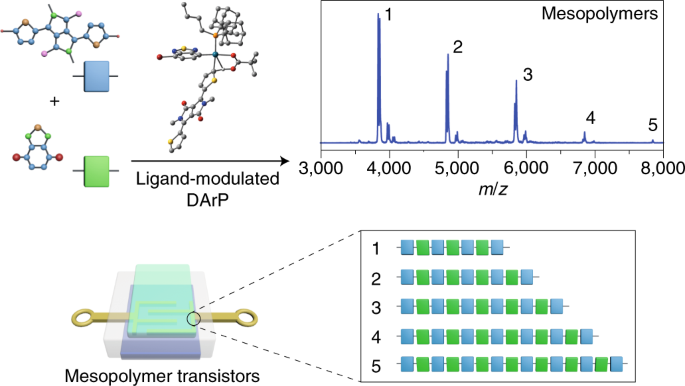
Mesopolymer synthesis by ligand-modulated direct arylation polycondensation towards n-type and ambipolar conjugated systems
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Conjugated polymers are attractive components for plastic electronics, but their structural defects, low solubility and batch-to-batch variation—mainly in terms of molecular weight
and dispersity—hinder practical applications. Here, we demonstrate that these issues can be circumvented by using conjugated mesopolymers, which have the advantages of both oligomers and
polymers. A diketopyrrolopyrrole monomer and a benzothiadiazole derivative react through direct arylation polycondensation, promoted by sterically hindered adamantyl ligand coordinated
palladium catalysts, to form mesopolymers. The reaction is facile, environmentally benign (it does not require tin or boron reagents) and occurs in high yields. The resulting mesopolymers
have a strictly alternating donor–acceptor structure, without detectable homocoupling and β-arylation defects, and exhibit number-averaged molecular weights (_M__n_) between 1 and 10 kDa.
They also show good solution processability and have significantly enhanced electron mobilities, which makes them n-type and ambipolar semiconductors, with advantages over their polymer
counterparts. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access
Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print
issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to
local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT
BEING VIEWED BY OTHERS GENERAL ROOM-TEMPERATURE SUZUKI–MIYAURA POLYMERIZATION FOR ORGANIC ELECTRONICS Article 29 January 2024 CONJUGATED POLYMERS BASED ON SELENOPHENE BUILDING BLOCKS Article
Open access 06 December 2022 EFFICIENT ROOM TEMPERATURE CATALYTIC SYNTHESIS OF ALTERNATING CONJUGATED COPOLYMERS VIA C-S BOND ACTIVATION Article Open access 10 January 2022 DATA
AVAILABILITY All relevant data supporting the findings of this study are available in this paper and its Supplementary Information. Synthetic procedures and characterization for all the new
compounds, description of the computational study and all copies of NMR spectra and GPC traces are provided in the Supplementary Information. All data are available from the corresponding
author upon reasonable request. REFERENCES * Shirakawa, H. et al. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)_x_. _J. Chem. Soc. Chem.
Commun._ 16, 578–580 (1977). Article Google Scholar * Chiang, C. K. et al. Electrical-conductivity in doped polyacetylene. _Phys. Rev. Lett._ 39, 1098–1101 (1977). Article CAS Google
Scholar * Hu, W. _Organic Optoelectronics_ (Wiley, Weinheim, 2012). * Geoghegan, M. & Hadziioannou, G. _Polymer Electronics_ (Oxford University Press, Oxford, 2013). * Tong, M. et al.
Higher molecular weight leads to improved photoresponsivity, charge transport and interfacial ordering in a narrow bandgap semiconducting polymer. _Adv. Funct. Mater._ 20, 3959–3965 (2010).
Article CAS Google Scholar * Kline, R. J., McGehee, M. D., Kadnikova, E. N., Liu, J. & Fréchet, J. M. Controlling the field-effect mobility of regioregular polythiophene by changing
the molecular weight. _Adv. Mater._ 15, 1519–1522 (2003). Article CAS Google Scholar * Hendriks, K. H. et al. Homocoupling defects in diketopyrrolopyrrole-based copolymers and their
effect on photovoltaic performance. _J. Am. Chem. Soc._ 136, 11128–11133 (2014). Article CAS Google Scholar * Wang, C., Dong, H., Hu, W., Liu, Y. & Zhu, D. Semiconducting
_π_-conjugated systems in field-effect transistors: a material odyssey of organic electronics. _Chem. Rev._ 112, 2208–2267 (2012). Article CAS Google Scholar * Jenkins, A., Kratochvil,
P., Stepto, R. & Suter, U. Glossary of basic terms in polymer science. _Pure Appl. Chem._ 68, 2287–2311 (1996). Article CAS Google Scholar * Nahid, M. M. et al. Unconventional
molecular weight dependence of charge transport in the high mobility n-type semiconducting polymer P(NDI2OD-T2). _Adv. Funct. Mater._ 27, 201604744 (2017). Article Google Scholar * Zhou,
N. et al. All-polymer solar cell performance optimized via systematic molecular weight tuning of both donor and acceptor polymers. _J. Am. Chem. Soc._ 138, 1240–1251 (2016). Article CAS
Google Scholar * Fukuta, S. et al. 2,2′-Bis(1,3,4-thiadiazole)-based _π_-conjugated copolymers for organic photovoltaics with exceeding 8% and its molecular weight dependence of device
performance. _Macromolecules_ 50, 891–899 (2017). Article CAS Google Scholar * Matsidik, R., Komber, H., Luzio, A., Caironi, M. & Sommer, M. Defect-free naphthalene diimide
bithiophene copolymers with controlled molar mass and high performance via direct arylation polycondensation. _J. Am. Chem. Soc._ 137, 6705–6711 (2015). Article CAS Google Scholar *
Facchetti, A., Vaccaro, L. & Marrocchi, A. Semiconducting polymers prepared by direct arylation polycondensation. _Angew. Chem. Int. Ed._ 51, 3520–3523 (2012). Article CAS Google
Scholar * Mercier, L. G. & Leclerc, M. Direct (hetero)arylation: a new tool for polymer chemists. _Acc. Chem. Res._ 46, 1597–1605 (2013). Article CAS Google Scholar * Okamoto, K.,
Zhang, J., Housekeeper, J. B., Marder, S. R. & Luscombe, C. K. C-H arylation reaction: atom efficient and greener syntheses of _π_-conjugated small molecules and macromolecules for
organic electronic materials. _Macromolecules_ 46, 8059–8078 (2013). Article CAS Google Scholar * Berrouard, P. et al. Synthesis of 5-alkyl [3,4-_c_] thienopyrrole-4,6-dione-based
polymers by direct heteroarylation. _Angew. Chem. Int. Ed._ 51, 2068–2071 (2012). Article CAS Google Scholar * Dudnik, A. S. et al. Tin-free direct C–H arylation polymerization for high
photovoltaic efficiency conjugated copolymers. _J. Am. Chem. Soc._ 138, 15699–15709 (2016). Article CAS Google Scholar * Grenier, F., Goudreau, K. & Leclerc, M. Robust direct
(hetero)arylation polymerization in biphasic conditions. _J. Am. Chem. Soc._ 139, 2816–2824 (2017). Article CAS Google Scholar * Yi, Z., Wang, S. & Liu, Y. Design of high-mobility
diketopyrrolopyrrole-based _π_-conjugated copolymers for organic thin-film transistors. _Adv. Mater._ 27, 3589–3606 (2015). Article CAS Google Scholar * Zaumseil, J. & Sirringhaus, H.
Electron and ambipolar transport in organic field-effect transistors. _Chem. Rev._ 107, 1296–1323 (2007). Article CAS Google Scholar * Sonar, P., Singh, S. P., Li, Y., Soh, M. S. &
Dodabalapur, A. A low-bandgap diketopyrrolopyrrole-benzothiadiazole-based copolymer for high-mobility ambipolar organic thin-film transistors. _Adv. Mater._ 22, 5409–5413 (2010). Article
CAS Google Scholar * Campeau, L.-C., Parisien, M., Leblanc, M. & Fagnou, K. Biaryl synthesis via direct arylation: establishment of an efficient catalyst for intramolecular processes.
_J. Am. Chem. Soc._ 126, 9186–9187 (2004). Article CAS Google Scholar * Campeau, L.-C., Parisien, M., Jean, A. & Fagnou, K. Catalytic direct arylation with aryl chlorides, bromides,
and iodides: intramolecular studies leading to new intermolecular reactions. _J. Am. Chem. Soc._ 128, 581–590 (2006). Article CAS Google Scholar * Gildner, P. G. & Colacot, T. J.
Reactions of the 21st century: two decades of innovative catalyst design for palladium-catalyzed cross-couplings. _Organometallics_ 34, 5497–5508 (2015). Article CAS Google Scholar *
Zapf, A., Ehrentraut, A. & Beller, M. A new highly efficient catalyst system for the coupling of nonactivated and deactivated aryl chlorides with arylboronic acids. _Angew. Chem. Int.
Ed._ 39, 4153–4155 (2000). Article CAS Google Scholar * Yang, J. et al. Bis-diketopyrrolopyrrole moiety as a promising building block to enable balanced ambipolar polymers for flexible
transistors. _Adv. Mater._ 29, 1606162 (2017). Article Google Scholar * Park, J. H., Jung, E. H., Jung, J. W. & Jo, W. H. A fluorinated phenylene unit as a building block for
high-performance n-type semiconducting polymer. _Adv. Mater._ 25, 2583–2588 (2013). Article CAS Google Scholar * Sonar, P. et al. High mobility organic thin film transistor and efficient
photovoltaic devices using versatile donor–acceptor polymer semiconductor by molecular design. _Energy Environ. Sci._ 4, 2288–2296 (2011). Article CAS Google Scholar * Kronemeijer, A. J.
et al. A selenophene-based low-bandgap donor–acceptor polymer leading to fast ambipolar logic. _Adv. Mater._ 24, 1558–1565 (2012). Article CAS Google Scholar * Yan, H. et al. A
high-mobility electron-transporting polymer for printed transistors. _Nature_ 457, 679–686 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank A.
Tenaglia for his suggestions. The authors acknowledge financial support from the Ministry of Science and Technology of China (2016YFB0401100 and 2017YFA0204503), the National Natural Science
Foundation of China (51725304, 51633006, 51703159, 51733004, 91433115 and 21875158), the Strategic Priority Research Program (XDB12030300 and XDB 12000000) of the Chinese Academy of
Sciences, the Youth Innovation Promotion Association of the Chinese Academy of Sciences and the National Program for Support of Top-notch Young Professionals. AUTHOR INFORMATION Author notes
* The authors contributed equally: Zhenjie Ni, Hanlin Wang and Huanli Dong. AUTHORS AND AFFILIATIONS * Beijing National laboratory for Molecular Sciences, Key Laboratory of Organic Solids,
Institute of Chemistry, Chinese Academy of Sciences, Beijing, China Zhenjie Ni, Hanlin Wang, Huanli Dong, Qiang Zhao & Wenping Hu * Tianjin Key Laboratory of Molecular Optoelectronic
Sciences, School of Science, Tianjin University, Tianjin, China Yanfeng Dang, Xiaotao Zhang & Wenping Hu * Collaborative Innovation Center of Chemical Science and Engineering, Tianjin,
China Wenping Hu Authors * Zhenjie Ni View author publications You can also search for this author inPubMed Google Scholar * Hanlin Wang View author publications You can also search for this
author inPubMed Google Scholar * Huanli Dong View author publications You can also search for this author inPubMed Google Scholar * Yanfeng Dang View author publications You can also search
for this author inPubMed Google Scholar * Qiang Zhao View author publications You can also search for this author inPubMed Google Scholar * Xiaotao Zhang View author publications You can
also search for this author inPubMed Google Scholar * Wenping Hu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.H. conceived the work.
Z.N. performed the synthetic experiments and characterization. H.W. and Q.Z. performed the device fabrication and analysis. Y.D. performed the computational study, X.Z. provided several
monomers. H.D. directed the synthesis and device fabrication. Z.N. and H.W. wrote the manuscript. W.H. provided overall supervision. CORRESPONDING AUTHOR Correspondence to Wenping Hu. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Synthetic procedures; Characterization, including NMR spectra and GPC traces;
Computational details RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Ni, Z., Wang, H., Dong, H. _et al._ Mesopolymer synthesis by ligand-modulated
direct arylation polycondensation towards n-type and ambipolar conjugated systems. _Nature Chem_ 11, 271–277 (2019). https://doi.org/10.1038/s41557-018-0200-y Download citation * Received:
22 May 2018 * Accepted: 30 November 2018 * Published: 28 January 2019 * Issue Date: March 2019 * DOI: https://doi.org/10.1038/s41557-018-0200-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative