CAS PubMed PubMed Central Google Scholar * Liu, J., Qian, C. & Cao, X. Post-translational modification
control of innate immunity. _Immunity_ 45, 15–30 (2016). PubMed Google Scholar * Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation.
_J. Biol. Chem._ 287, 36617–36622 (2012). CAS PubMed PubMed Central Google Scholar * Han, S. et al. Lipopolysaccharide primes the NALP3 inflammasome by inhibiting its ubiquitination and
degradation mediated by the SCFFBXL2 E3 ligase. _J. Biol. Chem._ 290, 18124–18133 (2015). CAS PubMed PubMed Central Google Scholar * Song, H. et al. The E3 ubiquitin ligase TRIM31
attenuates NLRP3 inflammasome activation by promoting proteasomal degradation of NLRP3. _Nat. Commun._ 7, 13727 (2016). CAS PubMed PubMed Central Google Scholar * Yan, Y. et al. Dopamine
controls systemic inflammation through inhibition of NLRP3 inflammasome. _Cell_ 160, 62–73 (2015). CAS PubMed Google Scholar * Py, B. F., Kim, M.-S., Vakifahmetoglu-Norberg, H. &
Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. _Mol. Cell_ 49, 331–338 (2013). CAS PubMed Google Scholar * Rodgers, M. A. et al. The linear
ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. _J. Exp. Med._ 211, 1333–1347 (2014). CAS PubMed PubMed Central Google Scholar * Song, N. et al. NLRP3
phosphorylation is an essential priming event for inflammasome activation. _Mol. Cell_ 68, 185–197 (2017). CAS PubMed Google Scholar * Zhang, Z. et al. Protein kinase D at the Golgi
controls NLRP3 inflammasome activation. _J. Exp. Med._ 214, 2671–2693 (2017). CAS PubMed PubMed Central Google Scholar * Stutz, A. et al. NLRP3 inflammasome assembly is regulated by
phosphorylation of the pyrin domain. _J. Exp. Med._ 214, 1725–1736 (2017). CAS PubMed PubMed Central Google Scholar * Spalinger, M. R. et al. NLRP3 tyrosine phosphorylation is controlled
by protein tyrosine phosphatase PTPN22. _J. Clin. Invest._ 126, 1783–1800 (2016). PubMed PubMed Central Google Scholar * Mortimer, L., Moreau, F., MacDonald, J. A. & Chadee, K. NLRP3
inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. _Nat. Immunol._ 17, 1176 (2016). CAS PubMed Google Scholar * Guo, C. et al. Bile acids
control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. _Immunity_ 45, 802–816 (2016). CAS PubMed Google Scholar * Barry, R. et al. SUMO-mediated regulation
of NLRP3 modulates inflammasome activity. _Nat. Commun._ 9, 3001 (2018). PubMed PubMed Central Google Scholar * Indramohan, M., Stehlik, C. & Dorfleutner, A. COPs and POPs patrol
inflammasome activation. _J. Mol. Biol._ 430, 153–173 (2018). CAS PubMed Google Scholar * Bedoya, F., Sandler, L. L. & Harton, J. A. Pyrin-only protein 2 modulates NF-kappaB and
disrupts ASC:CLR interactions. _J. Immunol._ 178, 3837–3845 (2007). CAS PubMed Google Scholar * Ratsimandresy, R. A. et al. The PYRIN domain-only protein POP2 inhibits inflammasome
priming and activation. _Nat. Commun._ 8, 15556 (2017). CAS PubMed PubMed Central Google Scholar * Periasamy, S. et al. Pyrin-only protein 2 limits inflammation but improves protection
against bacteria. _Nat. Commun._ 8, 15564 (2017). CAS PubMed PubMed Central Google Scholar * de Almeida, L. et al. The PYRIN domain-only protein POP1 inhibits inflammasome assembly and
ameliorates inflammatory disease. _Immunity_ 43, 264–276 (2015). PubMed PubMed Central Google Scholar * Dinarello, C. A., Simon, A. & Van Der Meer, J. W. Treating inflammation by
blocking interleukin-1 in a broad spectrum of diseases. _Nat. Rev. Drug Discov._ 11, 633 (2012). CAS PubMed PubMed Central Google Scholar * Ozaki, E., Campbell, M. & Doyle, S. L.
Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. _J. Inflamm. Res._ 8, 15 (2015). CAS PubMed PubMed Central Google Scholar * Brydges, S. D. et al.
Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. _Immunity_ 30, 875–887 (2009). CAS PubMed PubMed Central Google Scholar * Laliberte, R. E.
et al. Glutathione S-transferase omega 1–1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1β posttranslational processing. _J. Biol.
Chem._ 278, 16567–16578 (2003). CAS PubMed Google Scholar * Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. _Nat.
Med._ 21, 248–255 (2015). THIS STUDY SHOWS THAT THE SMALL MOLECULE MCC950 SPECIFICALLY INHIBITS NLRP3 INFLAMMASOME ACTIVATION AND IS EFFECTIVE IN NLRP3-ACTIVATED MOUSE DISEASE MODELS. CAS
PubMed PubMed Central Google Scholar * Dempsey, C. et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1
mice. _Brain Behav. Immun._ 61, 306–316 (2017). CAS PubMed Google Scholar * Ismael, S., Nasoohi, S. & Ishrat, T. MCC950, the selective NLRP3 inflammasome inhibitor protects mice
against traumatic brain injury. _J. Neurotrauma_ 35, 1294–1303 (2018). PubMed PubMed Central Google Scholar * van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces
atherosclerotic lesion development in apolipoprotein E–deficient mice — brief report. _Arterioscler. Thromb. Vasc. Biol._ 37, 1457–1461 (2017). PubMed Google Scholar * Monnerat, G. et al.
Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. _Nat. Commun._ 7, 13344 (2016). CAS PubMed PubMed Central Google Scholar * Van Hout, G. P. et al. The
selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. _Eur. Heart J._ 38, 828–836 (2016). Google Scholar
* Zhai, Y. et al. Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. _Molecules_ 23, 522 (2018). PubMed Central Google Scholar *
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. _J. Hepatol._ 66, 1037–1046 (2017). CAS PubMed PubMed Central Google
Scholar * Perera, A. P. et al. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. _Sci. Rep._ 8, 8618 (2018).
PubMed PubMed Central Google Scholar * Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. _J. Exp. Med._ 214, 3219–3238 (2017). CAS
PubMed PubMed Central Google Scholar * Cocco, M. et al. Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. _J. Med.
Chem._ 60, 3656–3671 (2017). CAS PubMed Google Scholar * Darakhshan, S. & Pour, A. B. Tranilast: a review of its therapeutic applications. _Pharmacol. Res._ 91, 15–28 (2015). CAS
PubMed Google Scholar * Huang, Y. et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. _EMBO Mol. Med._ 10, e8689 (2018). PubMed PubMed Central Google Scholar
* Ma, Z., Hu, C. & Zhang, Y. Therapeutic effect of Rabdosia rubescens aqueous extract on chronic pharyngitis and its safety [Chinese]. _Zhong Nan Da Xue Xue Bao Yi Xue Ban_ 36, 170–173
(2011). CAS PubMed Google Scholar * He, H. et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. _Nat. Commun._ 9, 2550 (2018). PubMed PubMed Central
Google Scholar * Baldwin, A. G. et al. Boron-based inhibitors of the NLRP3 inflammasome. _Cell Chem. Biol._ 24, 1321–1335 (2017). CAS PubMed PubMed Central Google Scholar * MacKenzie,
S. H., Schipper, J. L. & Clark, A. C. The potential for caspases in drug discovery. _Curr. Opin. Drug Discov. Develop._ 13, 568–576 (2010). CAS Google Scholar * Duewell, P. et al.
NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. _Nature_ 464, 1357–1361 (2010). CAS PubMed PubMed Central Google Scholar * Martinon, F.,
Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. _Nature_ 440, 237–241 (2006). CAS PubMed Google Scholar *
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. _Nat. Immunol._ 11, 897–904 (2010). CAS
PubMed PubMed Central Google Scholar * Mulay, S. R. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. _J. Clin. Invest._ 123, 236–246 (2013).
CAS PubMed Google Scholar * Lai, M. et al. The NLRP3-caspase 1 inflammasome negatively regulates autophagy via TLR4-TRIF in prion peptide-infected microglia. _Front. Aging Neurosci._ 10,
116 (2018). PubMed PubMed Central Google Scholar * Niemi, K. et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. _J. Immunol._
186, 6119–6128 (2011). CAS PubMed Google Scholar * Babelova, A. et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. _J. Biol. Chem._
284, 24035–24048 (2009). CAS PubMed PubMed Central Google Scholar * Yamasaki, K. et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan,
an endogenous trigger of inflammation in response to injury. _J. Biol. Chem._ 284, 12762–12771 (2009). CAS PubMed PubMed Central Google Scholar * Baron, L. et al. The NLRP3 inflammasome
is activated by nanoparticles through ATP, ADP and adenosine. _Cell Death Dis._ 6, e1629 (2015). CAS PubMed PubMed Central Google Scholar * Martinon, F., Agostini, L., Meylan, E. &
Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. _Curr. Biol._ 14, 1929–1934 (2004). CAS PubMed Google Scholar * Abdul-Sater, A.
A. et al. Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome. _EMBO Rep._ 14, 900–906 (2013). CAS PubMed PubMed Central Google Scholar * Sha, W. et al. Human NLRP3
inflammasome senses multiple types of bacterial RNAs. _Proc. Natl Acad. Sci. USA_ 111, 16059–16064 (2014). CAS PubMed PubMed Central Google Scholar * Kailasan Vanaja, S. et al. Bacterial
RNA:DNA hybrids are activators of the NLRP3 inflammasome. _Proc. Natl Acad. Sci. USA_ 111, 7765–7770 (2014). PubMed PubMed Central Google Scholar * Schweneker, K. et al. The
mycobacterial cord factor adjuvant analogue trehalose-6,6′-dibehenate (TDB) activates the Nlrp3 inflammasome. _Immunobiology_ 218, 664–673 (2013). CAS PubMed Google Scholar * Greaney, A.
J., Leppla, S. H. & Moayeri, M. Bacterial exotoxins and the inflammasome. _Front. Immunol._ 6, 570 (2015). PubMed PubMed Central Google Scholar * Gurcel, L., Abrami, L., Girardin, S.,
Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. _Cell_ 126, 1135–1145 (2006). CAS
PubMed Google Scholar * Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. _Nature_ 440, 228–232 (2006). CAS PubMed Google Scholar * Mathur, A.
et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. _Nat. Microbiol._ 4, 362–374 (2019). CAS PubMed Google Scholar
* Lamkanfi, M., Malireddi, R. K. & Kanneganti, T. D. Fungal zymosan and mannan activate the cryopyrin inflammasome. _J. Biol. Chem._ 284, 20574–20581 (2009). CAS PubMed PubMed
Central Google Scholar * Kankkunen, P. et al. (1,3)-beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. _J. Immunol._ 184, 6335–6342 (2010). CAS PubMed
Google Scholar * He, Y. et al. 3, 4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 activation by blocking assembly of the inflammasome. _J. Biol. Chem._ 289, 1142–1150 (2013). PubMed PubMed
Central Google Scholar * Marchetti, C. et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation.
_Proc. Natl Acad. Sci. USA_ 115, E1530–E1539 (2018). CAS PubMed PubMed Central Google Scholar * Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct
inhibitors of the inflammasome. _J. Biol. Chem._ 285, 9792–9802 (2010). CAS PubMed PubMed Central Google Scholar * Shim, D.-W. et al. BOT-4-one attenuates NLRP3 inflammasome activation:
NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. _Sci. Rep._ 7, 15020 (2017). PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS This Review was supported by the National Center for Advancing Translational Sciences, US National Institutes of Health (NIH), through grant KL2TR002490 awarded to K.V.S.
and by the NIH through grants AI029564, CA156330, DK094779, AI109965 and AI067798 awarded to J.P.-Y.T. The content is solely the responsibility of the authors and does not necessarily
represent the official views of the NIH. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medicine, Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill,
NC, USA Karen V. Swanson * Oral and Craniofacial Biomedicine Program, School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Meng Deng * Lineberger
Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Meng Deng & Jenny P.-Y. Ting * Department of Genetics, University of North Carolina at
Chapel Hill, Chapel Hill, NC, USA Jenny P.-Y. Ting * Institute for Inflammatory Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Jenny P.-Y. Ting * Center for
Translational Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA Jenny P.-Y. Ting Authors * Karen V. Swanson View author publications You can also search for this
author inPubMed Google Scholar * Meng Deng View author publications You can also search for this author inPubMed Google Scholar * Jenny P.-Y. Ting View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS All the authors contributed equally to all aspects of the article. CORRESPONDING AUTHOR Correspondence to Jenny P.-Y. Ting.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. GLOSSARY * Leucine-rich repeat domain (LRR domain). In Toll-like receptors (TLRs), the LRR domain mediates the detection of microbial
components; it may serve a similar role in certain NLRs (NACHT–LRR proteins). The LRR domain of NLRs and TLRs is structurally similar. It consists of leucine-rich amino acid strands forming
a peptide loop. The loops occur as tandem repeats that together form a coil or solenoid and contain constant sequences, as well as unique insertions or variable residues for each ligand. *
AIM2 A sensor that combines with the adaptor protein ASC and the protease caspase 1 to form the AIM2 inflammasome. It senses cytosolic double-stranded DNA from bacteria or viruses or from
mislocalized self-DNA and contributes to infection defence. * P2X purinoceptor 7 (P2X7). An ATP-gated cation channel that is expressed by haematopoietic cells and participates in cell
proliferation and apoptosis. It belongs to the family of purinoceptors for ATP and is responsible for the ATP-dependent activation of NLRP3 (NOD-, LRR- and pyrin domain-containing protein
3). * Caecal ligation and puncture An experimental model of peritonitis in rodents, in which the caecum is ligated and then punctured, thereby forming a small hole. This leads to leakage of
intestinal bacteria into the peritoneal cavity and subsequent peritoneal infection. * Mitophagy The selective removal of mitochondria by macroautophagy under conditions of nutrient
starvation or mitochondrial stress. * Oxidative stress Cells continuously produce reactive oxygen species (ROS) such as hydrogen peroxide or superoxide anions. Under physiological
conditions, mitochondria are the main source, and cellular antioxidants ensure that the redox equilibrium is maintained. During inflammatory responses (and in cancer), excessive production
of ROS leads to a metabolic condition known as oxidative stress, which can lead to apoptosis and necrosis. * Autophagy A cytoplasmic bulk degradation system in which cytoplasmic cargo is
targeted and is typically sequestered in double-membrane vesicles, leading to subsequent fusion with the lysosome. This process is essential for the response to starvation because it
facilitates the recycling of cellular components. In addition, autophagy can be targeted to intracellular bacteria to restrict their growth. * Urate crystal model A mouse model of
crystal-induced peritonitis that activates the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome. * Pyroptosis A lytic, inflammatory form of programmed cell death that is
triggered by cleavage of gasdermin D by the inflammatory caspase 1, 4, 5 or 11. It is characterized by cytoplasmic swelling, early plasma membrane rupture and nuclear condensation. The
cytoplasmic content is released into the extracellular space, and this is thought to augment inflammatory and repair responses. * Neutrophil extracellular traps (NETs). Fibrous networks that
are released into the extracellular environment by neutrophils. They are composed mainly of DNA but also contain proteins from neutrophil granules. NETs act as a mesh that traps
microorganisms and exposes them to neutrophil-derived effector molecules. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Swanson, K.V., Deng, M. &
Ting, J.PY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. _Nat Rev Immunol_ 19, 477–489 (2019). https://doi.org/10.1038/s41577-019-0165-0 Download citation *
Published: 29 April 2019 * Issue Date: August 2019 * DOI: https://doi.org/10.1038/s41577-019-0165-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative