Oxic corrosion model for kaeri reference disposal system via o2 consumption reactions and mixed-potential theory
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The durability of copper (Cu) canisters against corrosion is critical for the licensing of deep geological repositories. Assessing oxic corrosion, a primary degradation mechanism,
is essential for ensuring the reliability of such repositories. Due to the complex interactions influencing oxic corrosion, a comprehensive model is necessary for evaluating Cu canister
corrosion. This study develops a model for the KAERI Reference Disposal System (KRS), incorporating mixed-potential theory with key O2 consumption reactions, including Cu corrosion, Cu(I)
oxidation, FeS2 oxidation, aerobic microbial activity, and O2 dissolution and consumption. Simulation of 11 scenarios revealed that the representative KRS case would experience a maximum
corrosion depth of 9.3 μm on the Cu canister after 2.3 years due to oxic corrosion, under conditions that are unfavorable for the initiation of pitting corrosion. These results suggest that
oxic corrosion is not a threat to the isolation of spent nuclear fuels in KRS. SIMILAR CONTENT BEING VIEWED BY OTHERS COUPLED MIXED-POTENTIAL AND THERMAL-HYDRAULICS MODEL FOR LONG-TERM
CORROSION OF COPPER CANISTERS IN DEEP GEOLOGICAL REPOSITORY Article Open access 08 April 2023 EXPLORING THE INTERPLAY OF UNDER-DEPOSIT CORROSION AND MICROBIOLOGICALLY INFLUENCED CORROSION IN
THE PRESENCE OF DEPOSITS WITH VARIED ELECTRICAL CONDUCTIVITIES Article Open access 03 March 2025 CORROSION PROCESSES AFFECTING COPPER-COATED USED FUEL CONTAINERS FOR THE DISPOSAL OF SPENT
NUCLEAR FUEL: CRITICAL REVIEW OF THE STATE-OF-KNOWLEDGE Article Open access 04 December 2024 INTRODUCTION Deep geological repositories (DGRs) are attracting widespread interest as they offer
a permanent disposal solution for hazardous spent nuclear fuels (SNFs) from operational nuclear fission power plants, contributing significantly to the achievement of net-zero emissions. To
enhance safety, a DGR typically incorporates two main barrier systems: an engineered barrier and a natural barrier1,2,3,4,5,6,7. The engineered barrier consists of canisters for containing
SNFs and a bentonite backfill to seal the disposal holes and tunnels. Within these canisters, SNF is secured in a cast iron insert, while the outer shell is covered with Cu. These canisters
are then placed into disposal holes and sealed with bentonite, which is surrounded by host rock serving as the natural barrier. Failure of the DGR system can arise from corrosion. Given long
enough time required to isolate high-level wastes safely, preventing Cu canister corrosion indefinitely without human intervention is impractical. Consequently, corrosion emerges as a
critical concern in the licensing and construction process of DGRs. Recent literature has successfully evaluated the integrity of Cu canisters in DGRs against corrosion processes1,8,9.
However, despite these efforts, the precise impact of various reactions on oxic corrosion of Cu canisters remains partially understood. Such reactions include FeS2 oxidation, Cu oxidations,
Cu(I) oxidations, aerobic microbial activity, and the dissolution of O2 in groundwater5,7,9,10,11,12. It was reported that the Cu(I)-species oxidation rate is governed by a minor portion of
Cu(I)-species, such as Cu+ free, CuCl, CuCl3-2, and CuClOH-11. Also, the consumption of FeS2 on the FeS2 oxidation rate was not considered in the existing models which may lead to
underestimating the corrosion depth of the Cu canister1,9. Additionally, there are remaining questions about the interaction of corrosion products with bentonite. Maintaining bentonite
performance is one of the top priority for deep geological repositories, and there has been a lot of research to investigate the alteration of bentonite13,14,15. Despite of these efforts,
there are no models or experiments reporting the distribution of Cu corrosion products in the bentonite which is fundamental to analyze and predict the alteration of bentonite performance.
Simultaneously, the importance of pitting corrosion cannot be ignored and has received considerable attention in different national programs (Sweden, Finland, and Canada)5,16,17. For the
initiation of pitting corrosion, there are pre-requisites which are the formation of a passivation film and the breakage of the passivation film18. Hence, if the model can predict the
corrosion potential, then it will be a valuable tool to evaluate the possibility of pitting corrosion through a comparison of predicted corrosion potential and the reported pitting corrosion
potential. These complicated interaction phenomena are challenging to assess through experimental methods alone, requiring a numerical model that can consider multiphysics effects
simultaneously. Therefore, this research introduces a new multiphysics model to evaluate the effects of various reactions on the oxic corrosion of Cu canisters while calculating the
corrosion potentials and distribution of corrosion products in bentonite. This model is focused on the early oxic period in a deep geological repository. This model incorporates Cu(I)
oxidation, FeS2 oxidation, aerobic microbial activity, and the removal of O2,gas by groundwater via dissolution and consumption at the interface of engineered barrier and host rocks.
Simultaneously, the corrosion potential of the Cu canister is calculated by Mixed-potential theory. Also, the new oxidation kinetics of Cu(I) and FeS2 were implemented and validated with the
literature data while considering the minor portion of Cu(I)-species and the consumption of FeS2. Applied to the KAERI Reference disposal System (KRS), this model aims to elucidate the
evolution of O2 and its effects on Cu corrosion from the perspectives of corrosion potential and corrosion depth1. RESULTS AND DISCUSSION FATE OF O2 IN THE NEAR-FIELD As shown in Table 1, a
total of 11 cases were simulated for KRS. The CnSm cases (where n represents the weight contents of FeS2 in bentonite, and m represents the size of FeS2 particles) were designed to evaluate
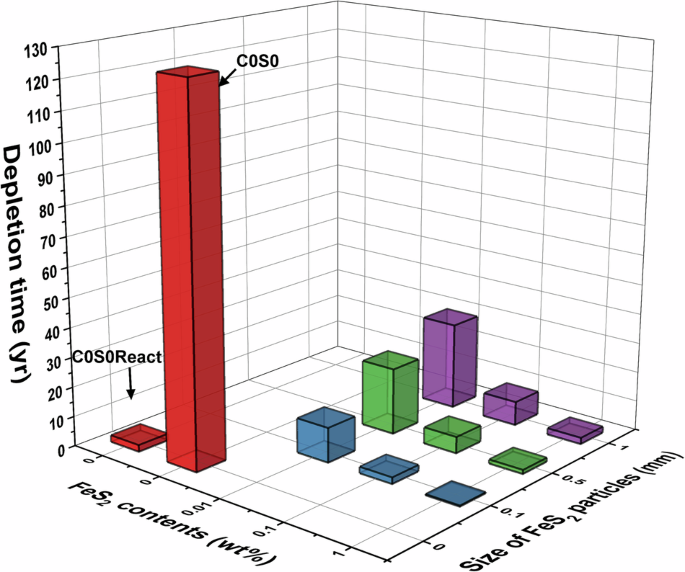
the effects of FeS2 contents and particle size on corrosion. Figs. 1 and 2 present the depletion time of O2 in the engineered barrier system (bentonite and backfilled materials) and the O2
consumption ratio by the five reactions, respectively. The depletion time was calculated for when less than 0.1%v/v of O2 remained in the engineered barrier system, allowing comparison with
the Mont Terri Full-scale Emplacement experiments12. Because the Mont Terri experiments as detector with a detection limit of 0.1%v/v to quantify the remaining O2 in the near-field, which
only can measure the O2,gas12. The depletion times ranged from 0.4 to 22.4 years for 9 cases (Except C0S0 and C0S0React). These 9 cases exhibited faster depletion times of O2 with higher
FeS2 contents (wt%) and smaller FeS2 particles due to the more rapid FeS2 oxidation kinetics, as described in Table 2. These cases show the FeS2 oxidation can be the primary reaction for O2
depletion, where 79.3–96.0% of O2 was reacted with FeS2. Interestingly, the predicted depletion times of O2 for C0.1S0.5 and C1S0.5 (5.6 and 1.4 years, respectively) are longer than the Mont
Terri Full-scale Emplacement (FE) experimental data (a half year)12,19. The Mont Terri used MX-80 bentonite, and the FeS2 contents and particle sizes (see Table 1) are situated between the
C0.1S0.5 and C1S0.5 cases. This discrepancy could be attributed to faster flow rates of gas and groundwater and the composition of groundwater in Mont Terri. The Mont Terri Full-scale
experiments were conducted in more porous host rock types (Opalinus Clay with 0.13-0.16 porosity) compared to these model cases (granite with 0.01 porosity)19. Additionally, the groundwater
may contain some other oxidizing agents, such as HS-, which can rapidly consume the O220,21. Tomonga et al. also pointed out that the fast depletion of O2 could be due to gas exchange from
the engineered barrier to host rocks during the Mont Terri experiment19. This comparison highlights the influence of host rock types and groundwater composition on the oxic corrosion of Cu
canisters, though further research is necessary for a full comparison the Mont Terri experiment and the numerical model. The C0S0 and C0S0React cases simulated 0 wt% FeS2 (KJ-II bentonite).
In these two cases, the depletion times of O2 are 124.7 and 2.3 years, respectively. The C0S0 case shows that Cu and Cu(I) oxidations are the predominant reactions (>94.7%), while
C0S0React demonstrates that the dissolved O2 reacted with reducing agents (such as HS-) in the ground water as the primary reaction (> 98.6%). The aerobic microbial activity contributes
only 2.6 and 0.1% of the total O2 consumption, respectively. The consumption of O2 in the groundwater shows a significant difference between the C0S0 and C0S0React cases. This difference
arises because the C0S0React case accounts for the consumption of O2 at the interface between the engineered barrier and host rocks, assuming that the groundwater of DGR contains other
oxidizing agents (such as HS-) which react with O21,22,23,24. Among these two cases, the C0S0React case is considered as the representative case for KRS by including the consumption of O2 in
the groundwater. This case suggests that the oxic period (O2 depletion time) can be shorter than 2.3 years for KRS, though it necessitates additional analysis through the combination of
numerical models and field experiments. This is because the C0S0React case assumes that the oxidizing agents (HS-) do not migrate into the engineered barrier system. The movements of
oxidizing agents can induce faster depletion of O2 in the engineered barriers23,25. In the previous research, we predicted that the O2 depletion time would require at least 100 years,
consistent with the expectation of CSM1,9. However, by incorporating the several different O2 consumption reactions, this model now predicts an O2 depletion time of 2.3 years. Due to this
short period (2.3 years), this model indicates that the long-cell action suggested by the previous research (Chae et al.) in the deep geological repository can be ignored1. Previous research
suggested that the long-cell action could accelerate the corrosion of Cu canister by less than 1 μm under conditions of 100 years of O2 remaining. This implies that the long-cell action
cannot be sustained beyond 2.3 years, and any resulting acceleration in corrosion depth should be even smaller than the previous research1. CORROSION DEPTH OF CU CANISTER Figure 3 presents
the calculated maximum corrosion depths (oxic phase) on the surface of the Cu canister from each case. The deepest corrosion depths, observed in each case, originate from the canister’s top
corner surface, exemplified by Case C0.1S0.5 (as depicted in Fig. 4). These corrosion depths vary from 1.8 to 531.7 μm, demonstrating a correlation with the O2 depletion times illustrated in
Fig. 1. This suggests that longer O2 depletion times promote increased O2 transport to the Cu surface. A significant portion of Cu corrosion is caused by O2 reduction, with over 99.7%
oxidation observed in all cases (see Fig. 3). Conversely, the majority of Cu(II) precipitates in the bentonite as CuO, rather than contributing to the further corrosion of the Cu canister.
For the KRS scenario, the expected maximum corrosion depth of the Cu canister is 9.3 µm for the oxic phase, which closely aligns with the findings from the C0S0React case. This case
simulates O2 consumption in the presence of dissolved HS- species at the interface between engineered barrier and granite host rock. The corrosion depth of 9.3 μm is comparable to the
best-estimate case for the KBS-3 deep geological repository, which is 6.4 um, and closely matches the 10 μm depth estimated for the Nuclear Waste Management Organization (NWMO) deep
geological repository, despite variations in conditions and geometries, as noted in refs. 9, 22. Such comparisons highlight the minimal impact of uniform oxic corrosion on Cu canister
integrity in the KRS, KBS-3, and NWMO deep geological repositories. This is further quantified by the respective corrosion percentages of only 0.02, 0.01, and 0.03% for Cu thicknesses of 50
(for both KRS and KBS-3) and 30 mm (for NWMO). Figure 4 demonstrates that the maximum corrosion depths on the Cu canister predominantly occur at the top edge, where corrosion is
significantly more extensive compared to the sides and bottom. This is attributed to the prolonged availability of O2 in the disposal tunnel, which ensures a continuous supply of O2 to the
top surface. As corrosion progresses, the side and bottom surfaces exhaust their O2 supply, whereas the top surface continues to receive O2 from the disposal tunnel. Although the top edge
may appear more susceptible to oxic corrosion, its greater thickness (~74 mm), although this value can be changed in the future, suggests a reduced susceptibility over time, mitigating
concerns regarding the canister’s structural integrity3. CORROSION POTENTIALS OF CU CANISTER Figure 5 illustrates the calculated corrosion potentials, highlighting the impact of various
reactions on the likelihood of localized corrosion on the Cu canister. The corrosion potentials begin at -0.31 VSCE, decreasing to below -0.5 VSCE for all cases except for the C0S0 case.
This observed trend towards low corrosion potentials, similar to those recorded in experiments conducted at approximately 50 °C with low O2 levels, was documented by He and Ahn26,27. They
reported notably lower corrosion potentials, ranging from -0.7 to -0.8 VSCE, under high temperature conditions (50 °C), elevated chloride concentrations (282 – 2820 mol·m-3), and extremely
low O2 concentration conditions (below 6E-6 mol·m-3). These experimental conditions closely mirror those in the simulated cases of this study, where the average temperature of the canister
surface exceeds 50 °C most of the time, as depicted in Fig. 5. An initial decrease in corrosion potentials (from -0.3 to -0.4 VSCE) coincides with O2 depletion (Fig. 5). The O2 concentration
near the canister becomes exceedingly low, resulting in a drop in the corrosion potential to between -0.3 to -0.4 VSCE. At approximately -0.4 VSCE, the corrosion potential is maintained
through the backward diffusion of Cu2+ from the bentonite, as demonstrated in Fig. 6 for the C0.01S0.1 case. While the reduction of Cu2+ ions could provide electrons for Cu oxidation, the
resultant cathodic currents are insufficient to sustain higher corrosion potentials throughout the simulation period. The second decline in corrosion potentials (-0.4 to -0.7 VSCE) results
from the depletion of Cu2+, leading to a further reduction in potential. The C0S0React case, representing the KRS condition, shows a corrosion potential below -0.5 VSCE after 50 years,
significantly lower than the experimentally determined pitting corrosion potentials. Previous studies by Allah et al. and Sridhar and Cragnolino reported pitting corrosion potentials ranging
from 0.2 to 1 VSCE (30–3160 mol m-3 Cl-, pH 7.26 – 13.0, 25 °C) and -0.024 to 0.628 VSCE (95 °C Cl-, 0.169 – 28.206 mol·m-3, pH 6.8 – 8.3)28,29. All simulated cases in this study exhibit
the corrosion potentials below these pitting corrosion potentials, suggesting a low likelihood of pitting corrosion occurring. This finding aligns with other modeling studies, indicating
that pitting corrosion is unlikely to pose a significant risk in deep geological repositories30,31. However, there can be more variation cases and it requires analyzing more cases with
meso-scale modeling to fully understand and predict the pitting corrosion of Cu canisters, incorporating the formation of oxide films. CORROSION PRODUCTS DISTRIBUTION IN BENTONITE Figure 7
presents the mass fraction of corrosion products distribution in the bentonite after 10 years of oxic corrosion. In Fig. 7, the calculated mass fractions of corrosion products in the
bentonite (KRS case – C0S0React) from the five different orientations (top, top-edge, side, bottom-edge, and bottom surface of Cu canister) are compared with experimentally reported values
from KURT (Kong et al.) for 10 years aerobic corrosion tests with 30 °C32. Interestingly, the model shows quite good agreement with the reported values, except for the data for 0.5 mm. This
trend indicates that corrosion products can diffuse out from the Cu surface by Cu(I)- and Cu(II)-chloride complexes. Besides, the higher contents of Cu at the 0.5 mm position could occur by
the detached Cu oxide layers from the Cu specimen, although the sampling method was not properly described in Kong et al. Also, Cu(I) and Cu(II) portions are calculated to be 29 and 71% in
bentonite from the simulation results. This trend also agrees with the trend of 10 years of experimental results from Kong et al, which show that Cu(II) is a major portion of Cu corrosion
products. Figure 8 shows the calculation results of corrosion products distribution in the bentonite after 50 years, where the oxic corrosion progress has ceased, for the KRS case. As can be
seen in Fig. 8, the precipitated Cu(I) and Cu(II) amount to 0.45 wt% at maximum in the fully saturated bentonite. Also, the distributed total corrosion products (Cu(I) and Cu(II)) and Cu(I)
are gradually decrease and become less than 0.1 wt% after 15 mm of bentonite. In contrast, the shape of Cu(II) distribution shows a peak around the 8 mm position in the bentonite. This
unique shape of Cu(II) distribution can be explained by the reaction of Cu(II) in eq. 2. As stated in eq. 2, Cu(II) can be formed by the oxidation reaction of Cu(I) by O2. In the bentonite,
the Cu(I) source originates from the oxidation of the Cu canister surface, and O2 is supplied from the bulk of the bentonite. Therefore, these two chemical species can have the highest
reaction rates somewhere in the middle of the bentonite. HIGHLIGHTS FROM MODEL RESULTS This study assessed the oxic corrosion of Cu canisters within the KRS concept through 11 cases,
considering various O2 consumption reactions in the near-field. The numerical simulations highlight the crucial role of O2 consumption reaction, particularly the interaction between the
groundwater and other oxidants. This interaction may account for the swift O2 depletion observed in the Mont Terri full-scale experiments, although further research is essential to
accurately replicate the conditions of Mont Terri full-scale experiments. Future studies should include an analysis of groundwater composition and accessory minerals in the host rocks to
better understand the mechanisms behind the rapid consumption of O2 in the Mont Terri experiments. The C0S0React case has been identified as representative of the KRS concept. According to
its findings, the expected corrosion duration is approximately 2.3 years, with a maximum Cu canister corrosion depth of 9.3 μm in KRS, closely aligning with the depths recorded for KBS-3 and
NWMO deep geological repositories (6.4 and 10 μm, respectively). Also, the long-cell action cannot be sustained for more than 2.3 years, and this have a very negligible effect on corrosion
depth compared to previous research (Chae et al.)1. Additionally, the oxic phase of KRS conditions does not show a higher corrosion potential than the experimentally reported film breakage
potential, which is one of the important factors for the initiation of pitting corrosion. These findings indicate that oxic corrosion may not pose a significant threat to the structural
integrity of Cu canisters, suggesting that validation of the Cu canister’s integrity in oxic conditions could be achievable within a few years under the KRS concept. The distribution of
corrosion products has been suggested based on the developed model, and it was compared with experimental data. This result can be useful for evaluating the alteration of bentonite
properties through interaction with corrosion products. Also, it can be used for evaluating the efficiency of retarding radionuclides from spent nuclear fuels. We believe this finding can
support the understanding of oxic corrosion of the Cu canister and its interaction with bentonite which is an important research topic to guarantee the performance of bentonite. Also, this
model was independently developed by the authors in South Korea considering the new kinetics equation for Cu(I) oxidation, FeS2 oxidation, and O2 consumption in groundwater. Nonetheless,
this model shows a similar corrosion depth of the Cu canister as the other national programs (SKB and NWMO). Therefore, we believe this model confirms corrosion damage on the Cu canister in
the oxic phase. We believe that this independent model can be a reference for other countries to compare with their own results. Long-term corrosion of the Cu canister can only be compared
with numerical results due to the required time of corrosion experiments. Throughout the analysis of this model, a pH-changing model that considers corrosion film formation is required to
reduce the uncertainty of the results while avoiding the underestimation of the corrosion of the Cu canister. However, this model does not currently predict pH value and the formation of a
corrosion film on the Cu surface. These pH and formation of passivation film may lead to localized corrosion through the film breakage with local acidic condition on the surface of the Cu
canister due to hydrolysis of Cu(I) species. In such cases, localized corrosion might occur and affect the performance of the Cu canister. This remaining challenge can be overcome by
combining a meso-scale model for pH changes with Cu corrosion. Therefore, it is recommended to develop such a model in the future to evaluate the corrosion of the Cu canister. Despite these
reassuring outcomes, uncertainties persist, primarily due to the absence of designated KRS sites, necessitating reliance on data and conditions from KBS-3 for bridging some gaps. The
potential influence of host rocks, acknowledged as significant, remains underexplored due to current data limitations, particularly concerning comprehensive applications and validations with
granite host rocks in KRS settings. The effects of microbiological organisms are quite complex, as suggested by Lee et al.33 and Little et al.34. These authors have shown the effects of
microbiological activity on corrosion potential and rates, and these aspects should be addressed in more detail to avoid underestimating the corrosion depth of Cu canister33,34. Therefore,
conducting future field experiments is crucial for refining the models with accurate conditions, thus bolstering the reliability of predictions regarding the extent of Cu canister corrosion
damage from oxic processes. METHODS CONSIDERED REACTIONS BETWEEN CHEMICAL REAGENTS IN DEEP GEOLOGICAL REPOSITORY The overall reactions (chemical, electrochemical, and aerobic microbial
activity) during the oxic period are summarized in Fig. 9. Trapped O2 acts as the major oxidizing agent of Cu corrosion for the oxic period17,22,35. Simultaneously, the trapped O2 can react
by the following reactions: Cu(I) oxidation, FeS2 oxidation, aerobic microbial activity, and dissolution in groundwater. The model considers these reactions comprehensively. Unlike the
existing corrosion models, this model implemented the contribution of a minor Cu(I)-species on Cu(I) oxidation kinetics based on Yuan et al. 11.The source term for Cu(I)-species is the
corrosion of Cu by the trapped O2, which is reduced while oxidizing the Cu to form Cu2O and Cu(I)-Cl- complexes in the Cl- containing ground water. Additionally, the Cu(I)-Cl- complex can be
further oxidized to form Cu(II) species, which can then be reduced back to Cu(I), perpetuating the cycle. During this cycle, minor species of Cu(I) can significantly contribute to the
overall kinetics due to their higher accessibility to O2 molecules as reported by Yuan et al.11. Thus, the minor Cu(I) species like Cu+ free, CuCl, CuCl3-2, and CuClOH- are also considered
in this model. The overall Cu(I) oxidation can be generalized into the reaction show as (1). $${Cu}\left(I\right)+0.25{O}_{2}\to {Cu}({II})$$ (1) FeS2, present in bentonite as one of several
accessory minerals, can alter geochemical environments through its reaction with O2, producing the formation of acid mine tailings10,36,37,38. The oxidation of FeS2 involves multiple
electrochemical steps, transitioning from -1 to +6 oxidation states of sulfur, forming various intermediate species. The overall FeS2 oxidation can be generalized into the reaction shown as
(2)10,39. Improvements have been achieved from the previous studies by incorporating a new approach to consider the consumption effects of FeS2 on the kinetics of FeS2
oxidation37,38,40,41,42,43,44. This model adopts the kinetics parameters of FeS2 oxidation systematically suggested by Williamson and Rimstidt10. $${Fe}{S}_{2}+3.5{O}_{2}+{H}_{2}O\to
2S{O}_{4}^{2-}+2{H}^{+}+F{e}^{2+}$$ (2) Deep geological repository systems have alkaline groundwater. Under the alkaline solution, numerous studies have identified sulfate ion (SO42-) as the
thermodynamically stable chemical species of sulfur45,46,47,48,49. Also, Manaka recently showed that sulfate ion is the stable form of pyrite oxidation after 3 years of pyrite oxidation in
bentonite49. Hence, we have considered SO42- as the final products of HS- oxidation. The bentonite and backfilled materials are porous mediums that contain aerobic microbes12,50,51. The
activity of these aerobic microbes can consume the trapped O2 in the near-field, eventually leading to the depletion of O2. Simultaneously, some of the O2 can dissolve in the
groundwater1,12,19,24,52. The dissolved O2 can be consumed by stronger oxidizing agents, such as HS-, from the host rocks. This model incorporates these phenomena, providing a comprehensive
view of the reaction processes. The explained reactions were incorporated in the model by using the kinetics equations in the section Kinetics of reactions. The Kinetics of reactions section
also includes all the parameters required for kinetics reactions, and the supplementary validation includes the validation of these kinetics reactions by comparing with literature data. The
given reaction kinetics equation in the Kinetics of reactions section is combined in the generalized reactive-transport equations which are provided in the Generalized governing equations
section. GENERALIZED GOVERNING EQUATIONS In our model, the authors employed a thermal-hydraulics-chemical (T-H-C) coupled model, which was developed by Chae et al. based on the MOOSE
framework1,53,54. A sequential coupling between thermal-hydraulics and chemical reactions was utilized to analyze the effects of thermal-hydraulic (T-H) conditions on chemical reactions.
Additionally, the chemical reactions were represented through Ordinary Differential Equation (ODE) to integrate with the transport equations of chemical species. The governing equations for
T-H behaviors are based on mass and energy conservation principles. The thermal governing equation is expressed as follows: $$\frac{\partial \left(\left(1-\phi \right){\rho
}_{{solid}}{c}_{{solid}}T+\phi S{\rho }_{{liquid}}{c}_{{liquid}}T\right)}{\partial t}=-\nabla \,{{\cdot }}\,\left(-\lambda \nabla T\right)-\nabla \,{{\cdot }}\,{hF}$$ (3) where \(\phi\) is
the porous medium porosity (dimensionless), \({\rho }_{{solid}}\) and \({\rho }_{{liquid}}\) are the densities of the solid and liquid parts of the porous medium (dimensionless), _c__solid_
and _c__liquid_ are the heat capacities [J· kg-1K-1], _S_ is the degree of saturation in the porous medium (dimensionless), _T_ is the system temperature [K], _t_ is simulation time [year],
\(\lambda\) is the thermal conductivity of the porous medium or target system [W·m-1·K-1], \(h\) is the specific enthalpy of a single phase (e.g., solution or porous fluid) [J·kg-1], and
\(F\) is the advective Darcy flux of a porous fluid [m·yr-1], which can be calculated by Darcy velocity of fluid in the hydraulics governing equations. The hydraulic behavior within the
porous medium (bentonite and backfilled materials) is modeled by the following equation: $$\frac{{\cdot\,}_{\partial M}}{\partial t}={{\nabla}} \,{{\cdot }}\,\left(\rho \frac{k}{\mu
}({{\nabla}} P-\rho g)\right)$$ (4) where _M_ is the mass density of the fluid [kg·m-3], \(\rho\) is the fluid density [kg m-3], \(k\) is the porous medium (bentonite and backfill)
permeability [m2], \(\mu\) is the fluid viscosity [N·m-2yr-1], _P_ is the porous medium pore pressure [Pa], and \(g\) is the gravitational acceleration [m s-2]. The reactive transport
equation (chemical), representing conservation of mass during chemical reactions and transports, is given by: $$\frac{d{C}_{{aq},i}}{{dt}}=\tau \phi \nabla \,\cdot\,
S{D}_{{aq},i}{C}_{{aq},i}+\phi S{R}_{j}$$ (5) where \(\phi\) is the porous medium porosity (dimensionless), \(S\) is the degree of saturation in bentonite and backfill materials
(dimensionless), _C__aq,i_ is the concentration of dissolved chemical species _i_ [mol·m-3], _D__aq,i_ is the diffusion coefficient of dissolved chemical species _i_ [m2·yr-1], _R__j_ is the
reaction rate for reaction _j_, [mol·m-3·s]. Also, the concentration of gas phase of O2 is considered by the similar equation: $$\frac{d{O}_{2,{gas}}}{{dt}}=\tau \phi \nabla \,\cdot\,
(1-S){D}_{{O}_{2,{gas}}}{O}_{2,{gas}}+{R}_{k}$$ (6) where \({O}_{2,{gas}}\) is the concentration of O2 in the gas phase [mol·m-3], \({D}_{{O}_{2,{gas}}}\) is the diffusion coefficient of O2
in the gas phase [m2·yr-1], and _R__k_ is the dissolved reaction from gas to aqueous [mol·m-3·s]. KINETICS OF REACTIONS (FES2 OXIDATION, CU(I) OXIDATION, AND MICROBIAL ACTIVITY) Cu(I) and
FeS2 oxidation were considered as the chemical reaction terms (_R__j_) in eq. 5. The equation for Cu(I) oxidation was adopted from Yuan et al. $${R}_{1}={k}_{1,0}\exp
\left(-\frac{{E}_{A,1}}{R}\left(\frac{1}{T}-\frac{1}{298.15}\right)\right){Cu}(I){O}_{2}$$ (7) where _k__1,0_ is a function of Cl- and pH [m3·mol-1·s-1], _E__A,1_ is the activation energy of
the Cu(I) oxidation reaction, [J mol-1], _R_ is the ideal gas constant, _T_ is the temperature of the bentonite or backfill materials [K], Cu(I) is the concentration of all Cu(I) species
[mol·m-3], and O2 is the concentration of O2 [mol m-3]11. The _k__1,0_ function was fitted by the following equation: $$\begin{array}{l}-{\log }_{10}{k}_{1,0}={A}_{1}\,\cdot\,
{pH}+{A}_{2}\,\cdot\, I+{A}_{3}\,\cdot\, C+{A}_{4}\,\cdot\, {pH}\,\cdot\, I+{A}_{5}\,\cdot\, {pH}* C\\+\,{A}_{6}\,\cdot\, I\,\cdot\, C+{A}_{7}\,\cdot\, {pH}\,\cdot\, C\,\cdot\,
I+{B}_{1}\,\cdot\, p{H}^{2}+\,{B}_{2}\,\cdot\, {I}^{2}+{B}_{3}\,\cdot\, {C}^{2}\\+\,{B}_{4}{\left({pH}\,\cdot\, I\,\cdot\, C\right)}^{-\frac{1}{2}}+\,{B}_{5}\,\cdot\, {\left({pH}\,\cdot\,
I\,\cdot\, C\right)}^{-\frac{1}{5}}+{B}_{6}\,\cdot\, \frac{{pH}}{I}+\,{B}_{7}\,\cdot\, \frac{I}{C}+{B}_{8}\,\cdot\, \frac{C}{{pH}}\\
+\,{B}_{9}\,\cdot\,p{H}^{2}\,\cdot\,I+{B}_{10}\,\cdot\,p{H}\,\cdot\,{C}^{2}+{B}_{11}\,\cdot\,{C}\,\cdot\,{I}^{2}+{B}_{12}{(p{H}\,\cdot\,{C}\,\cdot\,{I})}^{2} \end{array}$$ (8) where _k__1,0_
is kinetics constant [m3 mol-1 s-1], _C_ is log10_Cl__-_ and _Cl__-_ is the Cl- concentration [mol m-3] unit, and _I_ is the ionic strength of ground water [M]. All the parameters are given
in Table 3, and eq. 8 is valid for Cl- 0.1 M–0.7 M, pH 6.5–10, and ionic strength 0.7 – 5 M range. This equation was derived by combining kinetic constants (Table 4) of Cu+, CuCl, CuCl2-,
CuCl3-2, and CuClOH- with chemical speciation distributions by the following equation:
$$\begin{array}{l}{k}_{{app}}={{\rm{k}}}_{{{\rm{Cu}}}^{+}}{f}_{C{u}^{+}}+{{\rm{k}}}_{{\rm{CuCl}}}{f}_{{CuCl}}+{{\rm{k}}}_{{{\rm{CuCl}}}_{2}^{-}}{f}_{{{\rm{CuCl}}}_{2}^{-}}\\\qquad\quad+\,{{\rm{k}}}_{{{\rm{CuCl}}}_{3}^{-2}}{f}_{{{\rm{CuCl}}}_{3}^{-3}}+{{\rm{k}}}_{{{\rm{CuClOH}}}^{-}}{f}_{{CuClO}{H}^{-}}\end{array}$$
(9) where _k_ is the kinetics constant of the corresponding Cu(I) chemical species [m3 mol-1 s-1], _f_ is the fraction of the corresponding Cu(I) chemical species Cu(I) among soluble Cu(I)
species [mol·m-3]. The chemical speciation calculation was done by considering the Cu reactions as listed in Tables 4 and 5 through Specific Interaction Theory (SIT)55. The FeS2 oxidation
reaction rate was considered by: $${k}_{2}={k}_{2,0}\,\cdot\, {\left[\frac{{O}_{2}}{1000}\right]}^{0.5}{\left[\frac{{H}^{+}}{1000}\right]}^{-0.11}$$ (10) where _k__2,0_ is the kinetics
constant 1E-8.19 [mol0.61 kg0.39 m-2 s-1], _O__2_ is the concentration of O2 [mol∙m-3], and _H__+_ is the concentration of H+ [mol∙m-3]10. Here, the authors converted the concentration unit
from mol∙kg-1 to mol∙m-3 by dividing it by 1000 (water density 1000 kg m-3). Williamson and Rimstidt demonstrated that the FeS2 oxidation rate depends only on the concentration of O2 and H+
10. In a deep geological repository, the oxidation kinetics of FeS2 can be influenced by its consumption since significant amounts of FeS2 can be oxidized over the geological timescale used
for simulation. The authors derived the following kinetics equation to account for the effects of FeS2 consumption during oxidation: $${R}_{2}={k}_{2}\,\cdot\, {A}_{{Fe}{S}_{2}}\,\cdot\,
\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\,\cdot\, \frac{{m}_{{Fe}{S}_{2}}}{V}\,\cdot\, {\rho }_{{Fe}{S}_{2}}\,\cdot\, \exp
\left(-\frac{{E}_{A}}{R}\left(\frac{1}{T}-\frac{1}{298.15}\right)\right)$$ (11) where \({A}_{{Fe}{S}_{2}}\) is the specific surface area of FeS2 [m2 g-1], \({m}_{{Fe}{S}_{2}}\) is the total
mass of FeS2 in the system [g], _V_ is the total volume of the system that containing FeS2 [m3], \({\rho }_{{Fe}{S}_{2}}\) is the density of FeS2 [g mol-1],
\({c}_{{Fe}{S}_{2}}\left(t\right)\) is the concentration of remaining FeS2 at the calculation time _t_ [mol m-3], and \({c}_{{Fe}{S}_{2},0}\) is the concentration of the initial FeS2 [mol
m-3]. The term \(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\) is implemented to reflect the total mass reduction of FeS2 as it is consumed by the oxidation reaction,
equating the total mass change ratio to the concentration change for the same volume of solution. The specific surface area of FeS2 particles was assumed to be spherically shaped, and the
calculation of this area used the following equation: $${A}_{{Fe}{S}_{2}}={\left(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\right)}^{\frac{2}{3}}\,\cdot\,
\frac{{S}_{{{FeS}}_{2}}}{{\rho }_{{Fe}{S}_{2}}\,\cdot\, {V}_{{Fe}{S}_{2}}}$$ (12) where \({S}_{{{FeS}}_{2}}\) is the surface area of the sphere geometry [m2], \({\rho }_{{Fe}{S}_{2}}\) is
the density of FeS2 5.01E6 [g m-3], \({V}_{{Fe}{S}_{2}}\) is the volume of FeS2 [m3], \({c}_{{Fe}{S}_{2}}\left(t\right)\) is the concentration of remaining FeS2 at the calculation time _t_
[mol m-3], and \({c}_{{Fe}{S}_{2},0}\) is the concentration of initial FeS2 [mol m-3]. The term \({\left(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\right)}^{\frac{2}{3}}\)
on the right side of eq. 12 was implemented to account for the surface area reduction as FeS2 is consumed during the oxidation reactions. This term
\({\left(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\right)}^{\frac{2}{3}}\) is derived from a constant density of FeS2 which is $$\frac{{m}_{1}}{\pi
\frac{4}{3}{r}_{1}^{3}}=\frac{{m}_{2}}{\pi \frac{4}{3}{r}_{2}^{3}}$$ (13) where \({m}_{1}\) is initial mass, \({m}_{2}\) is changed mass after the oxidation reaction, \({r}_{1}\) is radius
of initial FeS2, \({r}_{2}\) is radius of FeS2 after the oxidation reaction. Therefore, the surface area change can be derived from eq. 13 into the following equation: $$4{\rm{\pi
}}\,\cdot\, {r}_{2}^{2}=4{\rm{\pi }}\,\cdot\, {r}_{1}^{2}\,\cdot\, {\left(\frac{{{\rm{m}}}_{2}}{{{\rm{m}}}_{1}}\right)}^{\frac{2}{3}}$$ (14) where \({m}_{1}\) and \({m}_{2}\) can be
calculated by concentration of FeS2: $$4{{\pi }}\,\cdot\, {r}_{2}^{2}=4{{\pi }}\,\cdot\,
{r}_{1}^{2}\,\cdot\,{\left(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\right)}^{\frac{2}{3}}$$ (15) The
\({\left(\frac{{c}_{{Fe}{S}_{2}}\left(t\right)}{{c}_{{Fe}{S}_{2},0}}\right)}^{\frac{2}{3}}\) term can express the surface change ratio during the oxidation reaction. Also, FeS2 is insoluble
in ground water pH range as can be seen in Fig. 10. FeS2 can be dissolved in three ways: oxidative dissolution, reductive dissolution, and chemical dissolution. Among these, the oxidative
dissolution is described as eq. 11. The other dissolution mechanisms are not possible or negligibly small for pH 8 – 10 and low temperature conditions (under 100 °C). The reductive
dissolution is only possible for high temperature (over 90 °C) with high H2 partial pressure (over 0.8 MPa) conditions which is impossible for the DGR case56,57. Therefore, in this model,
the authors neglect HS- producing from FeS2 in the ground water. The aerobic microbial activity was implemented as the pseudo first order reaction: $${R}_{3}=-{k}_{3}{O}_{2,{aq}}$$ (16)
where _k__3_ is the kinetic constant of aerobic microbial activity [mol m-3yr-1], O2,aq is the concentration of dissolved O2. The kinetic constant of aerobic microbial activity (_k__3_) was
set to 6.94E-3 [yr-1]9. Here, the authors adopted electrochemical reactions on the Cu surface using Butler-Volmer equations, which were suggested by King and Kolář (CSM) and adopted in the
previous research of the authors1,9. The sum of the total currents (in Ampere unit) of anodic and cathodic currents follows the mixed-potential theory. The densities of cathodic currents and
anodic currents (A m-2) were calculated at each quadrature point and multiplied by the surface area of each element (four or three quadrature points in each element). This is where the
local partial differential solutions were estimated for calculating the global solution (numerical results) by combining the local solutions to calculate the total currents (net zero). In
this model, if the total charge rate becomes lower than 10 C yr-1, then the model determines that the calculation results satisfy the mixed-potential theory and display the corrosion
potential. The Butler-Volmer equations (17–21) are summarized in Table 6, with the definitions of variables and parameters in Table 7 and Table 8. The overall corrosion depth was calculated
by the corrosion of Cu by O2 and Cu2+ as the following equation: $$d={\mathop{\displaystyle{\int} }\limits_{t=0}^{t}}\left\{\frac{{n}_{{O}_{2}}{\nabla }^{2}\left[{O}_{2}\right]\,\cdot\,
{{{\varnothing }}}_{{ben}}\,\cdot\, \tau \,\cdot\, {D}_{a{q}_{,{O}_{2}}}S\,\cdot\, {exp} \left(-\frac{{E}_{A,{O}_{2}}}{{RT}}\right)}{{\rho }_{{Cu}}}+\frac{{n}_{C{u}^{2+}}{\nabla
}^{2}\left[C{u}^{2+}\right]\,\cdot\, {{{\varnothing }}}_{{ben}}\,\cdot\, \tau \,\cdot\, {D}_{a{q}_{,C{u}^{2+}}}S\,\cdot\, {exp} \left(-\frac{{E}_{A,C{u}^{2+}}}{{RT}}\right)}{{\rho
}_{{Cu}}}\right\}d{t}^{{\prime} }$$ (17) where _d_ is the corrosion depth of Cu [m], \({\varnothing }_{{ben}}\) is the porosity of bentonite, \(\tau\) is the tortuosity of bentonite, \(D\)
is the diffusion coefficient of the chemical species O2 and Cu2+ [mol∙m2 ∙ s-1], S is the degree of saturation in the bentonite, _E__A_ is the activation energy for the diffusion of chemical
species O2 and Cu2+, _T_ is the temperature of bentonite [K], \(\rho\) is the density of Cu [mol∙m-3], and \(n\) are number of electrons transferred from a single O2 and Cu2+ species which
are 4 and 1 respectively. APPLICATION IN DEEP GEOLOGICAL REPOSITORY SYSTEM The developed model was applied to the KAERI Reference disposal System (KRS) under oxic conditions to evaluate the
integrity of the Cu canister2. As shown in Table 1, a total of 11 cases covered the possible combinations of FeS2 content and particle sizes as described in Table 958,59,60. The removal of
O2,gas by dissolving in groundwater was simulated in this model using eq. 5, 6. This approach was based on reports that the removal of O2 by dissolving it in groundwater enhances the
depletion of O2 inside bentonite and backfilled materials12,19. Moreover, the C0S0React case assumed that the dissolved O2 (O2,aq) was consumed in the groundwater by reacting with other
oxidizing agents (such as HS-) to simulate a more realistic case7,9,17,23,25,61. In this case, the concentration O2,aq was set to 0 mol∙m-3 at the interface of the engineered barrier and
natural barrier (granite host rock), which acts as the source term for oxidizing agents. The details of the initial conditions are provided in Table 10. The KRS was selected to simulate the
corrosion of Cu canisters under oxic corrosion conditions. Table 11 presents the physical properties of the bentonite and backfill materials, as well as the host rock for KRS52. The geometry
and mesh, illustrated in Fig. 11, depict a single disposal unit. The overall calculation was conducted for the single disposal unit in 2-Dimensions to save on computational costs. In the
model, rectangular meshes were employed to control mesh quality, especially the size of meshes perpendicular to the surface of the Cu canister. This perpendicular mesh size on the Cu surface
to the bentonite can influence the corrosion rate by altering the flux of chemical reagents. This approach allowed us to control the mesh quality on the Cu canister surface while also
saving computational costs. Additionally, the Cu corrosion rate was evaluated using mixed potential theory via Butler-Volmer equations, with further consideration given to Cu(II) reduction
on the Cu surface, as can be seen in Table 6. The derived equation 7 and 11 were applied to the KRS unit to analyze the overall effects of FeS2 and Cu(I) oxidation. The groundwater pH was
set to nine and remained constant throughout the simulation, following the conditions in DGR cases from Sweden and Finland (Olkiluoto and Forsmark), due to the absence of specific data for
KRS9,62,63. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. CODE AVAILABILITY The underlying code for
this study validation datasets is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author. REFERENCES * Chae, N. et al.
Coupled mixed-potential and thermal-hydraulics model for long-term corrosion of copper canisters in deep geological repository. _npj Mater. Degrad._ 7, 26 (2023). Article Google Scholar *
Lee, J., Cho, D., Choi, H. & Choi, J. Concept of a Korean reference disposal system for spent fuels. _J. Nucl. Sci. Technol._ 44, 1565–1573 (2007). Article CAS Google Scholar * Choi,
H.-J., Lee, J. Y. & Choi, J. Development of geological disposal systems for spent fuels and high-level radioactive wastes in Korea. _Nucl. Eng. Technol._ 45, 29–40 (2013). Article
Google Scholar * Briggs, S., McKelvie, J., Keech, P., Sleep, B. & Krol, M. Transient modelling of sulphide diffusion under conditions typical of a deep geological repository. _Corros.
Eng. Sci. Technol._ 52, 200–203 (2017). Article CAS Google Scholar * Hall, D. S., Behazin, M., Jeffrey Binns, W. & Keech, P. G. An evaluation of corrosion processes affecting
copper-coated nuclear waste containers in a deep geological repository. _Prog. Mater. Sci._ 118, 100766 (2021). Article CAS Google Scholar * Briggs, S., McKelvie, J., Sleep, B. &
Krol, M. Multi-dimensional transport modelling of corrosive agents through a bentonite buffer in a Canadian deep geological repository. _Sci. Total Environ._ 599–600, 348–354 (2017). Article
PubMed Google Scholar * King, F., Hall, D. S. & Keech, P. G. Nature of the near-field environment in a deep geological repository and the implications for the corrosion behaviour of
the container. _Corros. Eng. Sci. Technol._ 52, 25–30 (2017). Article CAS Google Scholar * Rashwan, T. L. et al. Exploring the governing transport mechanisms of corrosive agents in a
Canadian deep geological repository. _Sci. Total Environ._ 828, 153944 (2022). Article CAS PubMed Google Scholar * Svensk Kärnbränslehantering A. B. Copper Sulfide Model (CSM)-Model
improvements, sensitivity analysis, and results from the integrated sulfide project inter-model comparison exercise. _Technical Report SKB TR TR-18-04_ (2019). * Williamson, M. A. &
Rimstidt, J. D. The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. _Geochim. Cosmochim. Acta_ 58, 5443–5454 (1994). Article CAS Google Scholar * Yuan, X.,
Pham, A. N., Xing, G., Rose, A. L. & Waite, T. D. Effects of pH, chloride, and bicarbonate on Cu(I) oxidation kinetics at circumneutral pH. _Environ. Sci. Technol._ 46, 1527–1535
(2012). Article CAS PubMed Google Scholar * Giroud, N. et al. On the fate of oxygen in a spent fuel emplacement drift in Opalinus Clay. _Appl. Geochem._ 97, 270–278 (2018). Article CAS
Google Scholar * Marty, N. C. M., Fritz, B., Clément, A. & Michau, N. Modelling the long term alteration of the engineered bentonite barrier in an underground radioactive waste
repository. _Appl. Clay Sci._ 47, 82–90 (2010). Article CAS Google Scholar * Goo, J.-Y., Kim, J.-S., Kwon, J.-S. & Jo, H. Y. A literature review on studies of bentonite alteration by
cement-bentonite interactions. _Econ. Environ. Geol._ 55, 219–229 (2022). Article Google Scholar * Manjanna, J., Kozaki, T. & Sato, S. Fe(III)-montmorillonite: basic properties and
diffusion of tracers relevant to alteration of bentonite in deep geological disposal. _Appl. Clay Sci._ 43, 208–217 (2009). Article CAS Google Scholar * European Commission. State of the
Art document on the corrosion behaviour of container materials. _European Commission_ (2004). * Svensk Kärnbränslehantering A. B. An Update of the State-of-the-Art Report on the Corrosion of
Copper under Expected Conditions in a Deep Geologic Repository. _SKB TR-10-67_ (2010). * Obeyesekere. Trends in Oil and Gas Corrosion Research and Technologies: Pitting corrosion. _Woodhead
Publishing_. 215–248 (2017). * Tomonaga, Y. et al. On-line monitoring of the gas composition in the Full-scale Emplacement experiment at Mont Terri (Switzerland). _Appl. Geochem._ 100,
234–243 (2019). Article CAS Google Scholar * Vinsot, A. et al. CO2 data on gas and pore water sampled in situ in the Opalinus Clay at the Mont Terri rock laboratory. _Phys. Chem. Earth,
Parts A/B/C._ 33, S54–S60 (2008). Article Google Scholar * Office fédéral des eaux et de la géologie. Mont Terri Project - Geochemistry of Water in the Opalinus Clay Formation at the Mont
Terri Rock Laboratory. _Report No. BWGG-5-E_ (2003). * Keech, P. G., Behazin, M., Binns, W. J. & Briggs, S. An update on the copper corrosion program for the long-term management of used
nuclear fuel in Canada. _Mater. Corros._ 72, 25–31 (2021). Article CAS Google Scholar * Chae, N., Park, S. & Choi, S. Cross-verified Measurement of Sulfide Concentration in Anaerobic
Conditions Using Spectroscopic, Electrochemical, and Mass Spectrometric Methods. _J. Nucl. Fuel Cycle Waste Technol._ 21, 43–53 (2023). Article Google Scholar * King, F., Chen, J., Qin,
Z., Shoesmith, D. & Lilja, C. Sulphide-transport control of the corrosion of copper canisters. _Corros. Eng. Sci. Technol._ 52, 210–216 (2017). Article CAS Google Scholar * Chen, K.
Y. & Morris, J. C. Kinetics of Oxidation of Aqueous Sulfide by O2. _Environ. Sci. Technol._ 6, 529–537 (1972). Article CAS Google Scholar * He, X. & Ahn, T. Effects of chloride on
copper corrosion in extremely low-oxygen concentration conditions. in _NACE CORROSION_ NACE-2019 (NACE, 2019). * He, X., Ahn, T. & Gwo, J.-P. Corrosion of Copper as a Nuclear Waste
Container Material in Simulated Anoxic Granitic Groundwater. _Corrosion_ 74, 158–168 (2017). Article Google Scholar * Allah, A. G. G., Abou‐Romia, M. M., Badawy, W. A. & Rehan, H. H.
Effect of halide ions on passivation and pitting corrosion of copper in alkaline solutions. _Mater. Corros._ 42, 584–591 (1991). Article CAS Google Scholar * Sridhar, N. & Cragnolino,
G. A. Effects of Environment on Localized Corrosion of Copper-Based, High-Level Waste Container Materials. _CORROSION_ 49, 967–976 (1993). Article CAS Google Scholar * Briggs, S., Lilja,
C. & King, F. Probabilistic model for pitting of copper canisters. _Mater. Corros._ 72, 308–316 (2021). Article CAS Google Scholar * Nuclear Waste Management Organization. Review of
the NWMO copper corrosion allowance. _NWMO TR-2013-04_ (2013). * Kong, M., Lee, M., Kim, G.-Y., Jang, J. & Kim, J.-S. Characterization of Compacted Ca- and Na-Bentonite with Copper
Corrosion Products in the KAERI Underground Research Tunnel. _Minerals_ 13, 898 (2023). Article CAS Google Scholar * Lee, J. S., Ray, R. I., Little, B. J. & Lemieux, E. J. Evaluation
of Deoxygenation as a Corrosion Control Measure for Ballast Tanks. _Corrosion_ 61, 1173–1188 (2005). Article CAS Google Scholar * Little, B. J., Lee, J. S. & Ray, R. I. The influence
of marine biofilms on corrosion: A concise review. _Electrochim. Acta_ 54, 2–7 (2008). Article CAS Google Scholar * King, F., Litke, C. D., Quinn, M. J. & LeNeveu, D. M. The
measurement and prediction of the corrosion potential of copper in chloride solutions as a function of oxygen concentration and mass-transfer coefficient. _Corros. Sci._ 37, 833–851 (1995).
Article CAS Google Scholar * Salmon, S. U. Geochemical modelling of acid mine drainage in mill tailings: Quantification of kinetic processes from laboratory to field scale. _Doctoral
Dissertation_, _KTH Royal Institute of Technology_ (2003). * Xu, T., White, S. P., Pruess, K. & Brimhall, G. H. Modeling of Pyrite Oxidation in Saturated and Unsaturated Subsurface Flow
Systems. _Transp. Porous Media_ 39, 25–56 (2000). Article Google Scholar * Xu, Y. et al. A hydro-thermal-geochemical modeling framework to simulate reactive transport in a waste coal area
under amended and non-amended conditions. _Heliyon_ 6, e02803 (2020). Article PubMed Google Scholar * Dos Santos, E. C., De Mendonça Silva, J. C. & Duarte, H. A. Pyrite Oxidation
Mechanism by Oxygen in Aqueous Medium. _J. Phys. Chem. C._ 120, 2760–2768 (2016). Article Google Scholar * Alakangas, L., Lundberg, A. & Nason, P. Simulation of pyrite oxidation in
fresh mine tailings under near-neutral conditions. _J. Environ. Monit._ 14, 2245–2253 (2012). Article CAS PubMed Google Scholar * Molson, J. W., Fala, O., Aubertin, M. & Bussière, B.
Numerical simulations of pyrite oxidation and acid mine drainage in unsaturated waste rock piles. _J. Contam. Hydrol._ 78, 343–371 (2005). Article CAS PubMed Google Scholar * Tabelin,
C. et al. Prediction of acid mine drainage formation and zinc migration in the tailings dam of a closed mine, and possible countermeasures. _MATEC Web Conf._ 268, 6003 (2019). Article CAS
Google Scholar * King, F. & Behazin, M. A Review of the Effect of Irradiation on the Corrosion of Copper-Coated Used Fuel Containers. _Corrosion and Materials Degradation_ 2 678–707 at
https://doi.org/10.3390/cmd2040037 (2021). * Jodeiri Shokri, B., Doulati Ardejani, F., Ramazi, H. & Moradzadeh, A. Predicting pyrite oxidation and multi-component reactive transport
processes from an abandoned coal waste pile by comparing 2D numerical modeling and 3D geo-electrical inversion. _Int. J. Coal Geol._ 164, 13–24 (2016). Article CAS Google Scholar * Todd,
E. C., Sherman, D. M. & Purton, J. A. Surface oxidation of pyrite under ambient atmospheric and aqueous (pH = 2 to 10) conditions: electronic structure and mineralogy from X-ray
absorption spectroscopy. _Geochim. Cosmochim. Acta_ 67, 881–893 (2003). Article CAS Google Scholar * Bonnissel-Gissinger, P., Alnot, M., Ehrhardt, J.-J. & Behra, P. Surface Oxidation
of Pyrite as a Function of pH. _Environ. Sci. Technol._ 32, 2839–2845 (1998). Article CAS Google Scholar * Moses, C. O., Nordstrom, D., Herman, J. S. & Mills, A. Aqueous Pyrite
Oxidation by Dissolved Oxygen and by Ferric Iron. _Geochim. Cosmochim. Acta_ 51 (1985). * Guilinger, T. R., Schechter, R. S. & Lake, L. W. Kinetic study of pyrite oxidation in basic
carbonate solutions. _Ind. Eng. Chem. Res._ 26, 824–830 (1987). Article CAS Google Scholar * Manaka, M. Extent of pyrite oxidation by dissolved oxygen in alkaline solutions including
purified sodium bentonite. _Appl. Geochem._ 148, 105548 (2023). Article CAS Google Scholar * Martinez-Moreno, M. F. et al. Impact of compacted bentonite microbial community on the clay
mineralogy and copper canister corrosion: a multidisciplinary approach in view of a safe Deep Geological Repository of nuclear wastes. _J. Hazard. Mater._ 458, 131940 (2023). Article CAS
PubMed Google Scholar * Burzan, N. et al. Growth and Persistence of an Aerobic Microbial Community in Wyoming Bentonite MX-80 Despite Anoxic in situ Conditions. _Front. Microbiol_. 13,
(2022) * Lee, C., Cho, W.-J., Lee, J. & Kim, G. Y. Numerical Analysis of Coupled Thermo-Hydro-Mechanical (THM) Behavior at Korean Reference Disposal System (KRS) Using TOUGH2-MP/FLAC3D
Simulator. _J. Nucl. Fuel Cycle Waste Technol._ 17, 183–202 (2019). Article CAS Google Scholar * Permann, C. J. et al. MOOSE: Enabling massively parallel multiphysics simulation.
_SoftwareX_ 11, 100430 (2020). Article Google Scholar * Park, S. et al. Coupled 3D thermal-hydraulic code development for performance assessment of spent nuclear fuel disposal system.
_Nucl. Eng. Technol_. (2024) https://doi.org/10.1016/j.net.2024.04.043. * Adjustment, Estimation, and Uses of Equilibrium Reaction Constants in Aqueous Solution. _Chemistry International --
Newsmagazine for IUPAC_. 29, 14–15 (2007). * Truche, L., Berger, G., Destrigneville, C., Guillaume, D. & Giffaut, E. Kinetics of pyrite to pyrrhotite reduction by hydrogen in calcite
buffered solutions between 90 and 180 °C: Implications for nuclear waste disposal. _Geochim. Cosmochim. Acta_ 74, 2894–2914 (2010). Article CAS Google Scholar * Svensk
Kärnbränslehantering A. B. A Review of the Properties of Pyrite and the Implications for Corrosion of the Copper Canister. _SKB TR-13_–_19_ (2013). * Svensk Kärnbränslehantering A. B.
Mineralogy and sealing properties of various bentonites and smectite-rich clay materials. _SKB TR-06-30_ (2006). * MalGoBalGaeBitNaLa, Y., Heui-ju, C., Min-soo, L. & Seung-yeop, L.
Measurement of Properties of Domestic Bentonite for a Buffer of an HLW Repository. _J. Nucl. Fuel Cycle Waste Technol._ 14, 135–147 (2016). Article Google Scholar * Nuclear Waste
Management Organization. Review of the THMC Properties of MX-80 Bentonite. Nuclear Waste Management Organization. _NWMO TR-2019-07_ (2019). * Lefers, J. B., Koetsier, W. T. & Van Swaaij,
W. P. M. The oxidation of sulphide in aqueous solutions. _Chem. Eng. J._ 15, 111–120 (1978). Article CAS Google Scholar * Posiva Oy. On the Origin and Chemical Evolution of Ground Water
at the Olkiluoto Site. _POSIVA 96-04_ (1996). * Gimeno, M. J., Tullborg, E.-L., Nilsson, A.-C., Auqué, L. F. & Nilsson, L. Hydrogeochemical characterisation of the groundwater in the
crystalline basement of Forsmark, the selected area for the geological nuclear repositories in Sweden. _J. Hydrol._ 624, 129818 (2023). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This research was supported by the Institute for Korea Spent Nuclear Fuel (iKSNF) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science
and ICT (NRF-2021M2E1A1085194), and Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea
(No. 20224000000120). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Disposal Performance Demonstration Research Division, Korea Atomic Energy Research Institute, Daejeon, Republic of Korea
Nakkyu Chae & Seungjin Seo * Department of Nuclear Engineering, Seoul National University, Gwanak-gu, Seoul, Republic of Korea Nakkyu Chae, Seungjin Seo & Sungyeol Choi * Nuclear
Research Institute for Future Technology and Policy, Seoul National University, Gwanak-gu, Seoul, Republic of Korea Richard I. Foster & Sungyeol Choi * Institute of Engineering Research,
Seoul National University, Gwanak-gu, Seoul, Republic of Korea Sungyeol Choi Authors * Nakkyu Chae View author publications You can also search for this author inPubMed Google Scholar *
Seungjin Seo View author publications You can also search for this author inPubMed Google Scholar * Richard I. Foster View author publications You can also search for this author inPubMed
Google Scholar * Sungyeol Choi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.C. developed the numerical model and was a major
contributor in writing the manuscript. S.S. contributed to the multi-threads calculation of the developed model for reactive-transport phenomena. R.I.F. was data curation with a major
contributor in writing the manuscript. S.C. supervised the research and reviewed and edited the original draft. CORRESPONDING AUTHOR Correspondence to Sungyeol Choi. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission
under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chae, N., Seo, S., Foster, R.I. _et al._ Oxic corrosion model for KAERI
Reference disposal system via O2 consumption reactions and mixed-potential theory. _npj Mater Degrad_ 8, 80 (2024). https://doi.org/10.1038/s41529-024-00497-z Download citation * Received:
26 April 2024 * Accepted: 22 July 2024 * Published: 07 August 2024 * DOI: https://doi.org/10.1038/s41529-024-00497-z SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative