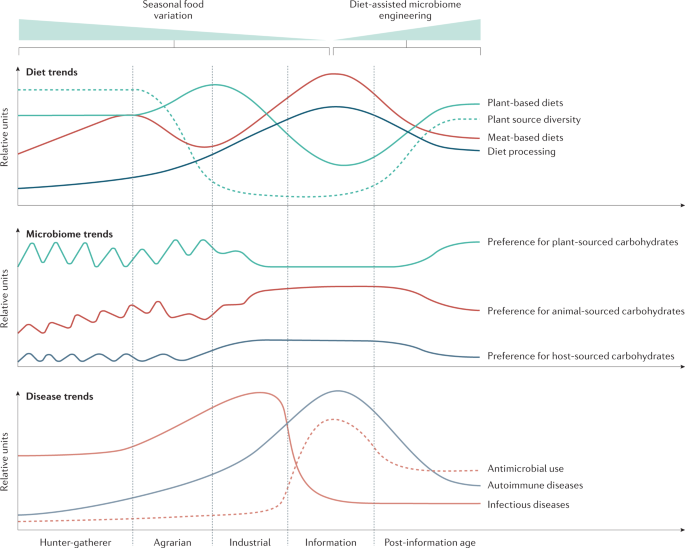
Leveraging diet to engineer the gut microbiome
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Autoimmune diseases, including inflammatory bowel disease, multiple sclerosis and rheumatoid arthritis, have distinct clinical presentations but share underlying patterns of gut
microbiome perturbation and intestinal barrier dysfunction. Their potentially common microbial drivers advocate for treatment strategies aimed at restoring appropriate microbiome function,
but individual variation in host factors makes a uniform approach unlikely. In this Perspective, we consolidate knowledge on diet–microbiome interactions in local inflammation, gut
microbiota imbalance and host immune dysregulation. By understanding and incorporating the effects of individual dietary components on microbial metabolic output and host physiology, we
examine the potential for diet-based therapies for autoimmune disease prevention and treatment. We also discuss tools targeting the gut microbiome, such as faecal microbiota transplantation,
probiotics and orthogonal niche engineering, which could be optimized using custom dietary interventions. These approaches highlight paths towards leveraging diet for precise engineering of
the gut microbiome at a time of increasing autoimmune disease. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS
OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn
more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to
full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our
FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS THE IMPACT OF THE GUT MICROBIOME ON EXTRA-INTESTINAL AUTOIMMUNE DISEASES Article 09 May 2022 ENGINEERING THE GUT
MICROBIOME Article 16 June 2023 MICROBIOTA IN INFLAMMATORY BOWEL DISEASE: MECHANISMS OF DISEASE AND THERAPEUTIC OPPORTUNITIES Article 10 March 2025 REFERENCES * Bach, J. F. The effect of
infections on susceptibility to autoimmune and allergic diseases. _N. Engl. J. Med._ 347, 911–920 (2002). PubMed Google Scholar * Markle, J. G. M. et al. Sex differences in the gut
microbiome drive hormone-dependent regulation of autoimmunity. _Science_ 339, 1084–1088 (2013). CAS PubMed Google Scholar * Inshaw, J. R. J., Cutler, A. J., Burren, O. S., Stefana, M. I.
& Todd, J. A. Approaches and advances in the genetic causes of autoimmune disease and their implications. _Nat. Immunol._ 19, 674–684 (2018). CAS PubMed Google Scholar * Thorburn, A.
N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. _Immunity_ 40, 833–842 (2014). CAS PubMed Google Scholar * Bach, J. F. The hygiene
hypothesis in autoimmunity: the role of pathogens and commensals. _Nat. Rev. Immunol._ 18, 105–120 (2018). CAS PubMed Google Scholar * Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. &
Elinav, E. Dysbiosis and the immune system. _Nat. Rev. Immunol._ 17, 219–232 (2017). CAS PubMed Google Scholar * Fugger, L., Jensen, L. T. & Rossjohn, J. Challenges, progress, and
prospects of developing therapies to treat autoimmune diseases. _Cell_ 181, 63–80 (2020). CAS PubMed Google Scholar * Ananthakrishnan, A. N. et al. Gut microbiome function predicts
response to anti-integrin biologic therapy in inflammatory bowel diseases. _Cell Host Microbe_ 21, 603–610.e3 (2017). CAS PubMed PubMed Central Google Scholar * Doherty, M. K. et al.
Fecal microbiota signatures are associated with response to ustekinumab therapy among Crohn’s disease patients. _mBio_ 9, e02120-17 (2018). PubMed PubMed Central Google Scholar * Scher,
J. U., Nayak, R. R., Ubeda, C., Turnbaugh, P. J. & Abramson, S. B. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. _Nat. Rev.
Rheumatol._ 16, 282–292 (2020). PubMed Google Scholar * Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. _Cell Res._ 30, 492–506
(2020). PubMed PubMed Central Google Scholar * Wasko, N. J., Nichols, F. & Clark, R. B. Multiple sclerosis, the microbiome, TLR2, and the hygiene hypothesis. _Autoimmun. Rev._ 19,
102430 (2020). CAS PubMed Google Scholar * Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host–microbiota interactions in immune-mediated diseases. _Nat. Rev. Microbiol._ 18, 521–538
(2020). CAS PubMed Google Scholar * Cekanaviciute, E. et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. _Proc. Natl
Acad. Sci. USA_ 114, 10713–10718 (2017). CAS PubMed PubMed Central Google Scholar * Khalili, H. et al. The role of diet in the aetiopathogenesis of inflammatory bowel disease. _Nat. Rev.
Gastroenterol. Hepatol._ 15, 525–535 (2018). CAS PubMed PubMed Central Google Scholar * Willett, W. et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from
sustainable food systems. _Lancet_ 393, 447–492 (2019). PubMed Google Scholar * Cordain, L. et al. Origins and evolution of the Western diet: health implications for the 21st century. _Am.
J. Clin. Nutr._ 81, 341–354 (2005). CAS PubMed Google Scholar * Kaoutari, A. E., Armougom, F., Gordon, J. I., Raoult, D. & Henrissat, B. The abundance and variety of
carbohydrate-active enzymes in the human gut microbiota. _Nat. Rev. Microbiol._ 11, 497–504 (2013). PubMed Google Scholar * Smits, S. A. et al. Seasonal cycling in the gut microbiome of
the Hadza hunter-gatherers of Tanzania. _Science_ 357, 802–806 (2017). CAS PubMed PubMed Central Google Scholar * Lombard, V., Golaconda, R. H., Drula, E., Coutinho, P. & Henrissat,
B. BT2824 – the carbohydrate-active enzymes database (CAZy) in 2013. _Nucleic Acids Res_ 42, D490–D495 (2014). CAS PubMed Google Scholar * Manzel, A. et al. Role of ‘western diet’ in
inflammatory autoimmune diseases. _Curr. Allergy Asthma Rep._ 14, 404 (2014). PubMed PubMed Central Google Scholar * Konijeti, G. G. et al. Efficacy of the autoimmune protocol diet for
inflammatory bowel disease. _Inflamm. Bowel Dis._ 23, 2054–2060 (2017). PubMed Google Scholar * Damas, O. M., Garces, L. & Abreu, M. T. Diet as adjunctive treatment for inflammatory
bowel disease: review and update of the latest literature. _Curr. Treat. Options Gastroenterol._ 17, 313–325 (2019). PubMed PubMed Central Google Scholar * Sonnenburg, E. D. et al.
Diet-induced extinctions in the gut microbiota compound over generations. _Nature_ 529, 212–215 (2016). CAS PubMed PubMed Central Google Scholar * Chandrasekaran, A. et al. The
autoimmune protocol diet modifies intestinal RNA expression in inflammatory bowel disease. _Crohns Colitis 360_ 1, otz016 (2019). PubMed PubMed Central Google Scholar * Klein, E. Y. et
al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. _Proc. Natl Acad. Sci. USA_ 115, e3463–e3470 (2018). CAS PubMed PubMed Central Google
Scholar * Postler, T. S. & Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. _Cell Metab._ 26, 110–130 (2017). CAS
PubMed PubMed Central Google Scholar * Camilleri, M. Leaky gut: mechanisms, measurement and clinical implications in humans. _Gut_ 68, 1516–1526 (2019). CAS PubMed Google Scholar *
Paray, B. A., Albeshr, M. F., Jan, A. T. & Rather, I. A. Leaky gut and autoimmunity: an intricate balance in individuals health and the diseased state. _Int. J. Mol. Sci._ 21, 9770
(2020). CAS PubMed Central Google Scholar * Li, B., Selmi, C., Tang, R., Gershwin, M. E. & Ma, X. The microbiome and autoimmunity: a paradigm from the gut–liver axis. _Cell. Mol.
Immunol._ 15, 595–609 (2018). PubMed PubMed Central Google Scholar * Márquez, A. et al. Meta-analysis of Immunochip data of four autoimmune diseases reveals novel single-disease and
cross-phenotype associations. _Genome Med._ 10, 97 (2018). PubMed PubMed Central Google Scholar * Kolodziejczyk, A. A., Zheng, D. & Elinav, E. Diet–microbiota interactions and
personalized nutrition. _Nat. Rev. Microbiol._ 17, 742–753 (2019). CAS PubMed Google Scholar * Inda, M. E., Broset, E., Lu, T. K. & de la Fuente-Nunez, C. Emerging Frontiers in
Microbiome Engineering. _Trends Immunol._ 40, 952–973 (2019). CAS PubMed Google Scholar * Ruder, W. C., Lu, T. & Collins, J. J. Synthetic biology moving into the clinic. _Science_
333, 1248–1252 (2011). CAS PubMed Google Scholar * O’Keefe, S. J. D. et al. Fat, fibre and cancer risk in African Americans and rural Africans. _Nat. Commun._ 6, 6342 (2015). PubMed
Google Scholar * Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. _Nat. Med._ 27, 321–332 (2021). CAS PubMed
PubMed Central Google Scholar * Parker, A., Fonseca, S. & Carding, S. R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. _Gut Microbes_
11, 135–157 (2020). PubMed Google Scholar * Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. _Cell_ 158,
705–721 (2014). CAS PubMed PubMed Central Google Scholar * Al Nabhani, Z. et al. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. _Immunity_
50, 1276–1288.e5 (2019). CAS PubMed Google Scholar * Kalbermatter, C., Fernandez Trigo, N., Christensen, S. & Ganal-Vonarburg, S. C. Maternal microbiota, early life colonization and
breast milk drive immune development in the newborn. _Front. Immunol._ 12, 683022 (2021). CAS PubMed PubMed Central Google Scholar * Bokulich, N. A. et al. Antibiotics, birth mode, and
diet shape microbiome maturation during early life. _Sci. Transl. Med._ 8, 343ra82 (2016). PubMed PubMed Central Google Scholar * Mahmoud, T. I. et al. Autoimmune manifestations in aged
mice arise from early-life immune dysregulation. _Sci. Transl. Med._ 8, 361ra137 (2016). PubMed PubMed Central Google Scholar * Vatanen, T. et al. The human gut microbiome in early-onset
type 1 diabetes from the TEDDY study. _Nature_ 562, 589–594 (2018). CAS PubMed PubMed Central Google Scholar * Lawson, M. A. E. et al. Breast milk-derived human milk oligosaccharides
promote Bifidobacterium interactions within a single ecosystem. _ISME J._ 14, 635–648 (2020). CAS PubMed Google Scholar * Fanning, S. et al. Bifidobacterial surface-exopolysaccharide
facilitates commensal-host interaction through immune modulation and pathogen protection. _Proc. Natl Acad. Sci. USA_ 109, 2108–2113 (2012). CAS PubMed PubMed Central Google Scholar *
Beaumont, M. et al. Gut microbiota derived metabolites contribute to intestinal barrier maturation at the suckling-to-weaning transition. _Gut Microbes_ 11, 1268–1286 (2020). CAS PubMed
PubMed Central Google Scholar * Zegarra-Ruiz, D. F. et al. A diet-sensitive commensal lactobacillus strain mediates TLR7-dependent systemic autoimmunity. _Cell Host Microbe_ 25, 113–127.e6
(2019). CAS PubMed Google Scholar * D’Hennezel, E., Abubucker, S., Murphy, L. O. & Cullen, T. W. Total lipopolysaccharide from the human gut microbiome silences toll-like receptor
signaling. _mSystems_ 2, e00046-17 (2017). PubMed PubMed Central Google Scholar * Fujiwara, M. et al. Enhanced TLR2 responses in multiple sclerosis. _Clin. Exp. Immunol._ 193, 313–326
(2018). CAS PubMed PubMed Central Google Scholar * Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. _Science_ 331, 337–341 (2011). CAS
PubMed Google Scholar * Ohkura, N. et al. Regulatory T cell-specific epigenomic region variants are a key determinant of susceptibility to common autoimmune diseases. _Immunity_ 52,
1119–1132.e4 (2020). CAS PubMed Google Scholar * Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. _Immunity_ 43, 727–738 (2015).
CAS PubMed PubMed Central Google Scholar * Cosorich, I. et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis.
_Sci. Adv._ 3, e1700492 (2017). PubMed PubMed Central Google Scholar * Buscarinu M. C. et al. Altered intestinal permeability in patients with relapsing–remitting multiple sclerosis: a
pilot study. _Mult. Scler_. 23, 442–446 (2017). PubMed Google Scholar * Zhang, X., Chen, B. D., Zhao, L. D. & Li, H. The gut microbiota: emerging evidence in autoimmune diseases.
_Trends Mol. Med._ 26, 862–873 (2020). CAS PubMed Google Scholar * Deehan, E. C. et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty
acid production. _Cell Host Microbe_ 27, 389–404.e6 (2020). CAS PubMed Google Scholar * Swanson, K. S. et al. The International Scientific Association for Probiotics and Prebiotics
(ISAPP) consensus statement on the definition and scope of synbiotics. _Nat. Rev. Gastroenterol. Hepatol._ 17, 687–701 (2020). PubMed PubMed Central Google Scholar * Hvas, C. L. et al.
Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. _Gastroenterology_ 156, 1324–1332.e3 (2019). PubMed Google Scholar *
Carmody, R. N. et al. Diet dominates host genotype in shaping the murine gut microbiota. _Cell Host Microbe_ 17, 72–84 (2015). CAS PubMed Google Scholar * Wu, G. D. et al. Comparative
metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. _Gut_ 65, 63–72 (2016). CAS PubMed Google Scholar * Venkataraman, A. et al.
Variable responses of human microbiomes to dietary supplementation with resistant starch. _Microbiome_ 4, 33 (2016). CAS PubMed PubMed Central Google Scholar * Sheflin, A. M., Melby, C.
L., Carbonero, F. & Weir, T. L. Linking dietary patterns with gut microbial composition and function. _Gut Microbes_ 8, 113–129 (2017). CAS PubMed Google Scholar * Vangay, P. et al.
US immigration westernizes the human gut microbiome. _Cell_ 175, 962–972.e10 (2018). CAS PubMed PubMed Central Google Scholar * Johnson, A. J. et al. Daily sampling reveals personalized
diet-microbiome associations in humans. _Cell Host Microbe_ 25, 789–802.e5 (2019). CAS PubMed Google Scholar * Makki, K., Deehan, E. C., Walter, J. & Bäckhed, F. The impact of dietary
fiber on gut microbiota in host health and disease. _Cell Host Microbe_ 23, 705–715 (2018). CAS PubMed Google Scholar * McNulty, N. P. et al. Effects of diet on resource utilization by a
model human gut microbiota containing bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. _PLoS Biol._ 11, e1001637 (2013). CAS PubMed PubMed Central Google
Scholar * Flint, H. J., Scott, K. P., Duncan, S. H., Louis, P. & Forano, E. Microbial degradation of complex carbohydrates in the gut. _Gut Microbes_ 3, 289–306 (2012). PubMed PubMed
Central Google Scholar * Tanes, C. et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. _Cell Host Microbe_ 29, 394–407.e5 (2021). CAS PubMed
PubMed Central Google Scholar * Déjean, G. et al. Synergy between cell surface glycosidases and glycan-binding proteins dictates the utilization of specific beta(1,3)-glucans by human gut
Bacteroides. _mBio_ 11, e00095-20 (2020). PubMed PubMed Central Google Scholar * Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and
enhances pathogen susceptibility. _Cell_ 167, 1339–1353.e21 (2016). CAS PubMed PubMed Central Google Scholar * Ananthakrishnan, A. N. Epidemiology and risk factors for IBD. _Nat. Rev.
Gastroenterol. Hepatol._ 12, 205–217 (2015). PubMed Google Scholar * So, D. et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and
meta-analysis. _Am. J. Clin. Nutr._ 107, 965–983 (2018). PubMed Google Scholar * Liu, F. et al. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but
reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. _Sci. Rep._ 7, 11789 (2017). PubMed PubMed Central Google Scholar * Valcheva, R. et al.
Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. _Gut Microbes_ 10, 334–357 (2019). CAS PubMed Google
Scholar * Salyers, A. A., West, S. E. H., Vercellotti, J. R. & Wilkins, T. D. Fermentation of mucins and plant polysaccharides by anaerobic bacteria from the human colon. _Appl.
Environ. Microbiol._ 34, 529–533 (1977). CAS PubMed PubMed Central Google Scholar * Chung, W. S. F. et al. Modulation of the human gut microbiota by dietary fibres occurs at the species
level. _BMC Biol._ 14, 3 (2016). PubMed PubMed Central Google Scholar * Goodman, A. L. et al. Extensive personal human gut microbiota culture collections characterized and manipulated in
gnotobiotic mice. _Proc. Natl Acad. Sci. USA_ 108, 6252–6257 (2011). CAS PubMed PubMed Central Google Scholar * Elzinga, J., van der Oost, J., de Vos, W. M. & Smidt, H. The use of
defined microbial communities to model host-microbe interactions in the human gut. _Microbiol. Mol. Biol. Rev._ 83, e00054-18 (2019). PubMed PubMed Central Google Scholar * Gibson, G. R.
et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. _Nat. Rev.
Gastroenterol. Hepatol._ 14, 491–502 (2017). PubMed Google Scholar * Singh, V. et al. Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer. _Cell_ 175,
679–394.e22 (2018). CAS PubMed PubMed Central Google Scholar * Yao, C. K. & Staudacher, H. M. The low-fibre diet: contender in IBD, or has it had its time? _Lancet Gastroenterol.
Hepatol._ 4, 339 (2019). PubMed Google Scholar * Wastyk, H. C. et al. Gut microbiota-targeted diets modulate human immune status. _Cell_ 184, 4137–4153 (2020). Google Scholar *
Ananthakrishnan, A. N. et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. _Gastroenterology_ 145, 970–977 (2013). CAS PubMed
Google Scholar * Andersen, V. et al. Fibre intake and the development of inflammatory bowel disease: a European prospective multi-centre cohort study (EPIC-IBD). _J. Crohns Colitis_ 12,
129–136 (2018). PubMed Google Scholar * Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients
with ulcerative colitis. _Gut_ 63, 1275–1283 (2014). CAS PubMed Google Scholar * Earley, H., Lennon, G., Coffey, J. C., Winter, D. C. & O’Connell, P. R. Colonisation of the colonic
mucus gel layer with butyrogenic and hydrogenotropic bacteria in health and ulcerative colitis. _Sci. Rep._ 11, 7262 (2021). CAS PubMed PubMed Central Google Scholar * Venegas, D. P. et
al. Short chain fatty acids (SCFAs) mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. _Front. Immunol._ 10, 277 (2019). CAS Google Scholar *
Wong, A. C. & Levy, M. New approaches to microbiome-based therapies. _mSystems_ 4, e00122-19 (2019). PubMed PubMed Central Google Scholar * Salminen, S. et al. The International
Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. _Nat. Rev. Gastroenterol. Hepatol._
https://doi.org/10.1038/s41575-021-00440-6 (2021). Article PubMed PubMed Central Google Scholar * Baxter, N. T. et al. Dynamics of human gut microbiota and short-chain fatty acids in
response to dietary interventions with three fermentable fibers. _mBio_ 10, e02566-18 (2019). PubMed PubMed Central Google Scholar * Sünderhauf, A. et al. Loss of mucosal p32/gC1qR/HABP1
triggers energy deficiency and impairs goblet cell differentiation in ulcerative colitis. _Cell. Mol. Gastroenterol. Hepatol._ 12, 229–250 (2021). PubMed PubMed Central Google Scholar *
Byndloss, M. X. et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. _Science_ 357, 570–575 (2017). CAS PubMed PubMed Central Google Scholar *
Zeng, M. Y., Inohara, N. & Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. _Mucosal Immunol._ 10, 18–26 (2017). CAS PubMed Google Scholar * Furusawa, Y. et
al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. _Nature_ 504, 446–450 (2013). CAS PubMed Google Scholar * Louis, P. & Flint, H. J.
Formation of propionate and butyrate by the human colonic microbiota. _Environ. Microbiol._ 19, 29–41 (2017). CAS PubMed Google Scholar * Duscha, A. et al. Propionic acid shapes the
multiple sclerosis disease course by an immunomodulatory mechanism. _Cell_ 180, 1067–1080.e16 (2020). CAS PubMed Google Scholar * Cohen, A. B. et al. Dietary patterns and self-reported
associations of diet with symptoms of inflammatory bowel disease. _Dig. Dis. Sci._ 58, 1322–1328 (2013). CAS PubMed Google Scholar * Levine, A. et al. Crohn’s disease exclusion diet plus
partial enteral nutrition induces sustained remission in a randomized controlled trial. _Gastroenterology_ 157, 440–450.e8 (2019). PubMed Google Scholar * Horwat, P. et al. Influence of
enteral nutrition on gut microbiota composition in patients with Crohn’s disease: a systematic review. _Nutrients_ 12, 2551 (2020). CAS PubMed Central Google Scholar * Walton, C. et al.
Enteral feeding reduces metabolic activity of the intestinal microbiome in Crohn’s disease: an observational study. _Eur. J. Clin. Nutr._ 70, 1052–1056 (2016). CAS PubMed Google Scholar *
Cignarella, F. et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. _Cell Metab._ 27, 1222–1235.e6 (2018). CAS PubMed PubMed Central Google
Scholar * Wang, H. et al. Dietary non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in IL-10 knockout mice. _J. Crohns Colitis_ 10, 1076–1086 (2016).
PubMed Google Scholar * Richards, J. L., Yap, Y. A., McLeod, K. H., MacKay, C. R. & Marinõ, E. Dietary metabolites and the gut microbiota: an alternative approach to control
inflammatory and autoimmune diseases. _Clin. Transl. Immunol._ 5, e82 (2016). Google Scholar * Macfarlane, G. T., Gibson, G. R., Beatty, E. & Cummings, J. H. Estimation of short-chain
fatty acid production from protein by human intestinal bacteria based on branched-chain fatty acid measurements. _FEMS Microbiol. Lett._ 101, 81–88 (1992). CAS Google Scholar * Llewellyn,
S. R. et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. _Gastroenterology_ 154, 1037–1046.e2 (2018). PubMed Google
Scholar * Kostovcikova, K. et al. Diet rich in animal protein promotes pro-inflammatory macrophage response and exacerbates colitis in mice. _Front. Immunol._ 10, 919 (2019). CAS PubMed
PubMed Central Google Scholar * Dallas, D. C. et al. Personalizing protein nourishment. _Crit. Rev. Food Sci. Nutr._ 57, 3313–3331 (2017). CAS PubMed PubMed Central Google Scholar *
Portune, K. J. et al. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. _Trends Food Sci. Technol._ 57, 213–232 (2016). CAS Google
Scholar * Gibson, G. R., Cummings, J. H. & Macfarlane, G. T. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative
colitis. _FEMS Microbiol. Lett._ 86, 103–111 (1991). CAS Google Scholar * Sridharan, G. V. et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. _Nat.
Commun._ 5, 5492 (2014). CAS PubMed Google Scholar * Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. _Cell Host Microbe_
23, 716–724 (2018). CAS PubMed Google Scholar * Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. _Nat.
Med._ 22, 598–605 (2016). CAS PubMed PubMed Central Google Scholar * Islam, J. et al. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon
receptor in mice. _J. Nutr. Biochem._ 42, 43–50 (2017). CAS PubMed Google Scholar * Kepert, I. et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic
airway disease. _J. Allergy Clin. Immunol._ 139, 1525–1535 (2017). CAS PubMed Google Scholar * Sonner, J. K. et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with
gut microbial ecology. _Nat. Commun._ 10, 4877 (2019). PubMed PubMed Central Google Scholar * Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and
colitis in Il10−/− mice. _Nature_ 487, 104–108 (2012). CAS PubMed PubMed Central Google Scholar * Watanabe, M., Fukiya, S. & Yokota, A. Comprehensive evaluation of the bactericidal
activities of free bile acids in the large intestine of humans and rodents. _J. Lipid Res._ 58, 1143–1152 (2017). CAS PubMed PubMed Central Google Scholar * Casadevall, A. The pathogenic
potential of a microbe. _mSphere_ 2, e00015-17 (2017). PubMed PubMed Central Google Scholar * Holscher, H. D. et al. Walnut consumption alters the gastrointestinal microbiota,
microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. _J. Nutr._ 148, 861–867 (2018). PubMed PubMed Central Google Scholar * Kim,
K. A., Gu, W., Lee, I. A., Joh, E. H. & Kim, D. H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. _PLoS ONE_ 7, e47713
(2012). CAS PubMed PubMed Central Google Scholar * Cani, P. D. et al. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional
glucagon-like peptide 1 receptor. _Diabetes_ 55, 1484–1490 (2006). CAS PubMed Google Scholar * Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in
humans. _Cell_ 165, 842–853 (2016). CAS PubMed PubMed Central Google Scholar * Steimle, A. et al. Weak agonistic LPS restores intestinal immune homeostasis. _Mol. Ther._ 27, 1974–1991
(2019). CAS PubMed PubMed Central Google Scholar * Ang, Q. Y. et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. _Cell_ 181, 1263–1275.e16
(2020). CAS PubMed PubMed Central Google Scholar * Lam, Y. Y. et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in
mice. _Obesity_ 23, 1429–1439 (2015). CAS PubMed Google Scholar * Wolters, M. et al. Dietary fat, the gut microbiota, and metabolic health–a systematic review conducted within the
MyNewGut project. _Clin. Nutr._ 38, 2504–2520 (2019). PubMed Google Scholar * De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children
from Europe and rural Africa. _Proc. Natl Acad. Sci. USA_ 107, 14691–14696 (2010). PubMed PubMed Central Google Scholar * Watson, H. et al. A randomised trial of the effect of omega-3
polyunsaturated fatty acid supplements on the human intestinal microbiota. _Gut_ 67, 1974–1983 (2018). CAS PubMed Google Scholar * Tindall, A. M., McLimans, C. J., Petersen, K. S.,
Kris-Etherton, P. M. & Lamendella, R. Walnuts and vegetable oils containing oleic acid differentially affect the gut microbiota and associations with cardiovascular risk factors:
follow-up of a randomized, controlled, feeding trial in adults at risk for cardiovascular disease. _J. Nutr._ 150, 806–817 (2020). PubMed Google Scholar * Swidsinski, A. et al. Reduced
mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. _Front. Microbiol._ 8, 1141 (2017). PubMed PubMed Central Google
Scholar * Kong, C. et al. Ketogenic diet alleviates colitis by reduction of colonic group 3 innate lymphoid cells through altering gut microbiome. _Signal. Transduct. Target. Ther._ 6, 154
(2021). CAS PubMed PubMed Central Google Scholar * Ni, F.-F. et al. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy.
_Seizure_ 38, 17–22 (2016). PubMed Google Scholar * Monteiro, C. A. et al. Ultra-processed foods: what they are and how to identify them. _Public. Health Nutr._ 22, 936–941 (2019). PubMed
Google Scholar * Zinöcker, M. K. & Lindseth, I. A. The western diet–microbiome–host interaction and its role in metabolic disease. _Nutrients_ 10, 365 (2018). PubMed Central Google
Scholar * Carmody, R. N. et al. Cooking shapes the structure and function of the gut microbiome. _Nat. Microbiol._ 4, 2052–2063 (2019). PubMed PubMed Central Google Scholar * Koppel, N.,
Rekdal, V. M. & Balskus, E. P. Chemical transformation of xenobiotics by the human gut microbiota. _Science_ 356, eaag2770 (2017). PubMed Google Scholar * Arcila, J. A. & Rose, D.
J. Repeated cooking and freezing of whole wheat flour increases resistant starch with beneficial impacts on in vitro fecal fermentation properties. _J. Funct. Foods_ 12, 230–236 (2015). CAS
Google Scholar * Lerner, A. & Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune
disease. _Autoimmun. Rev._ 14, 479–489 (2015). CAS PubMed Google Scholar * Obih, C. et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within
an academic IBD center. _Nutrition_ 32, 418–425 (2016). PubMed Google Scholar * Cox, S. R. et al. Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in
patients with quiescent inflammatory bowel disease in a randomized trial. _Gastroenterology_ 158, 176–188.e7 (2020). CAS PubMed Google Scholar * Chassaing, B. et al. Dietary emulsifiers
impact the mouse gut microbiota promoting colitis and metabolic syndrome. _Nature_ 519, 92–96 (2015). CAS PubMed PubMed Central Google Scholar * Stephen, A. M. et al. Dietary fibre in
Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. _Nutr. Res. Rev._ 30, 149–190 (2017). CAS PubMed Google Scholar * Logan,
M. et al. Analysis of 61 exclusive enteral nutrition formulas used in the management of active Crohn’s disease — new insights into dietary disease triggers. _Aliment. Pharmacol. Ther._ 51,
935–947 (2020). CAS PubMed PubMed Central Google Scholar * Brüssow, H. Problems with the concept of gut microbiota dysbiosis. _Microb. Biotechnol._ 13, 423–434 (2020). PubMed Google
Scholar * Volkova, A. & Ruggles, K. V. Predictive metagenomic analysis of autoimmune disease identifies robust autoimmunity and disease specific microbial signatures. _Front.
Microbiol._ 12, 621310 (2021). PubMed PubMed Central Google Scholar * Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. _Cell_ 163, 1079–1094 (2015). CAS
PubMed Google Scholar * Zmora, N. et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. _Cell_ 174,
1388–1405.e21 (2018). CAS PubMed Google Scholar * Korem, T. et al. Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. _Cell Metab._ 25,
1243–1253.e5 (2017). CAS PubMed Google Scholar * The Adaptive Platform Trials Coalition Adaptive platform trials: definition, design, conduct and reporting considerations. _Nat. Rev. Drug
Discov._ 18, 797–807 (2019). Google Scholar * Holzinger, D., Kessel, C., Omenetti, A. & Gattorno, M. From bench to bedside and back again: translational research in autoinflammation.
_Nat. Rev. Rheumatol._ 11, 573–585 (2015). CAS PubMed Google Scholar * Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. _Nat. Rev.
Microbiol._ 14, 20–32 (2016). CAS PubMed Google Scholar * Wei, Y. et al. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of
diversity of gut flora. _BMC Microbiol._ 16, 255 (2016). PubMed PubMed Central Google Scholar * Kearney, S. M., Gibbons, S. M., Erdman, S. E. & Alm, E. J. Orthogonal dietary niche
enables reversible engraftment of a gut bacterial commensal. _Cell Rep._ 24, 1842–1851 (2018). CAS PubMed PubMed Central Google Scholar * Shepherd, E. S., Deloache, W. C., Pruss, K. M.,
Whitaker, W. R. & Sonnenburg, J. L. An exclusive metabolic niche enables strain engraftment in the gut microbiota. _Nature_ 557, 434–438 (2018). CAS PubMed PubMed Central Google
Scholar * Quraishi, M. N. et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile
infection. _Aliment. Pharmacol. Ther._ 46, 479–493 (2017). CAS PubMed Google Scholar * de Groot, P. et al. Faecal microbiota transplantation halts progression of human new-onset type 1
diabetes in a randomised controlled trial. _Gut_ 70, 92–105 (2021). PubMed Google Scholar * Engen, P. A. et al. Single-arm, non-randomized, time series, single-subject study of fecal
microbiota transplantation in multiple sclerosis. _Front. Neurol._ 11, 978 (2020). PubMed PubMed Central Google Scholar * Van Beurden, Y. H. et al. Serendipity in refractory celiac
disease: full recovery of duodenal villi and clinical symptoms after fecal microbiota transfer. _J. Gastrointest. Liver Dis._ 25, 385–388 (2016). Google Scholar * Zeng, J. et al. Fecal
microbiota transplantation for rheumatoid arthritis: a case report. _Clin. Case Rep._ 9, 906–909 (2021). PubMed Google Scholar * Costello, S. P. et al. Effect of fecal microbiota
transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. _JAMA_ 321, 156–164 (2019). PubMed PubMed Central Google Scholar * Moayyedi, P. et
al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. _Gastroenterology_ 149, 102–109.e6 (2015). PubMed Google
Scholar * Paramsothy, S. et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. _Lancet_ 389, 1218–1228 (2017).
PubMed Google Scholar * Rossen, N. G. et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. _Gastroenterology_ 149, 110–118.e4
(2015). PubMed Google Scholar * Vaughn, B. P. et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. _Inflamm. Bowel Dis._ 22,
2182–2190 (2016). PubMed Google Scholar * Cui, B. et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: safety, feasibility, and efficacy trial results.
_J. Gastroenterol. Hepatol._ 30, 51–58 (2015). CAS PubMed Google Scholar * Philips, C. A. et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic
hepatitis: a pilot study. _Clin. Gastroenterol. Hepatol._ 15, 600–602 (2017). PubMed Google Scholar * Mullish, B. H., McDonald, J. A. K., Thursz, M. R. & Marchesi, J. R. Fecal
microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. _Hepatol_ 66, 1354–1355 (2017). Google Scholar * Kootte, R. S. et al.
Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. _Cell Metab._ 26, 611–619.e6 (2017). CAS PubMed
Google Scholar * Kang, D. W. et al. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. _Sci. Rep._ 9, 5821 (2019). PubMed PubMed Central Google
Scholar * Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. _Nature_ 569, 655–662 (2019). CAS PubMed PubMed Central Google Scholar *
Dailey, F. E., Turse, E. P., Daglilar, E. & Tahan, V. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. _Curr. Opin. Pharmacol._
49, 29–33 (2019). CAS PubMed Google Scholar * Wilson, B. C., Vatanen, T., Cutfield, W. S. & O’Sullivan, J. M. The super-donor phenomenon in fecal microbiota transplantation. _Front.
Cell. Infect. Microbiol._ 9, 2 (2019). CAS PubMed PubMed Central Google Scholar * Knox, N. C., Forbes, J. D., Van Domselaar, G. & Bernstein, C. N. The gut microbiome as a target for
IBD treatment: are we there yet? _Curr. Treat. Options Gastroenterol._ 17, 115–126 (2019). PubMed Google Scholar * Burrello, C. et al. Therapeutic faecal microbiota transplantation
controls intestinal inflammation through IL10 secretion by immune cells. _Nat. Commun._ 9, 5184 (2018). PubMed PubMed Central Google Scholar * Jang, Y. O. et al. Fecal microbial
transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. _Exp. Mol. Med._ 52, 1128–1139 (2020). CAS PubMed PubMed Central Google
Scholar * Anhê, F. F. et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. _Gut_
68, 453–464 (2019). PubMed Google Scholar * Petrof, E. O. et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut.
_Microbiome_ 1, 3 (2013). PubMed PubMed Central Google Scholar * Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. _Nature_ 565, 600–605
(2019). CAS PubMed Google Scholar * Sood, A. et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. _Clin. Gastroenterol.
Hepatol._ 7, 1202–1209 (2009). PubMed Google Scholar * Derwa, Y., Gracie, D. J., Hamlin, P. J. & Ford, A. C. Systematic review with meta-analysis: the efficacy of probiotics in
inflammatory bowel disease. _Aliment. Pharmacol. Ther._ 46, 389–400 (2017). CAS PubMed Google Scholar * Ganji-Arjenaki, M. & Rafieian-Kopaei, M. Probiotics are a good choice in
remission of inflammatory bowel diseases: a meta analysis and systematic review. _J. Cell. Physiol._ 233, 2091–2103 (2018). CAS PubMed Google Scholar * Shigemori, S. & Shimosato, T.
Applications of genetically modified immunobiotics with high immunoregulatory capacity for treatment of inflammatory bowel diseases. _Front. Immunol._ 8, 22 (2017). PubMed PubMed Central
Google Scholar * Sales-Campos, H., Soares, S. C. & Oliveira, C. J. F. An introduction of the role of probiotics in human infections and autoimmune diseases. _Crit. Rev. Microbiol._ 45,
413–432 (2019). PubMed Google Scholar * Flach, J., van der Waal, M. B., van den Nieuwboer, M., Claassen, E. & Larsen, O. F. A. The underexposed role of food matrices in probiotic
products: reviewing the relationship between carrier matrices and product parameters. _Crit. Rev. Food Sci. Nutr._ 58, 2570–2584 (2018). CAS PubMed Google Scholar * Cassani, L.,
Gomez-Zavaglia, A. & Simal-Gandara, J. Technological strategies ensuring the safe arrival of beneficial microorganisms to the gut: from food processing and storage to their passage
through the gastrointestinal tract. _Food Res. Int._ 129, 108852 (2020). CAS PubMed Google Scholar * Bezkorovainy, A. Probiotics: determinants of survival and growth in the gut. _Am. J.
Clin. Nutr._ 73 (Suppl. 2), 399–405 (2001). Google Scholar * Maldonado-Gómez, M. X. et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized
features of the resident microbiome. _Cell Host Microbe_ 20, 515–526 (2016). PubMed Google Scholar * Suez, J. et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by
probiotics and improved by autologous FMT. _Cell_ 174, 1406–1423.e16 (2018). CAS PubMed Google Scholar * Sorbara, M. T. & Pamer, E. G. Interbacterial mechanisms of colonization
resistance and the strategies pathogens use to overcome them. _Mucosal Immunol._ 12, 1–9 (2019). CAS PubMed Google Scholar * Champagne, C. P., Gardner, N. J. & Roy, D. Challenges in
the addition of probiotic cultures to foods. _Crit. Rev. Food Sci. Nutr._ 45, 61–84 (2005). CAS PubMed Google Scholar * Fujimori, S. et al. A randomized controlled trial on the efficacy
of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. _Nutrition_ 25, 520–525 (2009). PubMed Google Scholar * Amiriani,
T. et al. Effect of Lactocare® synbiotic on disease severity in ulcerative colitis: a randomized placebo-controlled double-blind clinical trial. _Middle East. J. Dig. Dis._ 12, 27–33 (2020).
PubMed PubMed Central Google Scholar * Furrie, E. et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative
colitis: a randomised controlled pilot trial. _Gut_ 54, 242–249 (2005). CAS PubMed PubMed Central Google Scholar * Steed, H. et al. Clinical trial: the microbiological and immunological
effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. _Aliment. Pharmacol. Ther._ 32, 872–883 (2010). CAS PubMed Google Scholar
* Zamani, B., Farshbaf, S., Golkar, H. R., Bahmani, F. & Asemi, Z. Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: a
randomised, double-blind, placebo-controlled trial. _Br. J. Nutr._ 117, 1095–1102 (2017). CAS PubMed Google Scholar * Zare Javid, A., Aminzadeh, M., Haghighi-Zadeh, M. H. &
Jamalvandi, M. The effects of synbiotic supplementation on glycemic status, lipid profile, and biomarkers of oxidative stress in type 1 diabetic patients. A placebo-controlled, double-blind,
randomized clinical trial. _Diabetes Metab. Syndr. Obes._ 13, 607–617 (2020). PubMed PubMed Central Google Scholar * Chen, L., Yang, T., Song, Y., Shu, G. & Chen, H. Effect of
xanthan-chitosan-xanthan double layer encapsulation on survival of Bifidobacterium BB01 in simulated gastrointestinal conditions, bile salt solution and yogurt. _LWT Food Sci. Technol._ 81,
274–280 (2017). CAS Google Scholar * Fratianni, F. et al. Ability of synbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated
gastrointestinal conditions. _J. Microencapsul._ 31, 299–305 (2014). CAS PubMed Google Scholar * Cook, M. T., Tzortzis, G., Charalampopoulos, D. & Khutoryanskiy, V. V.
Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. _Int. J. Pharm._ 466, 400–408 (2014). CAS PubMed Google Scholar * Hehemann, J. H. et al. Transfer of
carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. _Nature_ 464, 908–912 (2010). CAS PubMed Google Scholar * Pudlo, N. A. et al. Extensive transfer of genes for
edible seaweed digestion from marine to human gut bacteria. Preprint at _bioRxiv_ https://doi.org/10.1101/2020.06.09.142968 (2020). Article Google Scholar * Walter, J., Britton, R. A.
& Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. _Proc. Natl Acad. Sci. USA_ 108, 4645–4652 (2011). CAS PubMed
Google Scholar * Martínez, I. et al. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. _Cell Rep._ 11, 527–538 (2015). PubMed
Google Scholar * Mu, Q. et al. Control of lupus nephritis by changes of gut microbiota. _Microbiome_ 5, 73 (2017). PubMed PubMed Central Google Scholar * He, B. et al. Lactobacillus
reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. _Front. Immunol._ 10, 385 (2019). CAS PubMed PubMed Central Google Scholar
* De Moreno De Leblanc, A. et al. Evaluation of the biosafety of recombinant lactic acid bacteria designed to prevent and treat colitis. _J. Med. Microbiol._ 65, 1038–1046 (2016). PubMed
Google Scholar * Zeng, L. et al. An engineering probiotic producing defensin-5 ameliorating dextran sodium sulfate-induced mice colitis via Inhibiting NF-κB pathway. _J. Transl. Med._ 18,
107 (2020). CAS PubMed PubMed Central Google Scholar * Lerner, A., Matthias, T. & Aminov, R. Potential effects of horizontal gene exchange in the human gut. _Front. Immunol._ 8, 1630
(2017). PubMed PubMed Central Google Scholar * Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. _Lancet_ 393, 434–445 (2019).
CAS PubMed Google Scholar * Scher, J. U. et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. _eLi__fe_ 2, e01202 (2013). PubMed PubMed
Central Google Scholar * Kjeldsen-Kragh, J. et al. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. _Lancet_ 338, 899–902 (1991). CAS PubMed Google
Scholar * Stoll, M. L. Genetics, Prevotella, and the pathogenesis of rheumatoid arthritis. _Lancet Rheumatol._ 2, e375–e376 (2020). Google Scholar * Peltonen, R. et al. Faecal microbial
flora and disease activity in rheumatoid arthritis during a vegan diet. _Br. J. Rheumatol._ 36, 64–68 (1997). CAS PubMed Google Scholar * Charbonneau, M. R., Isabella, V. M., Li, N. &
Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. _Nat. Commun._ 11, 1738 (2020). CAS PubMed PubMed Central Google Scholar *
FitzGerald, M. J. & Spek, E. J. Microbiome therapeutics and patent protection. _Nat. Biotechnol._ 38, 806–810 (2020). CAS PubMed Google Scholar * Lloyd-Price, J., Abu-Ali, G. &
Huttenhower, C. The healthy human microbiome. _Genome Med._ 8, 51 (2016). PubMed PubMed Central Google Scholar * Schellekens, H. et al. Bifidobacterium longum counters the effects of
obesity: partial successful translation from rodent to human. _EBioMedicine_ 63, 103176 (2021). CAS PubMed Google Scholar * Fragiadakis, G. K. et al. Long-term dietary intervention
reveals resilience of the gut microbiota despite changes in diet and weight. _Am. J. Clin. Nutr._ 111, 1127–1136 (2020). PubMed PubMed Central Google Scholar * Genoni, A. et al. Long-term
Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. _Eur. J. Nutr._ 59, 1845–1848 (2020). CAS
PubMed Google Scholar * Saresella, M. et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. _Front. Immunol._ 8,
1391 (2017). PubMed PubMed Central Google Scholar * Laffin, M. et al. A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in
mice. _Sci. Rep._ 9, 12294 (2019). PubMed PubMed Central Google Scholar * Rodriguez-Palacios, A. et al. The artificial sweetener Splenda promotes gut proteobacteria, dysbiosis, and
myeloperoxidase reactivity in Crohn’s disease-like ileitis. _Inflamm. Bowel Dis._ 24, 1005–1020 (2018). PubMed PubMed Central Google Scholar * Grabinger, T. et al. Alleviation of
intestinal inflammation by oral supplementation with 2-fucosyllactose in mice. _Front. Microbiol._ 10, 1385 (2019). PubMed PubMed Central Google Scholar * Berer, K. et al. Dietary
non-fermentable fiber prevents autoimmune neurological disease by changing gut metabolic and immune status. _Sci. Rep._ 8, 10431 (2018). PubMed PubMed Central Google Scholar * Chen, K. et
al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. _Mol. Nutr. Food Res._ 61, 1601006
(2017). Google Scholar * Gudi, R. et al. Complex dietary polysaccharide modulates gut immune function and microbiota, and promotes protection from autoimmune diabetes. _Immunology_ 157,
70–85 (2019). CAS PubMed PubMed Central Google Scholar * Rosser, E. C. et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in
regulatory B cells. _Cell Metab._ 31, 837–851.e10 (2020). CAS PubMed PubMed Central Google Scholar * Zhang, T. et al. Sodium butyrate reduces colitogenic immunoglobulin A-coated bacteria
and modifies the composition of microbiota in IL-10 deficient mice. _Nutrients_ 8, 728 (2016). PubMed Central Google Scholar * Choi, S. C. et al. Gut microbiota dysbiosis and altered
tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. _Sci. Transl. Med._ 12, eaax2220 (2020). CAS PubMed PubMed Central Google Scholar * Alrafas, H. R., Busbee, P.
B., Nagarkatti, M. & Nagarkatti, P. S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. _J.
Leukoc. Biol._ 106, 467–480 (2019). CAS PubMed Google Scholar * Constante, M., Fragoso, G., Calvé, A., Samba-Mondonga, M. & Santos, M. M. Dietary heme induces gut dysbiosis,
aggravates colitis, and potentiates the development of adenomas in mice. _Front. Microbiol._ 8, 1809 (2017). PubMed PubMed Central Google Scholar * Lee, T. et al. Oral versus intravenous
iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. _Gut_ 66, 863–871 (2016). PubMed Google Scholar * Miranda, P. M. et al. High salt diet
exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. _Microbiome_ 6, 57 (2018). PubMed PubMed Central Google Scholar * Wilck, N. et al. Salt-responsive
gut commensal modulates TH17 axis and disease. _Nature_ 551, 585–589 (2017). CAS PubMed PubMed Central Google Scholar * Eaton, S. B. & Konner, M. Paleolithic nutrition: a
consideration of its nature and current implications. _N. Engl. J. Med._ 312, 283–289 (1985). CAS PubMed Google Scholar * Cordain, L. et al. Plant-animal subsistence ratios and
macronutrient energy estimations in worldwide hunter-gatherer diets. _Am. J. Clin. Nutr._ 71, 682–692 (2000). CAS PubMed Google Scholar * Diamond, J. Evolution, consequences and future of
plant and animal domestication. _Nature_ 418, 700–707 (2002). CAS PubMed Google Scholar * Burkitt, D. Related disease — related cause? _Lancet_ 294, 1229–1231 (1969). Google Scholar *
Burkitt, D. P., Walker, A. R. P. & Painter, N. S. Dietary fiber and disease. _JAMA_ 229, 1068–1074 (1974). CAS PubMed Google Scholar * Aries, V., Crowther, J. S., Drasar, B. S., Hill,
M. J. & Williams, R. E. Bacteria and the aetiology of cancer of the large bowel. _Gut_ 10, 334–335 (1969). CAS PubMed PubMed Central Google Scholar * Gu, P. & Feagins, L. A.
Dining with inflammatory bowel disease: a review of the literature on diet in the pathogenesis and management of IBD. _Inflamm. Bowel Dis._ 26, 181–191 (2020). PubMed Google Scholar *
Sabino, J., Lewis, J. D. & Colombel, J. F. Treating inflammatory bowel disease with diet: a taste test. _Gastroenterology_ 157, 295–297 (2019). PubMed Google Scholar * Hou, J. K., Lee,
D. & Lewis, J. Diet and inflammatory bowel disease: review of patient-targeted recommendations. _Clin. Gastroenterol. Hepatol._ 12, 1592–1600 (2014). PubMed Google Scholar * Moayyedi,
P., Simrén, M. & Bercik, P. Evidence-based and mechanistic insights into exclusion diets for IBS. _Nat. Rev. Gastroenterol. Hepatol._ 17, 406–413 (2020). CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by the following grants in the laboratory of M.S.D.: Luxembourg National Research Fund (FNR) CORE grants (C15/BM/10318186 and
C18/BM/12585940) to M.S.D.; M.B. was supported by a European Commission Horizon 2020 Marie Skłodowska-Curie Actions individual fellowship (897408); M.W. was supported by a Fulbright grant
for Visiting Scholars from the Commission for Educational Exchange between the United States of America, Belgium and Luxembourg; E.T.G. was supported by the Luxembourg National Research Fund
PRIDE (17/11823097) and the Fondation du Pélican de Mie et Pierre Hippert-Faber, under the aegis of the Fondation de Luxembourg. G.V.P. was supported by a fellowship from the W. Garfield
Weston Foundation and E.C.M. acknowledges the financial support from National Institutes of Health (DK118024). AUTHOR INFORMATION Author notes * These authors contributed equally: Mathis
Wolter, Erica T. Grant. AUTHORS AND AFFILIATIONS * Department of Infection and Immunity, Luxembourg Institute of Health, Esch-sur-Alzette, Luxembourg Mathis Wolter, Erica T. Grant, Marie
Boudaud, Alex Steimle & Mahesh S. Desai * Faculty of Science, Technology and Medicine, University of Luxembourg, Esch-sur-Alzette, Luxembourg Mathis Wolter & Erica T. Grant *
University of Michigan Medical School, Ann Arbor, MI, USA Gabriel V. Pereira & Eric C. Martens * Odense Research Center for Anaphylaxis, Department of Dermatology and Allergy Center,
Odense University Hospital, University of Southern Denmark, Odense, Denmark Mahesh S. Desai Authors * Mathis Wolter View author publications You can also search for this author inPubMed
Google Scholar * Erica T. Grant View author publications You can also search for this author inPubMed Google Scholar * Marie Boudaud View author publications You can also search for this
author inPubMed Google Scholar * Alex Steimle View author publications You can also search for this author inPubMed Google Scholar * Gabriel V. Pereira View author publications You can also
search for this author inPubMed Google Scholar * Eric C. Martens View author publications You can also search for this author inPubMed Google Scholar * Mahesh S. Desai View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors have contributed to the writing and editing of the manuscript. CORRESPONDING AUTHOR
Correspondence to Mahesh S. Desai. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Reviews
Gastroenterology & Hepatology_ thanks R. Carmody and the other, anonymous, reviewers for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wolter, M., Grant, E.T., Boudaud, M. _et al._ Leveraging diet to engineer the gut microbiome. _Nat Rev Gastroenterol Hepatol_ 18, 885–902
(2021). https://doi.org/10.1038/s41575-021-00512-7 Download citation * Accepted: 06 August 2021 * Published: 27 September 2021 * Issue Date: December 2021 * DOI:
https://doi.org/10.1038/s41575-021-00512-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative