Light-dominated selection shaping filamentous cyanobacterial assemblages drives odor problem in a drinking water reservoir
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Filamentous cyanobacteria have substantial niche overlap, and the causal mechanism behind their succession remains unclear. This has practical significance since several filamentous
genera are the main producers of the musty odorant 2-methylisoborneol (MIB), which lead to odor problems in drinking water. This study investigates the relationships between two filamentous
cyanobacteria, the MIB-producing genus _Planktothrix_ and the non-MIB-producing genus _Pseudanabaena_, in a drinking water reservoir. We firstly identified their niche characteristics based
on a monitoring dataset, combined this information with culture experiments and developed a niche-based model to clarify these processes. The results reveal that the optimal light
requirements of _Pseudanabaena_ (1.56 mol m−2 d−1) are lower than those of _Planktothrix_ (3.67 mol m−2 d−1); their light niche differentiation led to a fundamental replacement of
_Planktothrix_ (2013) by _Pseudanabaena_ (2015) along with MIB decreases in this reservoir during 2013 and 2015. This study suggests that light is a major driving force responsible for the
succession between filamentous cyanobacteria, and that subtle niche differentiation may play an important role in shaping the filamentous cyanobacterial assemblages that drives the MIB odor
problems in drinking water reservoirs. SIMILAR CONTENT BEING VIEWED BY OTHERS MULTIPLE ROLES OF BAMBOO AS A REGULATOR OF CYANOBACTERIAL BLOOM IN AQUATIC SYSTEMS Article Open access 31
January 2022 DYNAMIC BACTERIAL COMMUNITY RESPONSE TO _AKASHIWO SANGUINEA_ (DINOPHYCEAE) BLOOM IN INDOOR MARINE MICROCOSMS Article Open access 26 March 2021 COMPETITIVE INTERACTIONS AS A
MECHANISM FOR CHEMICAL DIVERSITY MAINTENANCE IN _NODULARIA SPUMIGENA_ Article Open access 26 April 2021 INTRODUCTION Odor problems in source water caused by 2-methylisoborneol (MIB), a
secondary metabolite of filamentous cyanobacteria in many reservoirs and lakes1, have been a common issue in the Northern Hemisphere, and have now been moving southward2,3,4,5,6,7. The major
MIB producers include _Oscillatoria_8,9,10, _Planktothrix_11, _Phormidium_10, _Pseudanabaena_12, _Lyngbya_13 and _Planktothricoides_14. It should be noted that MIB yield varies among
different strains11,15,16,17, and some strains of the known MIB-producing species are in some cases not even able to produce MIB8,13,18. Nonetheless, MIB occurrences and concentrations are
mainly determined by the presence and abundance of MIB-producing filamentous cyanobacteria in the aquatic environment. Nutrients, water temperature and light are essential factors governing
the growth and competition of phytoplankton. Recent studies have emphasized the importance of underwater light condition on their seasonal successions in both field investigations19,20 and
numeric models21,22,23. The cellular projected area (CPA, the two-dimensional area measurement by projecting cell shape on to a plane, as defined in22) has been proposed as a key indicator
of cellular light harvesting potential, and the specific CPA (CPA/V, normalized CPA by cell volume) could be used to indicate the optimum light requirements for various species with
different cell shapes22,24. For example, the bloom-forming cyanobacteria _Microcystis_ with a low specific CPA requires high light intensity and hence is usually observed in surface water,
particularly in the summer period, while filamentous cyanobacteria having a higher specific CPA tend to live in subsurface layers, where light intensity is usually low, but nutrient
availability is high25. The low-irradiance-tolerating characteristics of filamentous cyanobacteria have been verified by laboratory culture experiments26 and field investigation27. The light
niche differentiation between filamentous cyanobacteria and other phytoplankton enables us to model their succession based on ecological niche modeling25,28,29,30. However, little is known
about the competition between different filamentous cyanobacterial genera, since they are likely to have substantial niche overlap. Therefore, it is desirable to know whether the changes in
composition of filamentous cyanobacterial assemblages are deterministic (governed by niche differentiation) or stochastic (dominated by neutral theory). QCS Reservoir is a newly constructed
estuary reservoir used as the major drinking water resource for Shanghai, China. It directly imports highly turbid water from the Yangtze River, leading to underwater light conditions that
favors filamentous cyanobacteria rather than _Microcystis_7, and therefore has suffered from MIB odor problems since it was put into use in 2011. The filamentous cyanobacterium
_Planktothrix_ was the main MIB producer according to our previous study7. From 2011 to 2015, MIB concentrations showed a decreasing pattern along with the decrease of _Planktothrix_ cell
densities and the increase of another filamentous cyanobacterium, _Pseudanabaena_. We therefore hypothesize that their competition and succession might have great impact on MIB occurrence in
this reservoir. The aim of this study is to identify the driving forces responsible for the filamentous cyanobacterial assemblages, so that it can provide scientific basis to solve the
practical MIB problem in drinking water reservoirs. Accordingly, we identified their niche characteristics based on a monitoring dataset together with culture experiments, and developed a
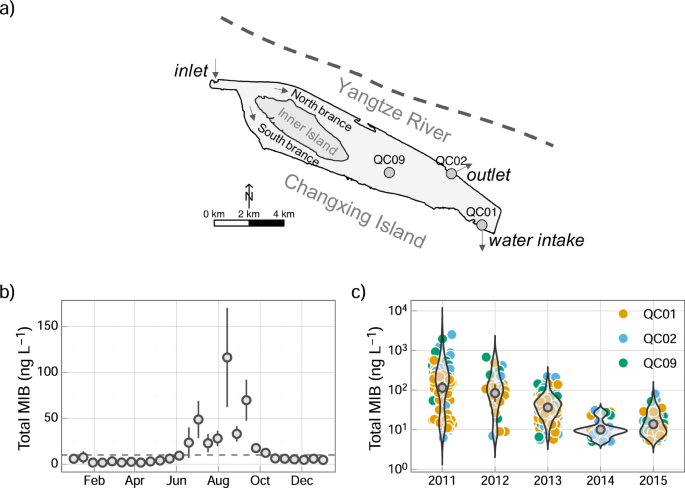
niche-based model to clarify these ecological processes. RESULTS MIB DYNAMICS IN QCS RESERVOIR MIB concentration of the river water (inlet) was rather low during the investigation
(Supplementary Fig. 1). Significant seasonal variation of MIB was observed in QCS Reservoir (Fig. 1b); higher MIB concentrations (mean: 49.2 ng L−1, range: 0.5–97.8 ng L−1) were mainly
observed during the period June to September (mean: 7.5 ng L−1, range: 0.5–12.3 ng L−1). The long-term development of MIB in June to September between 2011 and 2015 exhibited a significant
decrease (Fig. 1c). The mean concentrations in the first year were 101.0 ng L−1 (range: 0.5–257.0 ng L−1), equivalent to 6 times its human olfactory threshold (15 ng L−1,11), and thus
aroused great attention. However, in the following 2 years the mean MIB concentrations decreased to 34.2 ng L−1 (range: 0.5–107.0 ng L−1) and 29.4 ng L−1 (range: 0.5–66.4 ng L−1),
respectively. In 2014 and 2015, the concentrations further decreased to 6.2 ng L−1 (range: 0.5–15.6 ng L−1). TIME SERIES ANALYSIS OF FILAMENTOUS CYANOBACTERIA Four main filamentous
cyanobacteria were recorded during the investigation in QCS Reservoir (Supplementary Fig. 2); _Planktothrix_ (30.2%) and _Pseudanabaena_ (30.5%) exhibited higher occurrence frequencies than
_Phormidium_ (14.9%) and _Lyngbya_ (2.5%). _Lyngbya_ was only observed for eight samples, so it was not possible to identify the seasonality. _Planktothrix_ (_n_ = 175), _Pseudanabaena_ (_n_
= 168) and _Phormidium_ (_n_ = 88) were mainly observed during May to October (Supplementary Fig. 3). _Planktothrix_ was identified as the MIB producer in QCS Reservoir according to our
previous study7. _Microcystis_ dominated during August and September, which could affect the growth of filamentous cyanobacteria (Supplementary Fig. 3). Therefore, _Pseudanabaena_ was
considered as the most important competitor to _Planktothrix_ based on their seasonal distribution patterns (Supplementary Fig. 3) and their habitats. 20.5% of the variances of
_Planktothrix_ cell density could be explained by seasonal and long-term trend terms using the GAM model (Eq. (4), Supplementary Table 7, Supplementary Fig. 5). The model suggested that the
variance of _Planktothrix_ was dominated by strong seasonality (_p_ < 0.0001, Fig. 2a). During the investigation, no _Planktothrix_ were detected in February, March and April; the
earliest record of _Planktothrix_ was in May, with the mean density of 6.79 × 104 cell L−1 (0–2.04 × 105 cell L−1, 10–90% quantile, same hereinafter); the density increased in the following
3 months until late August, with a maximum of 1.01 × 106 cell L−1 (0–3.45 × 106 cell L−1); and subsequently decreased to 8.33 × 104 cell L−1 (0–1.35 × 105 cell L−1) in December and 2.78 ×
104 cell L−1 (0–3.89 × 104 cell L−1) in January. Besides, _Planktothrix_ also showed a long-term trend with a declining pattern (_p_ = 0.0915, Fig. 2b). The mean density during July to
September decreased by 93% from 1.95–2.42 × 106 cell L−1 in 2011 and 2012 to 1.40 × 105 cell L−1 in 2015. 47.7% of the variance of _Pseudanabaena_ could be explained by seasonal and
long-term trend terms (Supplementary Table 8, Supplementary Fig. 6), and a similar seasonal pattern (_p_ < 0.0001) of peak concentration (3.36 × 106 cell L−1, 0–1.306 × 107 cell L−1) in
early September (Fig. 2c). The long-term changes of _Pseudanabaena_ showed an opposite pattern (_p_ < 0.0001) to _Planktothrix_; this genus became more abundant after 2014 and has kept
increasing since then (Fig. 2d). Noted that, there was an early peak of _Pseudanabaena_ during April and June (Fig. 2c). LIMNOLOGICAL AND METEOROLOGICAL CHARACTERISTICS Figure 3 shows the
temporal distribution pattern of nutrients and meteorological parameters in QCS Reservoir. Nutrients including total nitrogen (TN), nitrate, ammonia and total phosphorus (TP) showed similar
seasonality (Supplementary Tables 1–4), with the lowest concentrations observed in August and September owing to sedimentation losses in the summer period. Regarding the inter-annual
dynamics, the TN and nitrate concentrations in 2014 were much higher than those in 2013 and 2015, ammonia showed a declining trend, while TP stayed almost unchanged between years.
Precipitation was mainly observed between May to September, highly correlated with air temperature and solar radiation (Supplementary Tables 5 and 6). It should be noted that the solar
radiation showed a declining trend from 2013 to 2015, possibly owing to the higher precipitation in the later years. Wind speed and relative humidity showed different seasonal patterns from
2013 to 2015. ECOLOGICAL NICHE MODELING OF _PLANKTOTHRIX_ AND _PSEUDANABAENA_ According to several published research31,32,33 and our previous culture experiments15,34 and field
studies7,11,35, we firstly selected water temperature, light availability, nutrients (including total nitrogen, nitrate, ammonia, total phosphorus), wind speed, and daily maximum air
temperature as the potential predictors of cyanobacterial abundance Six predictors were selected including water temperature, light availability, total N, total P, ammonia and wind speed,
according to linear models (LM1 and LM2) between these predictors (_X_) and _Planktothrix_ (\(Y_1 = \log _{10}\left( {N_1 + 1} \right)\), Supplementary Table 9) and _Pseudanabaena_ (\(Y_2 =
\log _{10}\left( {N_2 + 1} \right)\), Supplementary Table 10) associated with backward stepwise selection (Supplementary Tables 11 and 12) and variance inflation factor (VIF, Supplementary
Table 13). These predictors were subsequently classified into 4 groups based on the correlation analysis between each two predictors (Supplementary Fig. 7), which are (i) water temperature
and light availability (T&I); (ii) Total N and ammonia (TN&NH4); (iii) Total P (TP) and (iv) wind speed (WS). The interactions between the predictors within each group were
considered by modeling the interaction between T and I by bivariate tensor-product smoothers. GAM models for abundances of _Planktothrix_ (GAM1, Supplementary Table 14) and _Pseudanabaena_
(GAM2, Supplementary Table 15) were fitted with these predictors. The results suggested T&I and TN&NH4 were significantly correlated with the abundance of _Planktothrix_, while
T&I, TP and WS were significant correlated with that of _Pseudanabaena_. Based on the results above, the niche models of _Planktothrix_ (GAM3, Supplementary Table 16) and _Pseudanabaena_
(GAM4, Supplementary Table 17) were determined using their corresponding key explaining variables. In the temperature and light availability plane, both _Planktothrix_ and _Pseudanabaena_
exhibited greater abundance in high-temperature conditions (>20 °C). _Pseudanabaena_ could sustain higher abundance under lower light conditions (0.7–1.5 mol m−2 d−1, _p_ < 0.001)
compared to _Planktothrix_ (1.4~2.3 mol m−2 d−1, _p_ < 0.001), as illustrated in Fig. 4a and c. _Planktothrix_ was less abundant in high total N and ammonia conditions (_p_ = 0.007, Fig.
4b). While _Pseudanabaena_ was slightly more abundant in moderate total _P_ (_p_ = 0.064) and moderate wind speed (_p_ = 0.004) conditions. GROWTH CHARACTERISTICS OF _PLANKTOTHRIX_ AND
_PSEUDANABAENA_ UNDER DIFFERENT LIGHT DOSES Since _Planktothrix_ and _Pseudanabaena_ exhibited different growth potentials under different light conditions, a culture experiment was
performed to investigate the effect of light levels on their growth yield (Fig. 5a). The results suggested that light dose has a large impact: the cell density of _Planktothrix_ in
stationary phases (day 15–35) increased along with the light dose when it was <3.67 mol m−2 d−1, while the growth was inhibited under higher light dose. The optimum light dose for
_Pseudanabaena_ is 1.56 mol m−2 d−1, which was lower than that of _Planktothrix_. This suggested that photoinhibition exists for _Pseudanabaena_ when the light dose was >3.67 mol m−2 d−1.
RELATIONSHIP BETWEEN NICHE SPACE AND TEMPORAL TRAJECTORIES OF ENVIRONMENTAL FACTORS IN QCS RESERVOIR The focal niche spaces of _Planktothrix_ and _Pseudanabaena_ were determined with the
boundary defined by the 90% quantile of predicted abundances. The seasonal trajectories of environmental factors (water temperature & PAR) in QCS Reservoir are illustrated in Fig. 5b. In
2013, the trajectory went through the focal niche space of _Planktothrix_ in July and August, indicating that _Planktothrix_ had an advantage over _Pseudanabaena_ in this year. In the
following 2 years, especially for July and August, the trajectories followed different paths due to the lowered solar radiation and went through the niche space of _Pseudanabaena_ instead.
This probably enhanced the competitive ability of _Pseudanabaena_. DISCUSSION _Planktothrix_ was the main MIB producer in QCS Reservoir during 2011 and 2015, as identified in our previous
study7, and the synchronous declines of this genus and MIB (Figs. 1 and 2) supported this interpretation. The driving forces responsible for the _Planktothrix_ decline are therefore
important for understanding the odor problems of QCS Reservoir. Another filamentous cyanobacterium, isolated and identified as non-MIB-producing _Pseudanabaena_, showed an increasing trend
during the study period. Both _Planktothrix_ and _Pseudanabaena_ showed the same seasonal patterns in this reservoir; in particular, _Planktothrix_ was more abundant in the first 2 years
while _Pseudanabaena_ was more abundant afterwards (Fig. 2). Filamentous cyanobacteria tolerate low light36, and many studies have shown that they tend to grow in spring and/or autumn
seasons8,11,37. In this study, _Planktothrix_ and _Pseudanabaena_ were mainly observed during the summer period (July to September) and did not follow the typical seasonality of filamentous
cyanobacteria. We speculate that the unusually low water transparency in the reservoir (~40–60 NTU in turbidity, QC01) creates a habitat with low subsurface water light intensity, which
favors filamentous cyanobacteria but is inhospitable to heliophilic _Microcystis_. In addition, the absence of surface _Microcystis_ will also provide a more favorable underwater light
environment for filamentous cyanobacteria25. Light niche differentiation between filamentous cyanobacteria and _Microcystis_ can explain the competition between them, as reported in a study
of Miyun Reservoir25. However, the situation in QCS Reservoir is different, since the MIB-producing _Planktothrix_ has no competition from surface-blooming _Microcystis_ but rather from the
ecologically similar _Pseudanabaena_, during 2013 to 2015. The succession and/or competition between them are difficult to determine due to their niche overlap. The temporal dynamics of
limnological conditions in QCS Reservoir suggests different seasonal and inter-annual patterns (Fig. 3). The presence of nutrients in appropriate concentrations is one fundamental
requirement for net primary production and accumulation of phytoplankton biomass, while nutrients have been recently considered to be of limited value and to even be useless to shape the
phytoplankton dynamics if we focus on the genus level38. Culture studies have also observed the insignificant effect of nutrient concentrations on the growth of several filamentous
cyanobacteria strains including _Planktothrix agardhii_39 and _Phormidium_ sp.40. Although the nutrients (except TP) exhibited inter-annual changes in QCS Reservoir, the concentrations are
generally sufficient to support the observed biomasses of filamentous cyanobacteria. The 15th Workshop of the International Association for Phytoplankton Taxonomy and Ecology summarized a
series of research works, suggesting that the physical environment should be regarded as an important structuring tool for phytoplankton assemblages38; in particular, the light availability
has gathered increasing attention38. Filamentous cyanobacteria seem to have lower optimum temperatures compared to other cyanobacteria, e.g., _Planktothrix agardhii_ can grow better at 18–25
°C39,41,42,43,44, while the preferred temperature range of _Microcystis_ is higher (_Microcystis aeruginosa_: 24–34 °C45,46,47; _Microcystis wesenbergii_: 25–35 °C47; _Microcystis
ichthyoblabe_: 30–36 °C48), as summarized by49. Culture studies have shown that temperature is an important factor governing the growth of filamentous cyanobacteria when temperature varies
greatly (e.g. >5 °C, Supplementary Table 1). For example, the red-pigmented _Planktothrix rubescens_ has more competitive success at 15 °C, while the green-pigmented _Planktothrix
agardhii_ is more competitive at 25 °C42. No significant difference of water temperature was observed during July to September from 2013 to 2015 in QCS Reservoir, except the temperature of
August in 2015 higher than in 2013 and 2014 (Fig. 5b), suggesting temperature may not the major contributor regarding the replacement of _Pseudanabaena_ from _Planktothrix_. Nevertheless,
the role of temperature regarding the succession and/or competition still requires more specific study, since it usually correlated with light intensity so that it is hard to distinguish its
contribution. In general, a “light niche” specified by light intensity and spectral composition can promote phytoplankton species replacement. Growth rate responses to these different light
levels are among major traits that determine the ecological success of phytoplankton species50,51. In this study, our results as demonstrated in Fig. 4 indicated that these two genera still
have slight light niche differentiation, which probably is responsible for the replacement of _Planktothrix_ by _Pseudanabaena_ in later years. The light niche differentiation was also
verified by the culture experiment (Fig. 5a34), showing that the optimum light dose of _Planktothrix_ is lower than for _Pseudanabaena_, although these two genera were both recognized as
low-irradiance specialists26,27. Furthermore, we summarized the light preferences of 10 _Planktothrix_ strains and 2 _Pseudanabaena_ strains (Table 1), showing a consistent conclusion with
this study that the optimum light intensities of all _Pseudanabaena_ strains are lower than those of _Planktothrix_. Nevertheless, a more targeted comparison is needed to verify the
difference of light optimum between the two genera. The competitive advantage of _Planktothrix_ at high solar radiation conditions was weakened in 2014 and 2015 owing to the lowered solar
radiation (July–August: 1.27 ± 0.37 mol m−2 d−1 and 1.34 ± 0.41 mol m−2 d−1) compared that in 2013 (1.84 ± 0.33 mol m−2 d−1). On the other hand, _Pseudanabaena_ was promoted in lower light
conditions and hence outcompeted _Planktothrix_ in QCS Reservoir. Therefore, the subtle light niche differentiation of these two filamentous genera probably is the driving factor responsible
for the succession and/or competition between _Planktothrix_ and _Pseudanabaena_. Other factors may also play important roles on their succession, e.g., _Planktothrix_ posses gas vesicles,
while _Pseudanabaena_ not. The reservoir is well mixed during the whole year, and the depth of euphotic layer is relatively low due to high turbidity loading from Yangtze River. The gas
vesicles of _Planktothrix_ can provide buoyancy that enable the cells to perform vertical migrations or to maintain themselves in the euphotic zone52. Vellend’s new conceptual synthesis in
community ecology53 has identified four distinct processes including selection, drift, speciation and dispersal. Under this framework, the succession of _Planktothrix_ and _Pseudanabaena_ in
QCS Reservoir may dominate by the selection process, on the premise of the dispersal process that imported new _Pseudanabaena_ from the Yangtze River, although the supporting evidence for
this is limited. Owing to the decrease of irradiance of July and August in 2014 and 2015, we speculate that _Pseudanabaena_ had higher fitness than _Planktothrix_ and that this promoted the
replacement. Solar radiation seems an essential factor governing the competition between the filamentous cyanobacteria _Planktothrix_ and _Pseudanabaena_ in the present study, hence
adjusting the underwater light climate could be a possible measure to regulate the filamentous cyanobacteria composition. Besides, since MIB is mainly produced by filamentous cyanobacteria,
it is therefore possible to inhibit MIB-producing strains but enhance the non MIB-producing strains by adjusting the light climate. For instance, the non MIB-producing _Pseudanabaena_ is a
benign replacement for MIB-producing _Planktothrix_, by reducing the underwater light intensity by adjusting the water level54 or increasing the turbidity via flow management. The strategy
to control odor problems in QCS Reservoir is beyond of this study and will be discussed further in a subsequent publication. METHODS STUDY AREA AND LABORATORY ANALYSIS This is a follow-up
study for7. QCS Reservoir (32°27'N, 121°38'E), located in Changxing Island in the Yangtze estuary, is a newly built reservoir used as the drinking water resource for Shanghai. The
bathymetry map shows that the water depth in the reservoir varies from 2.7 m in the upstream area to 12.1 m in the downstream area, and an island in the upper section splits the water flow
into two branches (Fig. 1a). The hydraulic retention time is in the range of 21.3 ± 2.2 d (April) and 124.1 ± 8.9 (December), the mean water turbidity is in the range of 22.7 ± 23.8 NTU
(March) and 40 ± 44.5 NTU (December), and the mean water transparency is in the range of 55 ± 16 cm (September) and 80.35 ± 36.71 cm (March). The reservoir imports the water from Yangtze
River via inlet gate. Since the abundance of phytoplankton and the concentrations of odor compounds of the river water are very low, three routine sampling sites including QC01 (water
intake), QC02 and QC09 (reservoir center) located in the lower section of the reservoir were selected, as illustrated in Fig. 1a. Since the whole reservoir is well-mixed7 throughout the
year, 2 L surface water samples (0.5 m) at each site were collected using a Kemmerer water sampler for other water quality. Phytoplankton analysis was performed weekly from 2011 to 2015;
odorant identification and quantification were performed every day from 2011 to 2015; nutrients and other water quality were recorded every day since 2013. Physicochemical variables such as
water temperature, pH, dissolved oxygen (DO), turbidity, and conductivity were measured in-situ with a multi-parameter probe (YSI EXO2, Yellow Springs, Ohio, USA). Subsamples for MIB and
geosmin detection were added 10 mg L−1 HgCl2 to prevent biodegradation and stored in light-blocking bottles, and analyzed within 72 h using the solid phase micro-extraction (SPME) method
coupled with gas chromatography-mass spectrometry (GC-MS) (Agilent 6890/5975, Agilent Tech., USA)11. SPME was performed using an automated device (Combi PAL GC MultiFunction Autosampler, CTC
Analytics, Switzerland) as follows: samples were shaken at 65 °C for 20 min, then the SPME fiber was exposed in the head-space of the vial for 10 min in order to absorb the odor compounds.
The fiber was transferred to the injection port of the gas chromatograph and desorbed in the splitless mode at 250 °C for 3 min. Calibration standards for MIB (Supelco Inc.) were used.
2-isopropyl-3-methoxypyrazine (Supelco Inc.) was added to each sample as internal standard. This method has a detection limit of 1 ng L−1 for both compounds. Subsamples (1000 mL) for cell
enumeration were preserved with 5% Lugol’s iodine55 and left to settle for 48 h, then pre-concentrated 20× and kept in the dark until cell counting. The identification of cyanobacterial
species was carried out following56 and revised according to ref. 57. The phytoplankton were identified and enumerated using an upright microscope (Olympus BX53, Japan) following the
protocol established by58. The filamentous cyanobacteria abundances were quantified based on the length of each filament and the mean cell length of each strain. The number of cells in
colony species such as _Microcystis_ sp. was estimated based on colony volume and mean cell number per volume. The mean cell morphological characteristics including cell length, cell volume
etc. were determined according to >50 filaments/colonies of each strain using a in-house developed cell counting tool (CCT v1.4, https://drwater.rcees.ac.cn, in Chinese). The total global
radiation (_I__g_, MJ m−2 d−1) of Chongming Island (<20 km) was extracted from China Meteorological Data Service Center (CMDC)59. Photosynthetically Active Radiation (_PAR__E_, 400–700
nm, MJ m−2 d−1) values were determined by a simplified model (Eq. (1)), according to 30 years of estimations of total global radiation and photosynthetically active radiation (PAR) in
central China60. $$PAR_E = \frac{{1666.4}}{{3983.9}} \times I_g = 0.4183I_g$$ (1) The PAR quantum (_PAR__Q_, mol m−2·d−1) was estimated according to Eq. (2), where the coefficient 4.57 (μmol
m−2·s−1 per W·m−2) is adopted for the PAR of sky sunlight61. $$PAR_Q = \frac{{PAR_E \times 10^6}}{{24 \times 60 \times 60}} \times \frac{1}{{4.57}} \times \frac{{24 \times 60 \times
60}}{{10^6}} = \frac{{0.4183I_g}}{{4.57}} = 0.09153I_g$$ (2) CULTURE EXPERIMENTS OF FILAMENTOUS CYANOBACTERIA UNDER DIFFERENT LIGHT INTENSITIES Two filamentous cyanobacteria, _Planktothrix_
sp. (FACHB-1375) and _Pseudanabaena_ sp. (FACHB-1277), were obtained from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, FACHB, China. Culture experiments were
performed by growing the two genera in BG11 medium62 at 25 °C and 5 different light intensities (5, 17, 36, 85, 250 _μ_mol m−2 s−1, 12:12 h light:dark cycle) in accordance with measured
light intensities at different depths in the field. The triplicate samples were destructively collected from each set every 4 days over a 35 days’ culture period. Data for _Planktothrix_ has
been published in34. The light doses (_I__dose_, mol m−2 d−1) were calculated from the instantaneous light intensities (_I_, _μ_mol m−2 s−1) and daily radiation time (12 h) according to Eq.
(3). $$I_{dose} = \frac{{12 \times 60 \times 60}}{{10^6}}I = 0.0432I$$ (3) The growth yield of _Planktothrix_ and _Pseudanabaena_ were determined according to the quantiles (25%, 50%, 75%)
of cell densities observed within stationary phase (day 15–35). TIME SERIES ANALYSIS Generalized additive models (GAMs)63 were used to model the seasonal and long-term patterns of
environmental factors and filamentous cyanobacteria cell densities, as shown in Eq. (4). Thin plate spline (TS-spline)64 were used to represent the long-term trend terms; while cyclic cubic
splines, which have an additional constraint ensuring continuity between the beginning and the end of a year65, were used for the seasonal terms. $$y = \beta _0 +
f_{{{{\mathrm{seasonal}}}}}\left( {x_1} \right) + f_{{{{\mathrm{trend}}}}}\left( {x_2} \right) + \varepsilon ,\quad \varepsilon \sim N\left( {0,\sigma ^2} \right)$$ (4) where
\(f_{{{{\mathrm{seasonal}}}}}\) and \(f_{{{{\mathrm{trend}}}}}\) are smooth functions for the seasonal and interannual trend of environmental factors and cell densities; _x_1 denotes the
sampling week number and _x_2 denotes the sampling date in units of decimal years. To make it clear, R Language demonstration code is given in the supplementary material. To avoid
autocorrelation from observations of successive time series, which might result in negatively biased estimation of regression coefficients and residuals, a first-order autoregressive model
(AR(1), Eq. (5)) was employed for the error term. $$\varepsilon _i = \phi \varepsilon _{i - 1} + v_i$$ (5) Different model structures were compared with likelihood ratio tests and the Akaike
Information Criterion (AIC). ECOLOGICAL NICHE MODELING Ecological niche modeling (ENM), also known as species distribution modeling (SDM) uses computer algorithms to predict the
distribution of a species across geographic space and time using environmental data. Water temperature (_T_), pre-week PAR (_I_, the mean PAR of the week before the sampling date) and
nutrients (ammonia nitrogen, total nitrate, total phosphate) were used as predictors (_x__i_) of filamentous cyanobacterial abundances (_y_). Here, we use the Generalized Additive Model
(GAM) to model the abundances of the two filamentous cyanobacteria, as shown in Eq. (6). We use cell density to utilize more of the available information while many other studies use binary
absent/present data to represent the biotic response to environmental conditions. $${{{\mathrm{log}}}}_{10}\left( {1 + \left( {E\left( {y_j} \right)} \right)} \right. = \beta _0 + \mathop
{\sum}\limits_{j = 1}^J {\mathop {\sum}\limits_{k = 1}^K {\delta _{jk}b_{1j}\left( {x_1} \right)b_{2k}\left( {x_2} \right) + \varepsilon _j} }$$ (6) where _b_1 and _b_2 are basis functions,
_J_ and _K_ are corresponding basis dimensions and _δ_ is a matrix of unknown coefficients. Interactions among the indicators were evaluated by the tensor product (\(f_1\left( {x_1} \right)
\otimes f_2\left( {x_2} \right)\)66). ENM were performed following several steps as describe below: * 1. Correlation analysis between filamentous cyanobacteria abundances (including
_Planktothrix_ and _Pseudanabaena_) and environmental factors as the potential predictors according to the two linear models (name as LM1 and LM2) as summarized in Supplementary Tables 9 and
10; * 2. Backward Stepwise model simplification of LM1 and LM2 were performed to further sort out the possible predictors for both genus (Supplementary Tables 11 and 12); * 3. Variance
inflation factors (VIF) were computed for the predictors given by step 2 (Supplementary Table 13); * 4. Correlation coefficients among the predictors were calculated using Pearson method to
evaluate the potential interacting effects among predictors (Supplementary Fig. 6); * 5. Based on the results of step 4, the predictors were assembled accordingly with appropriate smooth
functions of GAM models for _Planktothrix_ (named as GAM1, Supplementary Table 14) and _Pseudanabaena_ (named as GAM2, Supplementary Table 15); * 6. ENMs (named as GAM3 and GAM4) were
optimized according to the importance of predictors in GAM1 and GAM2 (Supplementary Tables 16 and 17). Estimated abundances of targeted species versus environmental factors were illustrated
with contour maps. Quantile niche space was identified by the boundary defined by the 90% quantile of estimated abundances for each genus. STATISTICAL ANALYSIS AND ILLUSTRATION All data
analysis and illustration were performed using R 4.067. Data pretreatment and summary were performed using the DPLYR68 package in R, regression analysis including linear and generalized
linear models were performed using the STATS package67, generalized additive modeling was performed using the MGCV package69,70 quantile regression analysis was performed using the QUANTREG
package71; contour figures were created by the GRAPHICS package67, other figures were prepared using the GGPLOT2 package72. DATA AVAILABILITY The data that support the findings of this study
are available from the corresponding author upon reasonable request and with the permission from Shanghai Chengtou Raw Water Co. Ltd. REFERENCES * AWWA. _Algae: Source to Treatment_
(American Water Works Association, 2010). * Berglind, L., Holtan, H. & Skulberg, O. M. Case studies on off-flavours in some Norwegian lakes. _Wat. Sci. Tech._ 15, 199–209 (1983). Article
CAS Google Scholar * Izaguirre, G., Jungblut, A. & Neilan, B. A. Benthic cyanobacteria (oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in Southern
California. _Water Res._ 41, 492–498 (2007). Article CAS Google Scholar * Sun, D. et al. Occurrence of odor problems in drinking water of major cities across China. _Front. Environ. Sci._
8, 411–416 (2014). Article CAS Google Scholar * Suurnäkki, S. et al. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers
of these compounds. _Water Res._ 68, 56–66 (2015). Article CAS Google Scholar * Tsuchiya, Y. & Matsumoto, A. Identification of volatile metabolites produced by blue-green algae. _Wat.
Sci. Tech._ 20, 149 (1988). Article CAS Google Scholar * Su, M. et al. Identification of MIB producers and odor risk assessment using routine data: a case study of an estuary drinking
water reservoir. _Water Res._ 192, 116848 (2021). Article CAS Google Scholar * Izaguirre, G., Hwang, C. J., Krasner, S. W. & Mcguire, M. J. Geosmin and 2-methylisoborneol from
cyanobacteria in three water supply systems. _Appl. Environ. Microbiol._ 43, 708–714 (1982). Article CAS Google Scholar * Wu, J. & Jüttner, F. Differential partitioning of geosmin and
2-methylisoborneol between cellular constituents in _Oscillatoria tenuis_. _Arch. Microbiol._ 150, 580–583 (1988). Article CAS Google Scholar * Martin, J. F., Izaguirre, G. &
Waterstrat, P. A planktonic _Oscillatoria_ species from Mississippi Catfish Ponds that produces the off-flavor compound 2-methylisoborneol. _Water Res._ 25, 1447–1451 (1991). Article CAS
Google Scholar * Su, M. et al. MIB-producing cyanobacteria (_Planktothrix_ sp.) In a drinking water reservoir: distribution and odor producing potential. _Water Res._ 68, 444–453 (2015).
Article CAS Google Scholar * Izaguirre, G. & Taylor, W. D. A _Pseudanabaena_ species from Castaic Lake, California, that produces 2-methylisoborneol. _Water Res._ 32, 1673–1677
(1998). Article CAS Google Scholar * Tabachek, J. L. & Yurkowski, M. Isolation and identification of blue-green algae producing muddy odor metabolites, geosmin, and
2-methylisoborneol, in saline lakes in Manitoba. _J. Fish. Res. Board Can._ 33, 25–35 (1976). Article CAS Google Scholar * Lu, J. et al. Driving forces for the growth of MIB-producing
_Planktothricoides raciborskii_ in a low-latitude reservoir. _Water Res_. 220, 118670 (2022). Article CAS Google Scholar * Li, Z. et al. Earthy odor compounds production and loss in three
cyanobacterial cultures. _Water Res._ 46, 5165–5173 (2012). Article CAS Google Scholar * Sun, D. et al. Identification of causative compounds and microorganisms for musty odor occurrence
in the Huangpu River, China. _J. Environ. Sci._ 25, 460–465 (2013). Article CAS Google Scholar * Chiu, Y., Yen, H. & Lin, T. An alternative method to quantify 2-MIB producing
cyanobacteria in drinking water reservoirs: method development and field applications. _Environ. Res._ 151, 618–627 (2016). Article CAS Google Scholar * Wang, Z. et al. Establishment and
field applications of Real-time PCR methods for the quantification of potential MIB-producing cyanobacteria in aquatic systems. _J. Appl. Phycol._ 28, 325–333 (2015). Article CAS Google
Scholar * Filstrup, C. T., Heathcote, A. J., Kendall, D. L. & Downing, J. A. Phytoplankton taxonomic compositional shifts across nutrient and light gradients in temperate lakes. _Inland
Waters_ 6, 234–249 (2016). Article Google Scholar * Wang, H. et al. Light, but not nutrients, drives seasonal congruence of taxonomic and functional diversity of phytoplankton in a
eutrophic highland lake in China. _Front. Plant Sci._ 11, 179 (2020). Article CAS Google Scholar * Polimene, L. et al. Modelling a light-driven phytoplankton succession. _J. Plankton
Res._ 36, 214–229 (2013). Article CAS Google Scholar * Su, M., An, W., Yu, J., Pan, S. & Yang, M. Importance of underwater light field in selecting phytoplankton morphology in a
eutrophic reservoir. _Hydrobiologia_ 724, 203–216 (2014). Article CAS Google Scholar * Holtrop, T. et al. Vibrational modes of water predict spectral niches for photosynthesis in lakes
and oceans. _Nat. Ecol. Evol._ 5, 55–66 (2021). Article Google Scholar * Naselli-flores, L., Padisák, J. & Albay, M. Shape and size in phytoplankton ecology: do they matter?
_Hydrobiologia_ 578, 157–161 (2007). Article Google Scholar * Su, M. et al. Succession and interaction of surface and subsurface cyanobacterial blooms in oligotrophic/mesotrophic
reservoirs: A case study in Miyun Reservoir. _Sci. Total Environ._ 649, 1553–1562 (2019). Article CAS Google Scholar * Wang, Z. & Li, R. Effects of light and temperature on the odor
production of 2-methylisoborneol-producing _Pseudanabaena_ sp. and geosmin-producing _Anabaena ucrainica_ (cyanobacteria). _Biochem. Syst. Ecol._ 58, 219–226 (2015). Article CAS Google
Scholar * Halstvedt, C. B., Rohrlack, T., Andersen, T., Skulberg, O. & Edvardsen, B. Seasonal dynamics and depth distribution of _Planktothrix_ spp. In Lake Steinsfjorden (Norway)
related to environmental factors. _J. Plankton Res._ 29, 471–482 (2007). Article CAS Google Scholar * Tilman, D. _Resource Competition and Community Structure_. (Princeton University
Press, 1982). * Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. _Proc. Natl Acad. Sci. USA_
101, 10854 (2004). Article CAS Google Scholar * Warren, D. L. & Seifert, S. N. Ecological niche modeling in maxent: the importance of model complexity and the performance of model
selection criteria. _Ecol. Appl._ 21, 335–342 (2011). Article Google Scholar * Huisman, J. et al. Cyanobacterial blooms. _Nat. Rev. Microbiol._ 16, 471–483 (2018). Article CAS Google
Scholar * Paerl, H. W. & Huisman, J. Blooms like it hot. _Science_ 320, 57–58 (2008). Article CAS Google Scholar * Paerl, H. W., Fulton, R. S., Moisander, P. H. & Dyble, J.
Harmful freshwater algal blooms, with an emphasis on cyanobacteria. _Sci. World J._ 1, 76–113 (2001). Article CAS Google Scholar * Jia, Z. et al. Light as a possible regulator of
MIB-producing _Planktothrix_ in source water reservoir, mechanism and in-situ verification. _Harmful Algae_ 88, 101658 (2019). Article CAS Google Scholar * Su, M. et al. Ecological niche
and in-situ control of MIB producers in source water. _J. Environ. Sci._ 110, 119–128 (2021). Article CAS Google Scholar * Scheffer, M., Rinaldi, S., Gragnani, A., Mur, L. R. & Van
Nes, E. H. On the dominance of filamentous cyanobacteria in shallow, turbid lakes. _Ecology_ 78, 272–282 (1997). Article Google Scholar * Sugiura, N., Iwami, N., Inamori, Y., Nishimura, O.
& Sudo, R. Significance of attached cyanobacteria relevant to the occurrence of musty odor in Lake Kasumigaura. _Water Res._ 32, 3549–3554 (1998). Article CAS Google Scholar *
Zohary, T., Padisák, J. & Naselli-flores, L. Phytoplankton in the physical environment: Beyond nutrients, at the end, there is some light. _Hydrobiologia_ 639, 261–269 (2010). Article
CAS Google Scholar * Sivonen, K. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by _Oscillatoria agardhii_ strains. _Appl.
Environ. Microbiol._ 56, 2658–2666 (1990). Article CAS Google Scholar * Heath, M., Wood, S. A., Young, R. G. & Ryan, K. G. The role of nitrogen and phosphorus in regulating
_Phormidium_ sp. (Cyanobacteria) growth and anatoxin production. _FEMS Microbiol._ 92, fiw021 (2016). Article CAS Google Scholar * Foy, R. H. Interaction of temperature and light on the
growth rates of two planktonic _Oscillatoria_ species under a short photoperiod regime. _Br. Phycol. J._ 18, 267–273 (1983). Article Google Scholar * Oberhaus, L., Briand, J. F.,
Leboulanger, C., Jacquet, S. & Humbert, J. F. Comparative effects of the quality and quantity of light and temperature on the growth of _Planktothrix agardhii_ and _P. rubescens_. _J.
Phycol._ 43, 1191–1199 (2007). Article CAS Google Scholar * da Anunciação Gomes, A. M., de Oliveira e Azevedo, S. M. F. & Lürling, M. Temperature effect on exploitation and
interference competition among _Microcystis aeruginosa_, _Planktothrix agardhii_ and, _Cyclotella meneghiniana_. _Sci. World J._ 2015, 1–10 (2015). Article Google Scholar * Bright, D. I.
& Walsby, A. E. The daily integral of growth by _Planktothrix rubescens_ calculated from growth rate in culture and irradiance in Lake Zürich. _N. Phytol._ 146, 301–316 (2000). Article
Google Scholar * Ganf, G. G. Rates of oxygen uptake by the planktonic community of a shallow equatorial lake (Lake George, Uganda). _Oecologia_ 15, 17–32 (1974). Article CAS Google
Scholar * You, J., Mallery, K., Hong, J. & Hondzo, M. Temperature effects on growth and buoyancy of _Microcystis aeruginosa_. _J. Plankton Res_. 40, 16–28 (2017). Article Google
Scholar * Imai, H., Chang, K., Kusaba, M. & Nakano, S. Temperature-dependent dominance of _Microcystis_ (cyanophyceae) species: _M. aeruginosa_ and _M. wesenbergii_. _J. Plankton Res._
31, 171–178 (2009). Article Google Scholar * Mowe, M. A. D. et al. Rising temperatures may increase growth rates and microcystin production in tropical _Microcystis_ species. _Harmful
Algae_ 50, 88–98 (2015). Article CAS Google Scholar * Li, J., Amano, Y. & Machida, M. Temperature-dependent growth characteristics and dominance trends of the cyanobacterium
_Microcystis_ sp. and the diatom _Cyclotella meneghiniana_. _Hydrobiologia_ 849, 1677–1688 (2022). Article CAS Google Scholar * Litchman, E. & Klausmeier, C. A. Trait-based community
ecology of phytoplankton. _Annu. Rev. Ecol. Evol. Syst._ 39, 615–639 (2008). Article Google Scholar * Tezanos Pinto, P. & Litchman, E. Eco-physiological responses of nitrogen-fixing
cyanobacteria to light. _Hydrobiologia_ 639, 63–68 (2010). Article CAS Google Scholar * Porat, R., Teltsch, B., Mosse, R. A., Dubinsky, Z. & Walsby, A. E. Turbidity changes caused by
collapse of cyanobacterial gas vesicles in water pumped from Lake Kinneret into the Israeli national water carrier. _Water Res._ 33, 1634–1644 (1999). Article CAS Google Scholar *
Vellend, M. Conceptual synthesis in community ecology. _Q. Rev. Biol._ 85, 183–206 (2010). Article Google Scholar * Su, M. et al. Reducing production of taste and odor by deep-living
cyanobacteria in drinking water reservoirs by regulation of water level. _Sci. Total Environ._ 574, 1477–1483 (2017). Article CAS Google Scholar * Sherr, E. B. & Sherr, B. F. In
_Handbook of Methods in_ _Aquatic Microbial Ecology_. (eds Kemp, P. F) 207–212 (Lewis Publishers, 1993). * Komárek, J. & Anagnostidis, K. _Cyanoprokaryota 1_. _Susswasserflora Von
Mitteleuropa_ Vol. 19 (Deu, 1998). * Komarek, J., Kastovsky, J., Mares, J. & Johansen, J. R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic
approach. _Preslia_ 86, 295–335 (2014). Google Scholar * Martinez, M., Chakroff, R. & Pantastico, J. _Note: Direct Phytoplankton Counting Techniques, Using the Haemacytometer_
(_Philippine Agriculturist_, 1975). * CMDSC. _Data set of daily radiation in China_ (CMDC, 2018). * Ren, X., He, H., Zhang, L. & Yu, G. Global radiation, photosynthetically active
radiation, and the diffuse component dataset of China, 1981–2010. _Earth Syst. Sci. Data_ 10, 1217–1226 (2018). Article Google Scholar * Thimijan, R. W. & Heins, R. D. Photometric,
radiometric, and quantum light units of measure: a review of procedures for interconversion. _Hortscience_ 18, 818–822 (1983). Article Google Scholar * Castenholz, R. W. Culturing methods
for cyanobacteria. _Methods Enzymol_. 167, 68–93 (1988). Article CAS Google Scholar * Hastie, T. J. & Tibshirani, R. J. _Generalized Additive Models_ Vol. 43 (CRC Press, 1990). *
Wood, S. N. Thin-plate regression splines. _J. R. Stat. Soc. Ser. A. Stat. Soc. B_ 65, 95–114 (2003). Article Google Scholar * Adams, J. A. Cubic spline curve fitting with controlled end
conditions. _Comput. Aided Des. Appl._ 6, 2–9 (1974). Article Google Scholar * Wood, S. N., Scheipl, F. & Faraway, J. J. Straightforward intermediate rank tensor product smoothing in
mixed models. _Stat. Comput._ 23, 341–360 (2013). Article Google Scholar * R Core Team. _R: A Language and Environment for Statistical Computing_. (R Foundation for Statistical Computing,
2020). * Wickham, H., François, R., Henry, L. & Müller, K. _dplyr: A Grammar of Data Manipulation_. https://dplyr.tidyverse.org/ (2018). * Wood, S. N. Stable and efficient multiple
smoothing parameter estimation for generalized additive models. _J. Am. Stat. Assoc._ 99, 673–686 (2004). Article Google Scholar * Wood, S. N. Fast stable restricted maximum likelihood and
marginal likelihood estimation of semiparametric generalized linear models. _J. R. Stat. Soc. Ser. A. Stat. Soc. B_ 73, 3–36 (2011). Article Google Scholar * Koenker, R. _Quantreg:
Quantile Regression_ (Cambridge U. Press, 2017). * Wickham, H. _ggplot2: Elegant Graphics for Data Analysis_ (Springer-verlag New York, 2016). * Tonk, L. et al. The microcystin composition
of the cyanobacterium _Planktothrix agardhii_ changes toward a more toxic variant with increasing light intensity. _Appl. Environ. Microbiol._ 71, 5177–5181 (2005). Article CAS Google
Scholar * de Araujo Torres, C., Lürling, M. & Marinho, M. M. Assessment of the effects of light availability on growth and competition between strains of _Planktothrix agardhii_ and
_Microcystis aeruginosa_. _Microb. Ecol._ 71, 802–813 (2015). Article CAS Google Scholar * Fujimoto, N., Sudo, R., Sugiura, N. & Inamori, Y. Nutrient-limited growth of _Microcystis
aeruginosa_ and _Phormidium tenue_ and competition under various N:P supply ratios and temperatures. _Limnol. Oceanogr._ 42, 250–256 (1997). Article CAS Google Scholar * Muhetaer, G. et
al. Effects of light intensity and exposure period on the growth and stress responses of two cyanobacteria species: _Pseudanabaena galeata and Microcystis aeruginosa_. _Water_. 12, 407
(2020). Article CAS Google Scholar * Zhang, T., Li, L., Song, L. & Chen, W. Effects of temperature and light on the growth and geosmin production of _Lyngbya kuetzingii_ (cyanophyta).
_J. Appl. Phycol._ 21, 279–285 (2008). Article CAS Google Scholar * Anagnostidis, K. & Komárek, J. Modern approach to the classification system of cyanophytes. 3—oscillatoriales.
_Algological Stud./Arch. F.ür. Hydrobiologie, Suppl. Volumes_ 50–53, 327–472 (1988). Google Scholar Download references ACKNOWLEDGEMENTS This work was financially supported by the National
Key R&D Program of China (2018YFE0204101), the National Natural Science Foundation of China (51878649, 52030002), and Youth Innovation Promotion Association CAS. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Key Laboratory of Drinking Water Science and Technology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China Ming
Su, Zhiyong Yu, Jinping Lu, Tengxin Cao, Jianwei Yu, Yu Zhang & Min Yang * University of Chinese Academy of Sciences, Beijing, 100049, China Ming Su, Zhiyong Yu, Jinping Lu, Tengxin Cao,
Jianwei Yu, Yu Zhang & Min Yang * Shanghai Chengtou Raw Water Co. Ltd, Shanghai, 200125, China Yiping Zhu & Yichao Song * Department of Biosciences, University of Oslo, P.O. Box
1066 Blindern, 0316, Oslo, Norway Tom Andersen * National Engineering Research Center of China (South) for Urban Water, Shanghai, 200082, China Xianyun Wang Authors * Ming Su View author
publications You can also search for this author inPubMed Google Scholar * Yiping Zhu View author publications You can also search for this author inPubMed Google Scholar * Tom Andersen View
author publications You can also search for this author inPubMed Google Scholar * Xianyun Wang View author publications You can also search for this author inPubMed Google Scholar * Zhiyong
Yu View author publications You can also search for this author inPubMed Google Scholar * Jinping Lu View author publications You can also search for this author inPubMed Google Scholar *
Yichao Song View author publications You can also search for this author inPubMed Google Scholar * Tengxin Cao View author publications You can also search for this author inPubMed Google
Scholar * Jianwei Yu View author publications You can also search for this author inPubMed Google Scholar * Yu Zhang View author publications You can also search for this author inPubMed
Google Scholar * Min Yang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.S.: Funding acquisition, data analysis, writing, reviewing and
editing. Y.Z.: Data collation, laboratory testing. T.A.: Method guidance, reviewing and editing. X.W.: Laboratory testing. Z.Y.: Laboratory testing. J.L.: Data analysis. Y.S.: Sample
collection, laboratory testing. J.Y.: Reviewing. Y.Z.: Reviewing. M.Y.: funding acquisition, reviewing and editing. CORRESPONDING AUTHOR Correspondence to Min Yang. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Su, M., Zhu, Y., Andersen, T. _et al._ Light-dominated selection shaping
filamentous cyanobacterial assemblages drives odor problem in a drinking water reservoir. _npj Clean Water_ 5, 37 (2022). https://doi.org/10.1038/s41545-022-00181-2 Download citation *
Received: 11 March 2022 * Accepted: 28 July 2022 * Published: 23 August 2022 * DOI: https://doi.org/10.1038/s41545-022-00181-2 SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative