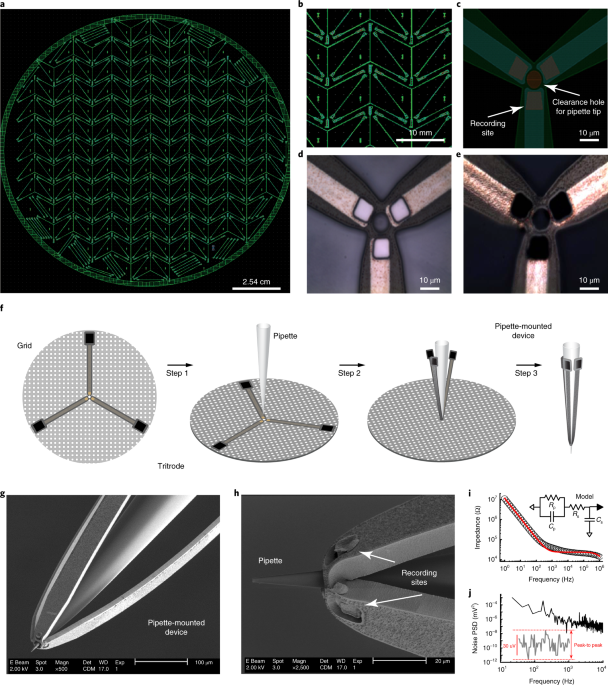
Multimodal in vivo brain electrophysiology with integrated glass microelectrodes
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Electrophysiology is the most used approach for the collection of functional data in basic and translational neuroscience, but it is typically limited to either intracellular or
extracellular recordings. The integration of multiple physiological modalities for the routine acquisition of multimodal data with microelectrodes could be useful for biomedical
applications, yet this has been challenging owing to incompatibilities of fabrication methods. Here, we present a suite of glass pipettes with integrated microelectrodes for the simultaneous
acquisition of multimodal intracellular and extracellular information in vivo, electrochemistry assessments, and optogenetic perturbations of neural activity. We used the integrated devices
to acquire multimodal signals from the CA1 region of the hippocampus in mice and rats, and show that these data can serve as ground-truth validation for the performance of spike-sorting
algorithms. The microdevices are applicable for basic and translational neurobiology, and for the development of next-generation brain–machine interfaces. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per
year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during
checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS IN VIVO
MICROELECTRODE ARRAYS FOR NEUROSCIENCE Article 08 May 2025 A SOFT, HIGH-DENSITY NEUROELECTRONIC ARRAY Article Open access 22 August 2023 IMPLANTABLE INTRACORTICAL MICROELECTRODES: REVIEWING
THE PRESENT WITH A FOCUS ON THE FUTURE Article Open access 05 January 2023 DATA AVAILABILITY The authors declare that all data supporting the findings of this study are available within the
paper and its Supplementary information. The raw data acquired in this study are available from the corresponding author on reasonable request. CODE AVAILABILITY The custom routines for
Matlab used in this work are available from the corresponding author. REFERENCES * Eccles, J. C. The synapse: from electrical to chemical transmission. _Ann. Rev. Neurosci._ 5, 325–339
(1982). Article CAS Google Scholar * Magee, J. C. Dendritic integration of excitatory synaptic input. _Nat. Rev. Neurosci._ 1, 181–190 (2000). Article CAS Google Scholar *
Schmidt-Hieber, C. & Nolan, M. F. Synaptic integrative mechanisms for spatial cognition. _Nat. Neurosci._ 20, 1483–1492 (2017). Article CAS Google Scholar * Harvey, C. D., Collman,
F., Dombeck, D. A. & Tank, D. W. Intracellular dynamics of hippocampal place cells during virtual navigation. _Nature_ 461, 941–946 (2009). Article CAS Google Scholar * Lee, D., Lin,
B. J. & Lee, A. K. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. _Science_ 337, 849–853 (2012). Article CAS Google Scholar * Long, M.
A., Jin, D. Z. & Fee, M. S. Support for a synaptic chain model of neuronal sequence generation. _Nature_ 468, 394–399 (2010). Article CAS Google Scholar * Tan, A. Y., Chen, Y.,
Scholl, B., Seidemann, E. & Priebe, N. J. Sensory stimulation shifts visual cortex from synchronous to asynchronous states. _Nature_ 509, 226–229 (2014). Article CAS Google Scholar *
Petersen, C. C. H. Whole-cell recording of neuronal membrane potential during behavior. _Neuron_ 95, 1266–1281 (2017). Article CAS Google Scholar * Poulet, J. F. A. & Petersen, C. C.
H. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. _Nature_ 454, 881–U836 (2008). Article CAS Google Scholar * Yuste, R. From the neuron
doctrine to neural networks. _Nat. Rev. Neurosci._ 16, 487–497 (2015). Article CAS Google Scholar * Buzsaki, G., Anastassiou, C. A. & Koch, C. The origin of extracellular fields and
currents—EEG, ECoG, LFP and spikes. _Nat. Rev. Neurosci._ 13, 407–420 (2012). Article CAS Google Scholar * Einevoll, G. T., Kayser, C., Logothetis, N. K. & Panzeri, S. Modelling and
analysis of local field potentials for studying the function of cortical circuits. _Nat. Rev. Neurosci._ 14, 770–785 (2013). Article CAS Google Scholar * Mazzoni, A., Logothetis, N. K.
& Panzeri, S. in _Principles of Neural Coding_ (eds Quiroga, R. D. & Panzeri, S.) 411–429 (CRC Press, 2013). * Buzsáki, G. Large-scale recording of neuronal ensembles. _Nat.
Neurosci._ 7, 446–451 (2004). Article Google Scholar * Lewicki, M. S. A review of methods for spike sorting: the detection and classification of neural action potentials. _Network_ 9,
R53–R78 (1998). Article CAS Google Scholar * Anastassiou, C. A., Perin, R., Buzsaki, G., Markram, H. & Koch, C. Cell type- and activity-dependent extracellular correlates of
intracellular spiking. _J. Neurophysiol._ 114, 608–623 (2015). Article Google Scholar * Chorev, E. & Brecht, M. In vivo dual intra- and extracellular recordings suggest bidirectional
coupling between CA1 pyramidal neurons. _J. Neurophysiol_ 108, 1584–1593 (2012). Article Google Scholar * Harris, K. D., Henze, D. A., Csicsvari, J., Hirase, H. & Buzsaki, G. Accuracy
of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. _J. Neurophysiol._ 84, 401–414 (2000). Article CAS Google Scholar * Henze, D. A. et
al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. _J. Neurophysiol._ 84, 390–400 (2000). Article CAS Google Scholar * Andrásfalvy, B. K. et al.
Quantum dot-based multiphoton fluorescent pipettes for targeted neuronal electrophysiology. _Nat. Methods_ 11, 1237–1241 (2014). Article Google Scholar * Canales, A. et al.
Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. _Nat. Biotechnol._ 33, 277–284 (2015). Article CAS Google Scholar *
LeChasseur, Y. et al. A microprobe for parallel optical and electrical recordings from single neurons in vivo. _Nat. Methods_ 8, 319–325 (2011). Article CAS Google Scholar * Katz, Y.,
Yizhar, O., Staiger, J. & Lampl, I. Optopatcher—an electrode holder for simultaneous intracellular patch-clamp recording and optical manipulation. _J. Neurosci. Methods_ 214, 113–117
(2013). Article Google Scholar * Wise, K. D. et al. Microelectrodes, microelectronics, and implantable neural microsystems. _Proc. IEEE_ 96, 1184–1202 (2008). Article CAS Google Scholar
* Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. _Nature_ 551, 232–236 (2017). * O’Keefe, J. & Recce, M. L. Phase relationship between
hippocampal place units and the EEG theta rhythm. _Hippocampus_ 3, 317–330 (1993). Article Google Scholar * Wilson, M. A. & McNaughton, B. L. Dynamics of the hippocampal ensemble code
for space. _Science_ 261, 1055–1058 (1993). Article CAS Google Scholar * Felix, S. H. et al. Insertion of flexible neural probes using rigid stiffeners attached with biodissolvable
adhesive. _J. Vis. Exp._ 79, e50609 (2013). * Fu, T. M. et al. Stable long-term chronic brain mapping at the single-neuron level. _Nat. Methods_ 13, 875–882 (2016). Article CAS Google
Scholar * Stieglitz, T., Beutel, H., Schuettler, M. & Meyer, J. U. Micromachined, polyimide-based devices for flexible neural interfaces. _Biomed. Microdevices_ 2, 283–294 (2000).
Article Google Scholar * Robinson, D. A. The electrical properties of metal microelectrodes. _Proc. IEEE_ 56, 1065–1071 (1968). Article CAS Google Scholar * Madisen, L. et al. A toolbox
of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. _Nat. Neurosci._ 15, 793–802 (2012). Article CAS Google Scholar * Robinson, D. L., Venton, B. J.,
Heien, M. L. A. V. & Wightman, R. M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. _Clin. Chem._ 49, 1763–1773 (2003). Article CAS Google Scholar *
Hamid, A. A. et al. Mesolimbic dopamine signals the value of work. _Nat. Neurosci._ 19, 117–126 (2016). Article CAS Google Scholar * Lebedev, M. A. & Nicolelis, M. A. Brain-machine
interfaces: past, present and future. _Trends Neurosci._ 29, 536–546 (2006). Article CAS Google Scholar * Bittner, K. C. et al. Conjunctive input processing drives feature selectivity in
hippocampal CA1 neurons. _Nat. Neurosci._ 18, 1133–1142 (2015). Article CAS Google Scholar * Bittner, K. C., Milstein, A. D., Grienberger, C., Romani, S. & Magee, J. C. Behavioral
time scale synaptic plasticity underlies CA1 place fields. _Science_ 357, 1033–1036 (2017). Article CAS Google Scholar * Izhikevich, E. M., Desai, N. S., Walcott, E. C. &
Hoppensteadt, F. C. Bursts as a unit of neural information: selective communication via resonance. _Trends Neurosci._ 26, 161–167 (2003). Article CAS Google Scholar * Li, C. Y. T., Poo,
M. M. & Dan, Y. Burst spiking of a single cortical neuron modifies global brain state. _Science_ 324, 643–646 (2009). Article CAS Google Scholar * Lisman, J. E. Bursts as a unit of
neural information: making unreliable synapses reliable. _Trends Neurosci._ 20, 38–43 (1997). Article CAS Google Scholar * Rey, H. G., Pedreira, C. & Quiroga, R. Q. Past, present and
future of spike sorting techniques. _Brain Res. Bull._ 119, 106–117 (2015). Article Google Scholar * Neto, J. P. et al. Validating silicon polytrodes with paired juxtacellular recordings:
method and dataset. _J. Neurophysiol._ 116, 892–903 (2016). Article CAS Google Scholar * Wild, J., Prekopcsak, Z., Sieger, T., Novak, D. & Jech, R. Performance comparison of
extracellular spike sorting algorithms for single-channel recordings. _J. Neurosci. Methods_ 203, 369–376 (2012). Article Google Scholar * Quiroga, R. Q., Nadasdy, Z. & Ben-Shaul, Y.
Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. _Neural Comput._ 16, 1661–1687 (2004). Article Google Scholar * Kadir, S. N., Goodman, D. F. &
Harris, K. D. High-dimensional cluster analysis with the masked EM algorithm. _Neural Comput._ 26, 2379–2394 (2014). Article Google Scholar * Anastassiou, C. A., Perin, R., Markram, H.
& Koch, C. Ephaptic coupling of cortical neurons. _Nat. Neurosci._ 14, 217–223 (2011). Article CAS Google Scholar * Holt, G. R. & Koch, C. Electrical interactions via the
extracellular potential near cell bodies. _J. Comput. Neurosci._ 6, 169–184 (1999). Article CAS Google Scholar * Barbic, M., Moreno, A., Harris, T. D. & Kay, M. W. Detachable glass
microelectrodes for recording action potentials in active moving organs. _Am. J. Physiol. Heart Circ. Physiol._ 312, H1248–H1259 (2017). Article Google Scholar * Lee, A. K., Epsztein, J.
& Brecht, M. Head-anchored whole-cell recordings in freely moving rats. _Nat. Protoc._ 4, 385–392 (2009). Article CAS Google Scholar * Long, M. A. & Lee, A. K. Intracellular
recording in behaving animals. _Curr. Opin. Neurobiol._ 22, 34–44 (2012). Article CAS Google Scholar * Margrie, T. W., Brecht, M. & Sakmann, B. In vivo, low-resistance, whole-cell
recordings from neurons in the anaesthetized and awake mammalian brain. _Pflügers Arch._ 444, 491–498 (2002). Article CAS Google Scholar * Vreeland, R. F. et al. Biocompatible PEDOT:
Nafion composite electrode coatings for selective detection of neurotransmitters in vivo. _Anal. Chem._ 87, 2600–2607 (2015). Article CAS Google Scholar * Atta, N. F., Galal, A. &
Ahmed, R. A. Poly(3,4-ethylene-dioxythiophene) electrode for the selective determination of dopamine in presence of sodium dodecyl sulfate. _Bioelectrochemistry_ 80, 132–141 (2011). Article
CAS Google Scholar * Tang, H., Lin, P., Chan, H. L. W. & Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. _Biosens. Bioelectron._ 26,
4559–4563 (2011). Article CAS Google Scholar * Hunt, D. L., Linaro, D., Si, B., Romani, S. & Spruston, N. A novel pyramidal cell type promotes sharp-wave synchronization in the
hippocampus. _Nat. Neurosci._ 21, 985–995 (2018). Article CAS Google Scholar * Cui, X. Y. & Martin, D. C. Electrochemical deposition and characterization of
poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. _Sens. Actuators B_ 89, 92–102 (2003). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We would like
thank A. Pais, D. Lee, V. Reddy, H. Esmailbeigi, B. Bowers, B. Biddle, J. Macklin, R. Patel, W. Sun, B. Barbarits, J. Venton, E. Privman, P. Ahamad and L. Coddington for their valuable
contributions to this study. We would also like to thank J. Markara and B. Andrasfalvy for helpful discussions. This work was supported by the Howard Hughes Medical Institute. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Howard Hughes Medical Institute, Janelia Research Campus, Ashburn, VA, USA David L. Hunt, Chongxi Lai, Richard D. Smith, Albert K. Lee, Timothy D.
Harris & Mladen Barbic Authors * David L. Hunt View author publications You can also search for this author inPubMed Google Scholar * Chongxi Lai View author publications You can also
search for this author inPubMed Google Scholar * Richard D. Smith View author publications You can also search for this author inPubMed Google Scholar * Albert K. Lee View author
publications You can also search for this author inPubMed Google Scholar * Timothy D. Harris View author publications You can also search for this author inPubMed Google Scholar * Mladen
Barbic View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.L.H., A.K.L., T.D.H. and M.B. conceived the project. T.D.H. supervised the
project. M.B. developed and fabricated all multimodal devices. A.K.L. and M.B. aquired data with the Patch-Tritrode. D.L.H. and C.L. analysed the Patch-Tritrode data. D.L.H. and M.B. aquired
data with the Patch-Silvertrode. D.L.H. analysed the Patch-Silvertrode data. R.D.S. and M.B. aquired Patch-Carbontrode data. D.L.H. and R.D.S. analysed the Patch-Carbontrode data. D.L.H.
and M.B. wrote the manuscript with input from all authors. CORRESPONDING AUTHORS Correspondence to David L. Hunt or Mladen Barbic. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary figures. REPORTING SUMMARY RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hunt, D.L.,
Lai, C., Smith, R.D. _et al._ Multimodal in vivo brain electrophysiology with integrated glass microelectrodes. _Nat Biomed Eng_ 3, 741–753 (2019). https://doi.org/10.1038/s41551-019-0373-8
Download citation * Received: 17 April 2018 * Accepted: 21 February 2019 * Published: 01 April 2019 * Issue Date: September 2019 * DOI: https://doi.org/10.1038/s41551-019-0373-8 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative