Formaldehyde initiates memory and motor impairments under weightlessness condition
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT During space flight, prolonged weightlessness stress exerts a range of detrimental impacts on the physiology and psychology of astronauts. These manifestations encompass depressive
symptoms, anxiety, and impairments in both short-term memory and motor functions, albeit the precise underlying mechanisms remain elusive. Recent studies have revealed that hindlimb
unloading (HU) animal models, which simulate space weightlessness, exhibited a disorder in memory and motor function associated with endogenous formaldehyde (FA) accumulation in the
hippocampus and cerebellum, disruption of brain extracellular space (ECS), and blockage of interstitial fluid (ISF) drainage. Notably, the impairment of the blood-brain barrier (BBB) caused
by space weightlessness elicits the infiltration of albumin and hemoglobin from the blood vessels into the brain ECS. However, excessive FA has the potential to form cross-links between
these two proteins and amyloid-beta (Aβ), thereby obstructing ECS and inducing neuron death. Moreover, FA can inhibit N-methyl-D-aspartate (NMDA) currents by crosslinking NR1 and NR2B
subunits, thus impairing memory. Additionally, FA has the ability to modulate the levels of certain microRNAs (miRNAs) such as miRNA-29b, which can affect the expression of aquaporin-4
(AQP4) so as to regulate ECS structure and ISF drainage. Especially, the accumulation of FA may inactivate the ataxia telangiectasia-mutated (ATM) protein kinase by forming cross-linking, a
process that is associated with ataxia. Hence, this review presents that weightlessness stress-derived FA may potentially serve as a crucial catalyst in the deterioration of memory and motor
abilities in the context of microgravity. SIMILAR CONTENT BEING VIEWED BY OTHERS THE EFFECTS OF REAL AND SIMULATED MICROGRAVITY ON CELLULAR MITOCHONDRIAL FUNCTION Article Open access 08
November 2021 ACCUMULATION OF FORMALDEHYDE CAUSES MOTOR DEFICITS IN AN IN VIVO MODEL OF HINDLIMB UNLOADING Article Open access 19 August 2021 BEHAVIORAL AND MULTIOMICS ANALYSIS OF 3D
CLINOSTAT SIMULATED MICROGRAVITY EFFECT IN MICE FOCUSING ON THE CENTRAL NERVOUS SYSTEM Article Open access 17 February 2025 INTRODUCTION The condition of weightlessness is a unique setting
encountered by individuals when they venture into space. In recent times, owing to the swift progress of the global space sector, the forthcoming establishment of advanced space stations
will furnish astronauts with a cutting-edge platform to engage in extended space missions. Consequently, ensuring the long-term well-being of astronauts in space has emerged as a central
area of concern in contemporary aerospace medicine research. Over the course of millions of years, human beings have developed physiological structures and functional characteristics that
are well-suited to the gravitational conditions of the Earth1. However, extended periods of weightlessness have been found to have inevitable physiological and psychological effects on the
human body2. Extensive research in aerospace medicine has demonstrated that weightlessness not only significantly impacts the physical well-being of astronauts, but also represents a crucial
factor in the development of brain damage among them3. The aforementioned phenomenon not only hampers the astronauts’ equilibrium performance, motor control, and short-term memory storage
capabilities, but also exhibits a high susceptibility to instigating neurological disorders, encompassing cognitive functions such as reaction, judgment, decision-making, and other cognitive
processes. Consequently, this significantly impairs the astronauts’ operational efficacy. Notably, the primordial gaseous molecules in the course of early evolution, such as: formaldehyde
(FA), carbon monoxide (CO), nitric oxide (NO), and hydrogen sulfide (H2S), exist in the brain and are considered to act as gaseous neuromodulators to regulate brain functions4,5. Endogenous
and active FA is mainly derived from the demethylation of sarcosine (SA), methylamine (MMA), DNA or histone via mitochondrial sarcosine dehydrogenase (SARDH), semicarbazide-sensitive amine
oxidase (SSAO) and demethylase6. Surprisingly, certain external stimuli such as hindlimb unloading (HU) simulating microgravity, spatial learning, and electrical stimulation, have been found
to elicit the generation of FA in the brain through the involvement of SSAO and SARDH4,7. Additionally, other stress also contributes to the endogenous production of FA8,9. It has been
found that stress can induce the accumulation of FA in the brain; especially, microgravity stress leads to an increase in FA content in the brains of the HU models mimicking the astronauts
in space4,7. However, when FA reaches a certain concentration, it does not continue to rise, but remains at a certain pathological concentration7. After astronauts return to Earth, the
microgravity stress disappears, the activity and expression contents of formaldehyde dehydrogenase (FDH) in the brain may return to normal levels10, thereby degrading excessive FA in the
brain11. In recent years, the advancement of magnetic resonance imaging (MRI) technology has facilitated the detection of water diffusion in the brain extracellular space (ECS) through the
utilization of tracers12. A high-resolution MRI technique for aquaporin-4 (AQP4) in vivo has been established, which greatly improves the sensitivity of MRI measurement of water molecule
transmembrane transport by specifically labeling and amplifying its magnetic resonance signal13. Consequently, this technique has become the preferred method for investigating the
microstructural characteristics of neural tissue. Notably, the glymphatic system removes brain interstitial solutes, with AQP4 being a key component14. AQP4’s involvement in the exchange of
cerebrospinal fluid (CSF) and interstitial fluid (ISF) has led to hypotheses about the impact of the excessive FA on AQP4 and the glymphatic system’s function. MicroRNAs (miRNAs) are a
notable group of endogenous non-coding single-stranded RNAs that have been observed to play a significant role in various neural functions. Many studies have confirmed that FA can regulate
the levels of miRNAs15,16,17. The use of drug delivery systems that target the lymphatic system and brain ECS to remove accumulated endogenous FA and regulate levels of microRNAs is crucial
for maintaining the cognitive health of astronauts and ensuring the success of future space exploration missions. Hence, this review presents a comprehensive examination of the advancements
in research pertaining to the possible molecular mechanisms underlying memory and motor dysfunctions induced by FA derived from space weightlessness, as elucidated in the preceding relevant
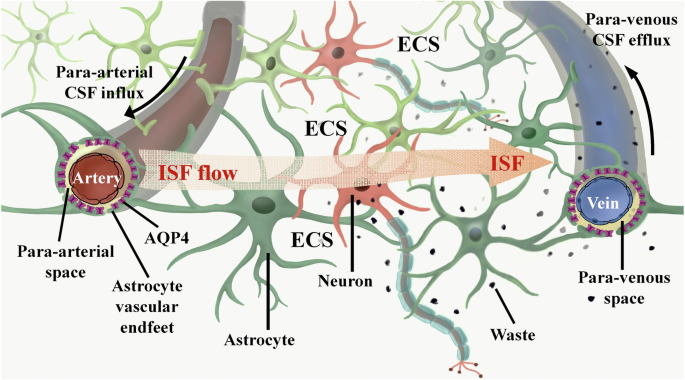
literatures. ROLE OF CSF-ISF EXCHANGE IN MAINTAINING BRAIN FUNCTIONS The brain ECS is a non-uniform spatial arrangement situated adjacent to the neural network, measuring ~38–64 nm in width.
It encompasses 15-20% of the brain’s total volume, surpassing the previously emphasized cerebral blood vessel space (3-5%)18. The ECS wall structure comprises the cell membranes of neurons,
astrocytes, oligodendrocytes, microglia, and other cellular components, in addition to the cerebral vascular wall. The brain ECS comprises ISF, extracellular matrix (ECM), and a variety of
essential nutrients, ions, and neurotransmitters necessary for the sustenance and operation of nerve cells19. Within the brain ECS, ISF facilitates the transportation of neurotransmitters
and nutrients to nerve cells, as well as the exchange of metabolic waste with the parenchyma20. Consequently, ISF plays a crucial role as a mediator for nutrient provision, waste
elimination, and intercellular communication within brain tissue. The primary physiological role of the human lymphatic system is the elimination of metabolites from the body. Lymphatic
vessels are distributed extensively throughout the body, contrary to the previous belief that the lymphatic system was absent in the brain21. Historically, it was thought that metabolites in
the brain were primarily eliminated through CSF circulation. The CSF, a transparent and colorless fluid, fills the ventricles of the brain and the subarachnoid space, serving to safeguard
the central nervous system against external shocks and maintain its normal metabolic functions22. Through the progression of scientific inquiry, researchers have discovered that the CSF
circulation, in isolation, is insufficient for the expeditious elimination of the majority of metabolites. Consequently, they embarked upon an investigation to ascertain the existence of
alternative pathways for metabolite removal within the brain. In 2012, it was found that there is the existence of a CSF-ISF convective system based on the perivascular space in the mouse
brain by using the method of two-photon in-vivo imaging to explore the dynamic flow process of CSF in the mouse brain23, further refining the mechanism of waste removal in the nervous
system24. This convective system employs AQP4 on the terminal feet of astrocytes to facilitate the transportation of amyloid-beta (Aβ) protein and metabolites to the CSF for clearance. It is
referred to as the glymphatic system due to its functional similarity to the peripheral lymphatic system25. However, there is an observed decline in the functionality of the brain’s
glymphatic system in various disease states, including traumatic brain injury, Alzheimer’s disease (AD) and Parkinson’s disease (PD)26,27,28. This suggests that the proper functioning of the
glymphatic system is crucial for maintaining the brain’s homeostasis. In brain tissue, ISF can undergo rapid transportation through the active or passive involvement of AQP4 located on the
astrocyte end-foot. This transportation process facilitates the translocation of ISF from the end of the astrocyte peduncle to the perivascular space. Subsequently, the ISF is exchanged with
the CSF and distributed to various locations29,30, ultimately draining into the peripheral lymphatic system31,32. Consequently, the intermingling and movement of the ISF and CSF within the
brain tissue establish a connection with the peripheral lymphatic system, which is widely acknowledged as the primary pathway for substance exchange and waste elimination in the brain
tissue33. Explorations into brain ECS and glymphatic system of the brain have yielded fresh insights into the underlying pathological mechanisms of brain disorders and hold promise as
prospective therapeutic targets for a range of brain diseases (Fig. 1). EFFECTS OF MICROGRAVITY-DERIVED FA ON COGNITIVE FUNCTIONS Gaseous FA, a colorless and volatile gas with an irritating
odor, is widely recognized for its use as a preservative34. It is noteworthy that primary gaseous FA has been regarded as the earliest and simplest form to emerge during the early stages of
Earth’s evolution, encompassing carbon, hydrogen, and oxygen elements within a small organic molecule35. In fact, FA serves as the primary precursor for numerous intricate organic compounds,
such as amino acids, RNA, DNA, and proteins36. It is widely recognized that FA is a well-established indoor air pollutant that has been observed to cause memory deficits in animals and
cognitive decline in humans37,38. Interestingly, FA is found in all vertebrate cells, potentially as a byproduct of various metabolic reactions such as methanol oxidation, DNA or histone
demethylation39. Recent research has demonstrated that endogenous FA is present in the cytoplasm, nucleus, and subcellular organelles of all organisms, and it plays a role as a gas signaling
molecule in the processes of learning and memory39,40. METABOLISM OF FA IN THE LIVING ORGANISM Unexpectedly, apart from the inhalation of exogenous FA, the human body also synthesizes
endogenous FA via diverse pathways, with enzyme-catalyzed reactions serving as the primary mechanism. For instance, the breakdown of endogenous amine substances, such as methylamine,
histamine, and polyamines, into FA is facilitated by SSAO. Additionally, FA is generated through DNA demethylation catalyzed by lysine demethylase (LSD), and mitochondrial cytochrome P450
enzymes oxidize exogenous compounds to produce FA (Fig. 2). Research has demonstrated that the stress-induced activation of SSAO, which is widely distributed in vascular cells, smooth muscle
cells, and adipocytes, facilitates the deamination of various exogenous and endogenous monoamines9. This enzymatic activity leads to the production and accumulation of endogenous FA41.
Numerous investigations have indicated that FA can disrupt the structural integrity of proteins, thereby impacting the physiological function of cells. Prolonged exposure to FA has been
associated with detrimental effects on multiple human systems, including the respiratory, digestive, immune, and neurological systems42. MICROGRAVITY STRESS INDUCES FA ACCUMULATION IN THE
BRAINS Microgravity is a significant stressor that can induce prolonged stress in astronauts. For example, the simulation of space weightlessness through HU for a continuous period of 2
weeks has been found to trigger the production and accumulation of FA in the hippocampus and cerebellum of mice models. This effect is achieved through the activation of both the SSAO and
SARDH4,7 (Fig. 3). During this period, significant sympathetic neurotransmitters, including adrenaline, experience a surge43, and adrenaline, facilitated by monoamine oxidase-A (MAO-A),
undergoes deamination resulting in the production of hydrogen peroxide (H2O2) and MMA44. MMA is readily metabolized by SSAO, leading to the generation of FA45,46. Concurrently, the metabolic
generation of H2O2 can directly inhibit the function of FDH10, thereby impeding the timely degradation of FA11. Consequently, this impediment results in the excessive buildup of endogenous
FA within brain tissues, subsequently instigating a pronounced state of oxidative stress and the generation of a substantial quantity of free radicals and other detrimental compounds.
Excessive free radicals in brain tissues, facilitated by the metal ion catalytic system, initiate an assault on the amino or imino groups of amino acid molecules47,48,49. This process
results in the conversion of amino acids into carbonyl derivatives, thereby causing structural disruption and functional impairment of protein molecules. Consequently, neuronal cells
experience structural damage, ultimately culminating in apoptosis or death. These detrimental effects on neuronal cells can manifest as symptoms including depression, anxiety, and memory
disorders50,51,52. MICROGRAVITY STRESS DAMAGES ECS STRUCTURE AND ISF DRAINAGE The hippocampus, situated between the thalamus and the medial temporal lobe, is an integral component of the
limbic system in the brain, primarily implicated in cognitive processes such as learning and memory53. Presently, it is widely accepted that the hippocampus primarily serves the purposes of
short-term memory consolidation and spatial orientation. Microgravity can disrupt the typical morphology of hippocampal neurons54, concurrently diminishing the levels of nerve growth factor
and brain derived neurotrophic factor (BDNF)55, thereby impeding the customary development and metabolic processes of hippocampal neurons56. BDNF, as a constituent of the neurotrophic factor
family57, plays a pivotal role in the formation of both short-term and long-term memory58. Evidence consistent with this is that application of FA solution can downregulate BDNF49. Notably,
microgravity in space modifies the convoluted nature of hippocampal ECS, resulting in disrupted ISF drainage and impaired neuromelanin sheaths. These alterations ultimately culminate in the
degeneration and demise of hippocampal neurons59. For example, the utilization of tracer-based MRI techniques revealed notable hippocampal impairment subsequent to the simulation of
microgravity conditions for a duration of 7 days in the HU model mice. This impairment was characterized by a pronounced deceleration in ISF drainage within hippocampal ECS, a reduction in
diffusion rate, and a simultaneous modification in the tortuosity of the ECS. Notably, such alterations are typically irreversible59 (Fig. 4). The occurrence of disturbed drainage of ISF in
the hippocampal ECS may lead to the accumulation of metabolic wastes that cannot be excreted, and the occurrence of strong oxidative stress in neuronal cells, which may influence the
expression of relevant proteins and trigger neuronal damage and death54. For example, HU simulated microgravity for 7 days can cause death of hippocampal neurons in rats, which indeed result
in memory impairments60. Unsurprisingly, the changes in the HU rat hippocampal CA1 region have been observed after 14-days tail suspension, because the mean area of the neurons, synaptic
gaps, and length of neuronal active zones in the hippocampus were markedly reduced61. FA IMPAIRS MEMORY BY SUPPRESSING NMDA RECEPTORS ACTIVITY Using the HU rat model, it has been observed
that the formation of the SNARE complex, which is linked to cognitive processes such as learning and memory, is partially hindered in a simulated microgravity environment for a continuous
period of 21 days62. Furthermore, the loss of β-synuclein was also observed in hippocampus from mice kept in simulated microgravity environment for 7 days, which serves as a molecular
chaperone and effectively prevents abnormal protein aggregation54,63. These findings provide evidence to support the view that microgravity triggers abnormal protein aggregation in
hippocampal neurons, thereby affecting the functions of learning and memory54. Although electric stimuli can elicit an elevation in the levels of endogenous FA (0.05 mM) and enhance memory
formation, injection of FA at a concentration of 450 μM can impair spatial memory in healthy mice64,65, it is possible that excessive FA may block N-methyl-D-aspartate receptors (NMDAR).
Meanwhile, it was observed that the level of FA in the cerebellum has exceeded 450 μM in the HU mouse model7, indicating that the level required to cause spatial memory impairment is indeed
consistent with microgravity simulation. A prior investigation has indicated the possibility of intermolecular cross-linking occurring between residue C79 of NR1 and lysine (K)79 of NR266,
both of which are subunits of the NMDAR. However, analysis of the 3D crystal structure of NR1/NR2B (PBD ID: 4PE5) reveals that the distance between C79 and K79 is ~4 Å67. It is
well-established that at a sufficiently high concentration, FA can act as a cross-linker for protein cysteine (C), K, and tyrosine (Y) residues68,69. This hypothesis posits that FA at a
concentration of 450 μM could potentially hinder the functioning of the NMDAR by forming a cross-link between C79 and K79. Notably, when a single point mutation was introduced in either NR1
C79 or NR2B K79, the inhibitory effect of FA on NMDA currents in transfected CHO cells was reversed4. These findings provide substantial evidence supporting the notion that an excessive
amount of FA can impede NMDAR activity through the cross-linking of NR1 and NR2B residues (Fig. 5). EFFECTS OF MICROGRAVITY ON MOTOR FUNCTION OF ASTRONAUTS Due to the significant weakening
of gravity in space, astronauts frequently encounter cephalad fluid shifting, resulting in notable changes in intracranial pressure70. The perception of their vestibular system is also
profoundly impacted, leading to imbalance and sensations of dizziness71. Meanwhile, muscular and skeletal alterations have been identified as potential primary factors contributing to the
decline in motor function among astronauts72,73. When exposed to microgravity, the musculoskeletal system lacks mechanical load, leading to muscle atrophy and bone loss, threatening the
safety of long-term missions and motor function of astronauts returning to Earth74. However, in the HU rat model, although muscle atrophy in the hind limbs had recovered after a 2-week
recovery period, the motor deficits were not reversed75,76. Recent studies have found that excessive FA in the muscle mainly induced gait instability but not motor disfunction7. Meanwhile,
anatomical observations showed that the cerebellum of rats has been damaged during space flight77. Astronauts usually recover from symptoms related to movement disorders and ataxia 30 days
after returning to Earth78,79, because microgravity stress disappears and the activity and expression contents of FDH in the brain may recover to the normal levels10, and lead to the
degradation of FA11. Although astronauts gradually regained their main motor function79, brain damage caused by changes in nanoscale neuronal structure still exists due to the acute
accumulation of FA in microgravity environment59,60,61, which is one of the more likely causes of astronaut motor dysfunction. With the widespread use of MRI technology, it has been found
that after astronauts return to Earth, the cerebellar structure of astronauts still undergoes changes after prolonged space flight80, including an increase in tissue density and gray matter
volume in regions such as the cerebellar vermis73,81. Due to the spatial resolution of MRI typically being at the micrometer level, it is unable to display changes in the nanoscale neuronal
structure caused by brain injury82. Hence, the precision instruments with nanometer resolution are better able to diagnose or detect space weightlessness-induced brain damage and monitor
brain recovery after astronauts return to Earth. CEREBELLAR DAMAGE INDUCES ATAXIA The cerebellum serves as a crucial motor regulatory hub within the human body, primarily tasked with the
maintenance of bodily equilibrium, regulation of muscle tone, and facilitation of voluntary movements. While voluntary movements are consciously initiated by the cerebral cortex, the
cerebellum assumes the responsibility of coordinating these intentional actions83. The cerebellum integrates afferent nerve impulses of both types and modulates the movement of associated
muscles via efferent fibers, thereby sustaining the coordination of voluntary movements. Consequently, cerebellar injury elicits symptoms of ataxia, characterized by impaired regulation of
muscle tone and coordination of voluntary movements. Ataxia is a motor disorder characterized by the disruption of autonomous trunk movements, resulting in the inability to maintain trunk
posture and balance despite normal muscle strength. The occurrence of ataxia can be attributed to abnormalities in subcortical motor structures, including the motor cortex, basal ganglia,
and cerebellum, with cerebellar ataxia being the predominant form84. In a manner akin to the manifestations of ataxia, astronauts encounter a progressive deterioration in their motor control
and coordination throughout extended periods of space travel, subsequently presenting symptoms upon reentry to the Earth, including impaired spatial orientation during ambulation85,
modified patterns of muscle activation86,87, and diminished motor coordination88. RELATIONSHIP BETWEEN CSF VOLUME AND ISF DRAINAGE In recent years, research has revealed that prolonged
spaceflight has led to modifications in the cerebellar structure of astronauts89, which plays a crucial role in regulating precise motor movements. Additionally, the vestibular system
responsible for maintaining equilibrium has also been affected to a certain degree90. Specifically, weightlessness has been observed to disrupt the connectivity of the vestibular nuclei,
resulting in diminished connectivity of the inferior cerebellar peduncle structures associated with space travel91. The astronauts exhibited a decrease in intrinsic connectivity in the right
insula and ventral posterior cingulate cortex, which corresponded to a decrease in functional connectivity between the left cerebellum and brain regions associated with motor functions92.
Following exposure to weightlessness in space, the expansion of CSF volume may exert pressure on the cerebellar parenchyma, potentially resulting in an elevation in the tortuosity of the ECS
within the cerebellum93. Consequently, this could impede or halt the ISF drainage, thereby hindering the exchange between CSF and ISF. Both microglia activation and cerebellar neuron death
contribute to the increase in tortuosity of ECS in the cerebellum7 (Fig. 6). MICROGRAVITY-RELATED OXIDATIVE STRESS INDUCES NEURON DEATH Previous research has shown that microgravity
influences the biological functions of mitochondria within cells, resulting in an increase in glycolysis, tricarboxylic acid cycle, reactive oxygen species levels, and NADPH oxidase
activity94. However, oxidative phosphorylation and components of the mitochondrial respiratory chain are downregulated in this context94. Simulated microgravity induced oxidative stress in
the cerebellar tissues of rats, resulting in a noteworthy elevation in reactive nitrogen species levels and a significant reduction in the overall antioxidant capacity within the cerebellar
tissues after 21 days of tail suspension95. Following space flight, a decline in cytochrome oxidase activity was observed in rat cerebellar Purkinje cells, leading to an upsurge in free
radical production and a decline in energy release, thereby impacting the proper functioning of the cerebellum96. Neuronal cells exhibited notable oxidative stress when subjected to
simulated microgravity97. Meanwhile, neural stem cells may exhibit autophagy related events like caused by endoplasmic reticulum stress98. Notably, H2O2, the production of oxidative stress,
has been found to induce FA generation99; subsequently, excessive FA induces the death of cerebellar neurons7. However, the damage to the cerebellum caused by microgravity is not persistent.
It has been shown that the structural changes in the cerebellar cortex of rats were most significant on the 21st day of tail suspension, but showed a trend of recovery on the 28th day with
adaptation100. IMPAIRMENTS OF ECS AND ISF BY MICROGRAVITY STRESS-DERIVED FA FA can quickly penetrate into cells and undergo chemical reactions with proteins or DNA molecules, forming
intermolecular cross-linking or intramolecular chemical modifications101. By nucleophilic addition reactions with groups such as amino or imino groups in protein molecules, FA forms new
covalent bonds between protein molecules and cross-links them102, thus altering the spatial structure of protein molecules. There have been studies in recent years suggesting that FA
cross-links not only proteins within the cell, but also proteins in the ECS, leading to abnormalities in the ECS structure. FA-CROSSLINKED ECM PROTEINS IN THE BRAIN ECS One of the important
structures for cells to sense gravity is the ECM in the ECS103, which includes components such as collagen, elastin, proteoglycans, aminoglycans, fibronectin, and laminin, while the
expression of ECM proteins in the ECS can be affected by weightlessness conditions104. Surprisingly, weightlessness can increase the amount of ECM proteins and cytoskeleton in the papillary
thyroid carcinoma cells105. Remarkably, excessive FA can fix brain tissues and lead to the hardening of tissue106. Especially, FA can crosslink ECM proteins107. Hence, FA-deposited ECM
protein in the ECS is a direct factor of increasing the tortuosity of brain ECS and impeding ISF drainage. FA-CROSSLINKED AΒ DEPOSITION IN THE BRAIN ECS In the context of prolonged
weightlessness, endogenous FA primarily originates from the oxidative deamination of methylamine catalyzed by SSAO. The presence of FA further intensifies intracellular oxidative stress8,
and causes harm to vascular endothelial cells108. Additionally, FA readily binds to cysteine and lysine sites on proteins109,110, leading to the formation of cross-linking products between
proteins and ultimately compromising the integrity of the ECS structure. Excessive FA have been observed to induce the aggregation of Aβ in vitro and in vivo by promoting the formation of Aβ
oligomers and protofibrils111. This characteristic allows FA to cross-link harmless Aβ monomer depositions in the brain ECS, leading to the formation of Aβ-related senile plaques (SP).
Recent study has found that sleep can scavenge Aβ from the brain to the peripheral blood; however, sleep disorders increase the risk of Aβ deposition in the brain112. Hence, Aβ-deposited in
the ECS not only impairs the ECS structure but also impedes ISF drainage in the brain6, ultimately resulting in the demise of neurons located deep within the brain, such as the hippocampal
neurons responsible for memory formation59. Under the condition of a disruption of the neuronal microenvironment, these neurons are unable to obtain adequate nourishment and effectively
eliminate metabolic waste products (Fig. 7). FA-CROSSLINKED ALBUMIN/HEMOGLOBIN DEPOSITION IN ECS Under physiological conditions, albumin contents in the plasma is much higher than that in
the ISF; while hemoglobin is mainly present in the red blood cells of the plasma113,114. It is worth mentioning that the disruption of the blood-brain barrier (BBB) induced by microgravity
in space triggers the penetration of albumin and hemoglobin from the blood vessels into the brain ECS115. When endothelial damage occurs in capillaries, resulting in increased vascular
permeability, there is a notable influx of albumin into the brain ECS116. Meanwhile, some red blood cells in the blood will also enter the ECS through the vascular wall, leading to
extravascular hemolysis and the release of a large amount of hemoglobin into the ECS117. Excessive FA has been found in the hippocampus and cerebellum after microgravity stress7. The
interaction between albumin and FA induces structural changes in albumin, leading to a reduction in the quantity of α-helices118. This alteration results in a relaxation of the albumin
framework and exposure of internal amino acids, potentially causing toxicity in organisms118. FA has been shown to possess the capacity to modify hemoglobin, generating a diverse combination
of modified hemoglobin, including adducts with cross-linked hemoglobin chains119. In the presence of a disrupted BBB after space flight, FA cross-linked hemoglobin/albumin could accumulate
in the brain ECS (Fig. 8). Although cross-linked proteins can activate microglia to enhance their phagocytic and proteolytic activities120, excessive activation may lead to more protein
cross-linking in brain ECS, causing a vicious cycle121. Cross-linked albumin/hemoglobin cannot break down under physiological conditions, blocking ISF drainage by taking up space in the
ECS109,110. ROLE OF MIRNAS IN REGULATING GENE EXPRESSION Multiple miRNAs have been identified in a wide range of organisms, including animals, plants, and viruses. Their discovery was
serendipitous, occurring during a study on lin-14 in the nematode Hidradenitis elegans122. These miRNAs belong to a highly conserved class of endogenous non-coding small molecule
single-stranded RNAs, which play a crucial role in post-transcriptional regulation of gene expression123,124. By binding to specific sites in the 3’ untranslated region of target genes and
engaging in complementary base pairing, miRNAs can modulate the degradation or translation of target mRNAs125 (Fig. 9). Despite constituting a mere 2% of the overall human gene count, miRNAs
are responsible for regulating at least 30% of human genes, with over 5300 human protein targets being subject to miRNA regulation by regulating different expression patterns126,127,128.
Numerous clinical studies have demonstrated that miRNAs play a crucial role in modulating central nervous system functions and are implicated in diverse biological processes129,130,
including neuronal cell metabolism, proliferation, apoptosis, and the pathogenesis of various neurodegenerative disorders129,130. For example, the downregulation of miR-205 may contribute to
an increase in the potential pathogenicity of LRRK2 protein in the brain of patients with sporadic PD131. Deficiency of miR-218-2 induces the deficits in the morphology and presynaptic
neurotransmitter release in the hippocampus, impairing the abilities of learning and memory132. In addition, the deficiency of miR-26a-3p in the dentate gyrus of the hippocampus can activate
the PTEN/PI3K/Akt signaling pathway, leading to neuronal deterioration and depressive- like behaviors133. The low levels of miR-132 upregulates ITPKB, leading to an increase in BACE1
activity and promoting the production of Aβ134 (Fig. 9). Recent studies have revealed that FA has the capacity to modify the regulatory role of miRNAs in gene expression15. Specifically, FA
has been found to disrupt the expression profiles of miRNAs in neuronal cells16, consequently leading to the dysregulation of certain miRNA expressions17, some of which may be implicated in
microgravity-induced brain injury. ROLE OF AQP4 IN REGULATING ECS STRUCTURE AND ISF DRAINAGE Aquaporin, a group of transmembrane proteins known for their water permeability, exhibits
widespread expression on cell membranes and primarily functions in the regulation of intra- and extracellular water and electrolyte balance in diverse tissues and organs of organisms135.
Among the 13 identified types of AQP in mammalian cell membranes, AQP1, AQP4, and AQP9 are particularly prevalent in brain tissues136. Recent studies have demonstrated that AQP4, a water
channel protein, exhibits extensive distribution in the brain137,138. This protein not only plays a crucial role in the regulation of brain tissue water metabolism but also exerts
significant influence on various processes such as CSF formation, glial lymphatic clearance, astrocyte activation and migration, and neural synaptic potential formation139. AQP4 typically
exhibits polarization and is distributed on astrocytes, forming channels primarily extending from the ends surrounding blood vessels to the entirety of the astrocyte membrane, as well as
glial cells located around the ventricles and beneath the pia mater140. Concurrently, astrocytes assume a crucial role in facilitating the proper functioning of neural processes within the
brain141. In addition to supplying nutrients to neuronal cells and mediating neurotransmitters, astrocytes also regulate the concentration of inorganic ions in the internal environment. In
AQP4 knockout mice, the volume fraction of brain ECS is increased142, leading to a reduction in the clearance of waste products through the ISF18. Consequently, the dysregulation of the
microenvironment results in neuron death. FA-REGULATED MIRNA LEVELS AND AQP4 EXPRESSION Previous studies has demonstrated that FA has the potential to impair the expression profiles of miRNA
in the neuronal cells16, thereby causing dysregulation in the certain miRNA expression, such as: miR-22, 26, 29, 125, 140, 142, 145, 203, 204, 320, 328, 344, 374, 485, 520, 1949, 3096,
etc15,16,17. Especially, the expression of AQP4 could be regulated by these miRNAs including: miR-19a143, 29b144, 130a145, 130b146, 224143, 320a147, thereby perturbing the homeostasis of the
brain’ ECS and ultimately culminating in neuronal demise. Notably, miRNA-29b, miRNA-145, and miRNA-320a have been identified as regulators of AQP4 expression within astrocytes148. The
upregulation of miRNA-29b has been found to enhance the expression of AQP4 in mouse brain tissue during cerebral ischemia, resulting in a reduction in the size of cerebral infarcts,
mitigating the extent of edema, and minimizing disruption of the BBB144. Moreover, miRNA-29 in astrocytes plays a role in glutamate signaling and mitigates brain cell damage caused by
excessive levels of glutamate149. In typical circumstances, the upregulation of miRNA-29b results in cerebral edema, thereby providing additional evidence of miRNA-29b’s mechanism of action
through direct targeting of AQP4 expression150. In a rat astrocyte primary culture model subjected to oxygen and glucose deprivation, miRNA-145 mitigates the detrimental consequences of
AQP4-induced astrocyte damage151. Furthermore, in the context of cerebral edema, miRNA-320a exhibits a down-regulatory effect on the expression of AQP4, resulting in an elevation in cerebral
infarct volume. Conversely, the utilization of an anti-miRNA-320a antibody to target miRNA-320a resulted in an upsurge in AQP4 expression and a reduction in infarct volume147. Previous
studies have demonstrated that miRNA-320a can interact with the mRNA coding for AQP4, leading to inhibition of its translation or degradation, ultimately resulting in a downregulation of
AQP4 expression152. Interestingly, recent studies have found that AQP4 is upregulated in the foot of hippocampal astrocytes as a result of space flight153; the findings may differ in other
brain regions, such as the cortex, where studies have demonstrated that there is no significant variation in AQP4 expression153. Weightlessness during spaceflight is highly likely to affect
the levels of miR-29b, miR-145, and miR-320a due to the accumulation of FA in most of brain regions148, which subsequently causes disruption of normal AQP4 expression in the astrocytes.
Hence, in certain regions of the brain, the decrease in AQP4 expression may hinder or slow down the ISF drainage of ECS in the brain, leading to neuronal death (Fig. 10). However, it is
important to recognize that this phenomenon may not be occurring universally throughout the brain148. ROLES OF ATM GENE IN ATAXIA OCCURENCE The consensus in the scientific community is that
cerebellar injury in humans results in the manifestation of ataxia, characterized by the organism’s inability to effectively regulate posture and balance, thereby leading to tremors and
motor instability. Similarly, astronauts commonly experience these aforementioned symptoms upon reentry to Earth154. The ataxia telangiectasia-mutated (ATM) gene plays a crucial role in
initiating DNA damage repair in cellular processes. It has been proven that ATM deficiency is strongly associated with ataxia155. This gene is located on the human chromosome 11q22-23 and
encodes a protein kinase called ATM protein, which consists of 3056 amino acids156. The ATM protein kinase, belonging to the phosphatidylinositol 3-kinase (PI3K) family, primarily governs
DNA double-strand breaks (DSBs) repair, cell cycle arrest, and apoptosis to uphold cellular genome stability and impede tumor initiation and progression157. Remarkably, FA can hinder DNA
replication and cause DSBs in dividing cells158. Even when human cells are subjected to relatively low levels of FA toxicity, the presence of DSBs leads to an exceptionally robust and
expeditious activation of the ATM pathway159, thereby initiating a sequence of intricate cascade reactions in downstream proteins. However, research has confirmed that the presence of DSBs
is not the cause of the main wave of ATM activation by FA159. The available evidence suggests that ATM protein kinases are frequently present as inactive dimers and are primarily located
within the nucleus of higher eukaryotic tissue cells. However, their presence in the cytoplasm and nucleus of neurons remains largely consistent160. Numerous studies have demonstrated that
mice lacking ATM protein kinase exhibit elevated levels of reactive oxygen species, particularly in the cerebellum, which is characterized by significant oxidative stress resulting in the
degeneration and death of cerebellar neurons. Furthermore, activation of ATM protein kinase in the cytoplasm protects cerebellar neurons from damage due to oxidative stress161,162,163, which
suggests that ATM protein kinase is closely related to the maintenance of cerebellar neurons’ vital activities. Especially, it has proven that inactivation of ATM protein kinase in
cerebellar neurons causes symptoms of ataxia and movement disorders164,165. It has been proven that overexpression of miRNA-203 can downregulate the expression of the ATM gene166. Notably,
exposure to FA can simultaneously downregulate the level of miRNA-20317, thereby reducing its inhibitory effect on ATM expression and enhancing the activation of ATM to repair DNA damage166.
This may be one of the possible reasons why FA activates the ATM pathway (Fig. 10). Furthermore, the accumulation of low FA doses can cause strong and rapid activation of ATM signals in
human cells159, which has a certain effect on protecting cerebellar neurons from damage. However, this study provides further evidence that the buildup of elevated levels of formaldehyde can
deactivate ATM protein kinase through covalent dimerization and the creation of larger crosslinks159, at concentrations similar to those observed in the microgravity simulation model
discussed earlier7. In summary, FA appears to be neuroprotective by upregulating the ATM gene at low levels, whereas at higher levels, it becomes toxic due to its ability to dimerize and
inactivate ATM protein kinase. CONCLUSION AND FUTURE PERSPECTIVES This review examines the effects of long-term space flight on astronaut health, specifically the impact of microgravity on
memory function and motor ability, with endogenous FA accumulation playing a crucial role. The animal studies simulating microgravity mentioned in this review indicate that after prolonged
exposure to microgravity, the concentration of FA in the hippocampus and cerebellum may abnormally increase4,7. This leads to pathological changes in these tissues similar to those seen in
specific neurodegenerative diseases59, ultimately resulting in a decline in memory and motor function7. Moreover, microgravity-induced FA could influence miRNA expression, impacting the
survival of nerve cells15,16,17. Hence, the regulation of FA metabolism has emerged as a promising target for drug treatment, offering novel insights and approaches for the advancement of
therapeutic medications for memory and motor deficits associated with neurodegenerative disorders111. To safeguard the enduring well-being of astronauts, global space centers have undertaken
a sequence of drug development investigations pertaining to the amelioration of cerebral impairment induced by microgravity in space. Despite advancements in comprehending the molecular
mechanisms that underlie the cognitive and motor impairments experienced by astronauts in weightless environments, the creation of efficacious pharmaceutical interventions remains elusive.
This challenge arises from the presence of the BBB, which restricts the entry and functionality of conventional synthetic and protein-based drugs within the central nervous system167.
However, the utilization of drug delivery mechanisms involving the lymphatic system and brain ECS presents a viable approach to circumvent the constraints imposed by the BBB, thereby
offering a novel avenue for investigating therapeutic interventions targeting brain disorders. Nanopackaged coenzyme Q10 at 30 nm can penetrate both BBB and brain ECS (38–64 nm) to scavenge
FA111, suggesting that nanomedicine may contribute to the improvement of astronaut health. In addition, due to its high stability in human fluids, miRNAs in the circulation can serve as
biomarkers for the diagnosis and prognosis of astronaut diseases168. Thus, this review provides new insights into the role of FA in memory and motor impairments, which will help researchers
design relevant drugs or other interventions to ensure the long-term health of astronauts in microgravity environment. REFERENCES * Prasad, B. et al. Influence of microgravity on apoptosis
in cells, tissues, and other systems in vivo and in vitro. _Int. J. Mol. Sci._ 21, https://doi.org/10.3390/ijms21249373 (2020). * Oluwafemi, F. A., Abdelbaki, R., Lai, J. C., Mora-Almanza,
J. G. & Afolayan, E. M. A review of astronaut mental health in manned missions: potential interventions for cognitive and mental health challenges. _Life Sci. Space Res._ 28, 26–31
(2021). Article Google Scholar * Roy-O’Reilly, M., Mulavara, A. & Williams, T. A review of alterations to the brain during spaceflight and the potential relevance to crew in
long-duration space exploration. _NPJ Microgravity_ 7, 5 (2021). Article PubMed PubMed Central Google Scholar * Ai, L. et al. Endogenous formaldehyde is a memory-related molecule in mice
and humans. _Commun. Biol._ 2, 446 (2019). Article CAS PubMed PubMed Central Google Scholar * Palasz, A., Menezes, I. C. & Worthington, J. J. The role of brain gaseous
neurotransmitters in anxiety. _Pharm. Rep._ 73, 357–371 (2021). Article CAS Google Scholar * Kou, Y., Zhao, H., Cui, D., Han, H. & Tong, Z. Formaldehyde toxicity in age-related
neurological dementia. _Ageing Res. Rev._ 73, 101512 (2022). Article CAS PubMed Google Scholar * Yao, D. et al. Accumulation of formaldehyde causes motor deficits in an in vivo model of
hindlimb unloading. _Commun. Biol._ 4, 933 (2021). Article CAS PubMed PubMed Central Google Scholar * Yu, P. H. Deamination of methylamine and angiopathy; toxicity of formaldehyde,
oxidative stress and relevance to protein glycoxidation in diabetes. _J. Neural Transm. Suppl._ 52, 201–216 (1998). Article CAS PubMed Google Scholar * Yu, P. H., Lai, C. T. & Zuo,
D. M. Formation of formaldehyde from adrenaline in vivo; a potential risk factor for stress-related angiopathy. _Neurochem Res._ 22, 615–620 (1997). Article CAS PubMed Google Scholar *
Zhang, J. et al. Illumination with 630 nm red light reduces oxidative stress and restores memory by photo-activating catalase and formaldehyde dehydrogenase in SAMP8 mice. _Antioxid. Redox
Signal._ 30, 1432–1449 (2019). Article PubMed Google Scholar * Jelski, W., Sani, T. A. & Szmitkowski, M. [Class III alcohol dehydrogenase and its role in the human body]. _Postepy
Hig. Med. Dosw._ 60, 406–409 (2006). Google Scholar * Han, H. et al. A novel MRI tracer-based method for measuring water diffusion in the extracellular space of the rat brain. _IEEE J.
Biomed. Health Inf._ 18, 978–983 (2014). Article Google Scholar * Jia, Y. et al. Transmembrane water-efflux rate measured by magnetic resonance imaging as a biomarker of the expression of
aquaporin-4 in gliomas. _Nat. Biomed. Eng._ 7, 236–252 (2023). Article CAS PubMed Google Scholar * Peng, S., Liu, J., Liang, C., Yang, L. & Wang, G. Aquaporin-4 in glymphatic system,
and its implication for central nervous system disorders. _Neurobiol. Dis._ 179, 106035 (2023). Article CAS PubMed Google Scholar * Rager, J. E., Smeester, L., Jaspers, I., Sexton, K.
G. & Fry, R. C. Epigenetic changes induced by air toxics: formaldehyde exposure alters miRNA expression profiles in human lung cells. _Environ. Health Perspect._ 119, 494–500 (2011).
Article CAS PubMed Google Scholar * Li, G., Yang, J. & Ling, S. Formaldehyde exposure alters miRNA expression profiles in the olfactory bulb. _Inhal. Toxicol._ 27, 387–393 (2015).
Article CAS PubMed Google Scholar * Rager, J. E. et al. Formaldehyde and epigenetic alterations: microRNA changes in the nasal epithelium of nonhuman primates. _Environ. Health
Perspect._ 121, 339–344 (2013). Article PubMed PubMed Central Google Scholar * Teng, Z. et al. The effect of aquaporin-4 knockout on interstitial fluid flow and the structure of the
extracellular space in the deep brain. _Aging Dis._ 9, 808–816, (2018). Article PubMed PubMed Central Google Scholar * Odackal, J. et al. Real-time Iontophoresis with tetramethylammonium
to quantify volume fraction and tortuosity of brain extracellular space. _J. Vis. Exp._ https://doi.org/10.3791/55755 (2017). * Lei, Y., Han, H., Yuan, F., Javeed, A. & Zhao, Y. The
brain interstitial system: anatomy, modeling, in vivo measurement, and applications. _Prog. Neurobiol._ 157, 230–246 (2017). Article PubMed Google Scholar * Aukland, K. & Reed, R. K.
Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. _Physiol. Rev._ 73, 1–78 (1993). Article CAS PubMed Google Scholar * Khasawneh, A. H., Garling, R. J.
& Harris, C. A. Cerebrospinal fluid circulation: what do we know and how do we know it? _Brain Circ._ 4, 14–18, (2018). Article PubMed PubMed Central Google Scholar * Iliff, J. J. et
al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. _Sci. Transl. Med._ 4, 147ra111 (2012). Article
PubMed PubMed Central Google Scholar * Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer’s disease. _Nat. Rev. Neurol._ 11, 457–470 (2015). Article
CAS PubMed PubMed Central Google Scholar * Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is
reduced in aged mice. _Nat. Commun._ 8, 1434 (2017). Article PubMed PubMed Central Google Scholar * Jullienne, A. et al. Chronic cerebrovascular dysfunction after traumatic brain injury.
_J. Neurosci. Res._ 94, 609–622 (2016). Article CAS PubMed PubMed Central Google Scholar * Harrison, I. F. et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s
disease model. _Brain_ 143, 2576–2593 (2020). Article PubMed PubMed Central Google Scholar * Sundaram, S. et al. Establishing a framework for neuropathological correlates and glymphatic
system functioning in Parkinson’s disease. _Neurosci. Biobehav Rev._ 103, 305–315 (2019). Article CAS PubMed PubMed Central Google Scholar * Xia, M., Yang, L., Sun, G., Qi, S. & Li,
B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. _Psychopharmacology_ 234, 365–379 (2017). Article CAS
PubMed Google Scholar * Munk, A. S. et al. PDGF-B is required for development of the glymphatic system. _Cell Rep._ 26, 2955–2969.e2953 (2019). Article CAS PubMed PubMed Central
Google Scholar * Pollay, M. The function and structure of the cerebrospinal fluid outflow system. _Cerebrospinal Fluid Res._ 7, 9 (2010). Article PubMed PubMed Central Google Scholar *
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. _Nat. Neurosci._ 21, 1380–1391 (2018). Article CAS PubMed PubMed Central
Google Scholar * Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. _J. Neurosci._ 33, 18190–18199 (2013). Article
CAS PubMed PubMed Central Google Scholar * Musiał, A., Gryglewski, R. W., Kielczewski, S., Loukas, M. & Wajda, J. Formalin use in anatomical and histological science in the 19th and
20th centuries. _Folia Med. Cracov._ 56, 31–40 (2016). PubMed Google Scholar * Pinto, J. P., Gladstone, G. R. & Yung, Y. L. Photochemical production of formaldehyde in Earth’s
primitive atmosphere. _Science_ 210, 183–185 (1980). Article CAS PubMed Google Scholar * Robertson, M. P., & Miller, S. L. An efficient prebiotic synthesis is of cytosine and uracil.
_Nature_ 375, 772–774 (1995). Article CAS PubMed Google Scholar * Lu, Z., Li, C. M., Qiao, Y., Yan, Y. & Yang, X. Effect of inhaled formaldehyde on learning and memory of mice.
_Indoor Air_ 18, 77–83 (2008). Article CAS PubMed Google Scholar * Kilburn, K. H., Warshaw, R. & Thornton, J. C. Formaldehyde impairs memory, equilibrium, and dexterity in histology
technicians: effects which persist for days after exposure. _Arch. Environ. Health_ 42, 117–120 (1987). Article CAS PubMed Google Scholar * Kalász, H. Biological role of formaldehyde,
and cycles related to methylation, demethylation, and formaldehyde production. _Mini Rev. Med. Chem._ 3, 175–192 (2003). Article PubMed Google Scholar * Zhuo, M., Small, S. A., Kandel, E.
R. & Hawkins, R. D. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. _Science_ 260, 1946–1950 (1993). Article CAS PubMed
Google Scholar * Bour, S. et al. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. _Biochimie_ 89, 916–925 (2007). Article
CAS PubMed Google Scholar * Ozen, O. A. et al. Effect of formaldehyde inhalation on Hsp70 in seminiferous tubules of rat testes: an immunohistochemical study. _Toxicol. Ind. Health_ 21,
249–254 (2005). Article CAS PubMed Google Scholar * Frankenhaeuser, M. Behavior and circulating catecholamines. _Brain Res._ 31, 241–262 (1971). Article CAS PubMed Google Scholar *
Edmondson, D. E. & Binda, C. Monoamine oxidases. _Subcell. Biochem._ 87, 117–139 (2018). Article CAS PubMed Google Scholar * Boor, P. J., Trent, M. B., Lyles, G. A., Tao, M. &
Ansari, G. A. Methylamine metabolism to formaldehyde by vascular semicarbazide-sensitive amine oxidase. _Toxicology_ 73, 251–258 (1992). Article CAS PubMed Google Scholar * Yu, P. H.,
Davis, B. A. & Boulton, A. A. Aliphatic propargylamines: potent, selective, irreversible monoamine oxidase B inhibitors. _J. Med. Chem._ 35, 3705–3713 (1992). Article CAS PubMed
Google Scholar * Lu, J., Miao, J., Su, T., Liu, Y. & He, R. Formaldehyde induces hyperphosphorylation and polymerization of Tau protein both in vitro and in vivo. _Biochim. Biophys.
Acta_ 1830, 4102–4116 (2013). Article CAS PubMed Google Scholar * Tong, Z. et al. Accumulated hippocampal formaldehyde induces age-dependent memory decline. _Age_ 35, 583–596 (2013).
Article PubMed Google Scholar * Li, X. et al. Hydrogen sulfide ameliorates cognitive dysfunction in formaldehyde-exposed rats: involvement in the upregulation of brain-derived
neurotrophic factor. _Neuropsychobiology_ 79, 119–130 (2020). Article CAS PubMed Google Scholar * Li, Y. et al. Effects of formaldehyde exposure on anxiety-like and depression-like
behavior, cognition, central levels of glucocorticoid receptor and tyrosine hydroxylase in mice. _Chemosphere_ 144, 2004–2012 (2016). Article CAS PubMed Google Scholar * Yue, X. et al.
New insight into Alzheimer’s disease: light reverses Aβ-obstructed interstitial fluid flow and ameliorates memory decline in APP/PS1 mice._Alzheimers Dement._ 5, 671–684 (2019). Google
Scholar * Malek, F. A., Möritz, K. U. & Fanghänel, J. Formaldehyde inhalation & open field behaviour in rats. _Indian J. Med. Res._ 118, 90–96 (2003). CAS PubMed Google Scholar *
Sekeres, M. J., Winocur, G. & Moscovitch, M. The hippocampus and related neocortical structures in memory transformation. _Neurosci. Lett._ 680, 39–53 (2018). Article CAS PubMed
Google Scholar * Sarkar, P. et al. Proteomic analysis of mice hippocampus in simulated microgravity environment. _J. Proteome Res._ 5, 548–553 (2006). Article CAS PubMed PubMed Central
Google Scholar * Santucci, D. et al. Evaluation of gene, protein and neurotrophin expression in the brain of mice exposed to space environment for 91 days. _PLoS ONE_ 7, e40112 (2012).
Article CAS PubMed PubMed Central Google Scholar * Leal, G., Comprido, D. & Duarte, C. B. BDNF-induced local protein synthesis and synaptic plasticity. _Neuropharmacology_ 76,
639–656 (2014). PT C. Article CAS PubMed Google Scholar * Alonso, M. et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation.
_Hippocampus_ 12, 551–560 (2002). Article CAS PubMed Google Scholar * Radecki, D. T., Brown, L. M., Martinez, J. & Teyler, T. J. BDNF protects against stress-induced impairments in
spatial learning and memory and LTP. _Hippocampus_ 15, 246–253 (2005). Article CAS PubMed Google Scholar * Gao, Y. et al. Early changes to the extracellular space in the hippocampus
under simulated microgravity conditions. _Sci. China Life Sci._ 65, 604–617 (2022). Article CAS PubMed Google Scholar * Sun, X. Q., Xu, Z. P., Zhang, S., Cao, X. S. & Liu, T. S.
Simulated weightlessness aggravates hypergravity-induced impairment of learning and memory and neuronal apoptosis in rats. _Behav. Brain Res._ 199, 197–202 (2009). Article PubMed Google
Scholar * Ranjan, A., Behari, J. & Mallick, B. N. Cytomorphometric changes in hippocampal CA1 neurons exposed to simulated microgravity using rats as model. _Front. Neurol._ 5, 77
(2014). Article PubMed PubMed Central Google Scholar * Wang, Y. et al. Effects of simulated microgravity on the expression of presynaptic proteins distorting the GABA/glutamate
equilibrium–A proteomics approach. _Proteomics_ 15, 3883–3891 (2015). Article CAS PubMed Google Scholar * Takamatsu, Y. et al. Protection against neurodegenerative disease on Earth and
in space. _NPJ Microgravity_ 2, 16013 (2016). Article PubMed PubMed Central Google Scholar * Heck, H. D., White, E. L. & Casanova-Schmitz, M. Determination of formaldehyde in
biological tissues by gas chromatography/mass spectrometry. _Biomed. Mass Spectrom._ 9, 347–353 (1982). Article CAS PubMed Google Scholar * Tong, Z. Q. et al. Urine formaldehyde level is
inversely correlated to mini mental state examination scores in senile dementia. _Neurobiol. Aging_ 32, 31–42, (2011). Article CAS PubMed Google Scholar * Lee, C. H. & Gouaux, E.
Amino terminal domains of the NMDA receptor are organized as local heterodimers. _PLoS ONE_ 6, e19180 (2011). Article CAS PubMed PubMed Central Google Scholar * Karakas, E. &
Furukawa, H. Crystal structure of a heterotetrameric NMDA receptor ion channel. _Science_ 344, 992–997 (2014). Article CAS PubMed PubMed Central Google Scholar * Metz, B. et al.
Identification of formaldehyde-induced modifications in proteins: reactions with insulin. _Bioconjug. Chem._ 17, 815–822 (2006). Article CAS PubMed Google Scholar * Toews, J., Rogalski,
J. C., Clark, T. J. & Kast, J. Mass spectrometric identification of formaldehyde-induced peptide modifications under in vivo protein cross-linking conditions. _Anal. Chim. Acta_ 618,
168–183 (2008). Article CAS PubMed Google Scholar * Ly, V., Velichala, S. R. & Hargens, A. R. Cardiovascular, lymphatic, and ocular health in space. _Life_ 12,
https://doi.org/10.3390/life12020268 (2022). * Carriot, J., Mackrous, I. & Cullen, K. E. Challenges to the vestibular system in space: how the brain responds and adapts to microgravity.
_Front. Neural Circuits_ 15, 760313 (2021). Article PubMed PubMed Central Google Scholar * Koppelmans, V. et al. Brain plasticity and sensorimotor deterioration as a function of 70 days
head down tilt bed rest. _PLoS ONE_ 12, e0182236 (2017). Article PubMed PubMed Central Google Scholar * Koppelmans, V., Bloomberg, J. J., Mulavara, A. P. & Seidler, R. D. Brain
structural plasticity with spaceflight. _NPJ Microgravity_ 2, 2 (2016). Article PubMed PubMed Central Google Scholar * Juhl, O. J. T. et al. Update on the effects of microgravity on the
musculoskeletal system. _NPJ Microgravity_ 7, 28 (2021). Article PubMed PubMed Central Google Scholar * Ishihara, A. et al. Comparison of the response of motoneurons innervating perineal
and hind limb muscles to spaceflight and recovery. _Muscle Nerve_ 23, 753–762 (2000). Article CAS PubMed Google Scholar * Tajino, J. et al. Discordance in recovery between altered
locomotion and muscle atrophy induced by simulated microgravity in rats. _J. Mot. Behav._ 47, 397–406 (2015). Article PubMed Google Scholar * Holstein, G. R., Kukielka, E. &
Martinelli, G. P. Anatomical observations of the rat cerebellar nodulus after 24 h of spaceflight. _J. Gravit. Physiol._ 6, P47–P50 (1999). CAS PubMed Google Scholar * Reschke, M. F.
& Clément, G. Vestibular and sensorimotor dysfunction during space flight. _Curr. Pathobiol. Rep._ 6, 177–183 (2018). Article CAS Google Scholar * Macaulay, T. R. et al. Developing
proprioceptive countermeasures to mitigate postural and locomotor control deficits after long-duration spaceflight. _Front. Syst. Neurosci._ 15, 658985 (2021). Article PubMed PubMed
Central Google Scholar * Salazar, A. P. et al. Neural working memory changes during a spaceflight analog with elevated carbon dioxide: a pilot study. _Front. Syst. Neurosci._ 14, 48
(2020). Article CAS PubMed PubMed Central Google Scholar * Roberts, D. R. et al. Structural brain changes following long-term 6° head-down tilt bed rest as an analog for spaceflight.
_Am. J. Neuroradiol._ 36, 2048–2054 (2015). Article CAS PubMed PubMed Central Google Scholar * Lemberskiy, G. et al. Characterization of prostate microstructure using water diffusion
and NMR relaxation. _Front. Phys._ 6, https://doi.org/10.3389/fphy.2018.00091 (2018). * Thach, W. T., Goodkin, H. P. & Keating, J. G. The cerebellum and the adaptive coordination of
movement. _Annu. Rev. Neurosci._ 15, 403–442 (1992). Article CAS PubMed Google Scholar * Marsden, J. F. Cerebellar ataxia. _Handb. Clin. Neurol._ 159, 261–281 (2018). Article PubMed
Google Scholar * Glasauer, S. et al. Spatial orientation during locomotion [correction of locomation] following space flight. _Acta Astronaut._ 36, 423–431 (1995). Article CAS PubMed
Google Scholar * Layne, C. S., McDonald, P. V. & Bloomberg, J. J. Neuromuscular activation patterns during treadmill walking after space flight. _Exp. Brain Res._ 113, 104–116 (1997).
Article CAS PubMed Google Scholar * Layne, C. S. et al. Adaptation of neuromuscular activation patterns during treadmill walking after long-duration space flight. _Acta Astronaut._ 43,
107–119 (1998). Article CAS PubMed Google Scholar * Kozlovskaya, I. B., Grigoriev, A. I. & Stepantzov, V. I. Countermeasure of the negative effects of weightlessness on physical
systems in long-term space flights. _Acta Astronaut._ 36, 661–668 (1995). Article CAS PubMed Google Scholar * Van Ombergen, A. et al. The effect of spaceflight and microgravity on the
human brain. _J. Neurol._ 264, 18–22 (2017). Article PubMed PubMed Central Google Scholar * Cebolla, A. M. et al. Cerebellar contribution to visuo-attentional alpha rhythm: insights from
weightlessness. _Sci. Rep._ 6, 37824 (2016). Article CAS PubMed PubMed Central Google Scholar * Lee, J. K. et al. Spaceflight-associated brain white matter microstructural changes and
intracranial fluid redistribution. _JAMA Neurol._ 76, 412–419, (2019). Article PubMed PubMed Central Google Scholar * Demertzi, A. et al. Cortical reorganization in an astronaut’s brain
after long-duration spaceflight. _Brain Struct. Funct._ 221, 2873–2876 (2016). Article PubMed Google Scholar * Van Ombergen, A. et al. Brain tissue-volume changes in cosmonauts. _N. Engl.
J. Med._ 379, 1678–1680 (2018). Article PubMed Google Scholar * Nguyen, H. P., Tran, P. H., Kim, K. S. & Yang, S. G. The effects of real and simulated microgravity on cellular
mitochondrial function. _NPJ Microgravity_ 7, 44 (2021). Article CAS PubMed PubMed Central Google Scholar * Chen, H. L. et al. Simulated microgravity-induced oxidative stress in
different areas of rat brain. _Sheng Li Xue Bao_ 61, 108–114 (2009). CAS PubMed Google Scholar * Krasnov, I. B. & Krasnikov, G. V. Purkinje’s cells in the vestibular and
proprioceptive segments of rat’s cerebellum following 14-day space flight. _Aviakosm Ekol. Med._ 43, 43–47 (2009). CAS Google Scholar * Qu, L., Yang, T., Yuan, Y., Zhong, P. & Li, Y.
Protein nitration increased by simulated weightlessness and decreased by melatonin and quercetin in PC12 cells. _Nitric Oxide_ 15, 58–63 (2006). Article PubMed Google Scholar * Carpo, N.,
Tran, V., Biancotti, J. C., Cepeda, C. & Espinosa-Jeffrey, A. Space flight enhances stress pathways in human neural stem cells. _Biomolecules_ 14, https://doi.org/10.3390/biom14010065
(2024). * Tao, R. et al. In situ imaging of formaldehyde in live mice with high spatiotemporal resolution reveals aldehyde dehydrogenase-2 as a potential target for Alzheimer’s disease
treatment. _Anal. Chem._ 94, 1308–1317 (2022). Article CAS PubMed Google Scholar * Bi, L. et al. Ultrastructural changes in cerebral cortex and cerebellar cortex of rats under simulated
weightlessness. _Space Med. Med. Eng._ 17, 180–183 (2004). Google Scholar * Sutherland, B. W., Toews, J. & Kast, J. Utility of formaldehyde cross-linking and mass spectrometry in the
study of protein-protein interactions. _J. Mass Spectrom._ 43, 699–715 (2008). Article CAS PubMed Google Scholar * Bolt, H. M. Experimental toxicology of formaldehyde. _J. Cancer Res.
Clin. Oncol._ 113, 305–309 (1987). Article CAS PubMed Google Scholar * Häder, D. P., Braun, M., Grimm, D. & Hemmersbach, R. Gravireceptors in eukaryotes-a comparison of case studies
on the cellular level. _NPJ Microgravity_ 3, 13 (2017). Article PubMed PubMed Central Google Scholar * Grimm, D. et al. The fight against cancer by microgravity: the multicellular
spheroid as a metastasis model. _Int. J. Mol. Sci._ 23, https://doi.org/10.3390/ijms23063073 (2022). * Infanger, M. et al. Simulated weightlessness changes the cytoskeleton and extracellular
matrix proteins in papillary thyroid carcinoma cells. _Cell Tissue Res._ 324, 267–277 (2006). Article CAS PubMed Google Scholar * Abe, M., Takahashi, M., Horiuchi, K. & Nagano, A.
The changes in crosslink contents in tissues after formalin fixation. _Anal. Biochem._ 318, 118–123 (2003). Article CAS PubMed Google Scholar * Andreeva, N. V. & Belyavsky, A. V.
Formaldehyde fixation of extracellular matrix protein layers for enhanced primary cell growth. _Bio Protoc._ 7, e2374 (2017). Article PubMed PubMed Central Google Scholar * Zhang, Y. et
al. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. _J. Cell Mol. Med._ 25, 5358–5371 (2021). Article CAS PubMed PubMed
Central Google Scholar * Metz, B. et al. Identification of formaldehyde-induced modifications in proteins: reactions with model peptides. _J. Biol. Chem._ 279, 6235–6243 (2004). Article
CAS PubMed Google Scholar * Gubisne-Haberle, D., Hill, W., Kazachkov, M., Richardson, J. S. & Yu, P. H. Protein cross-linkage induced by formaldehyde derived from
semicarbazide-sensitive amine oxidase-mediated deamination of methylamine. _J. Pharm. Exp. Ther._ 310, 1125–1132 (2004). Article CAS Google Scholar * Fei, X. et al. Degradation of FA
reduces Aβ neurotoxicity and Alzheimer-related phenotypes. _Mol. Psychiatry_ 26, 5578–5591 (2021). Article CAS PubMed Google Scholar * Kang, J. E. et al. Amyloid-beta dynamics are
regulated by orexin and the sleep-wake cycle. _Science_ 326, 1005–1007 (2009). Article CAS PubMed PubMed Central Google Scholar * Taguchi, K., Okamoto, Y., Matsumoto, K., Otagiri, M.
& Chuang, V. T. G. When albumin meets liposomes: a feasible drug carrier for biomedical applications. _Pharmaceuticals_ 14, https://doi.org/10.3390/ph14040296 (2021). * Malka, R.,
Delgado, F. F., Manalis, S. R. & Higgins, J. M. In vivo volume and hemoglobin dynamics of human red blood cells. _PLoS Comput. Biol._ 10, e1003839 (2014). Article PubMed PubMed Central
Google Scholar * Bellone, J. A., Gifford, P. S., Nishiyama, N. C., Hartman, R. E. & Mao, X. W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma
radiation on behavior and blood-brain barrier integrity. _NPJ Microgravity_ 2, 16019 (2016). Article PubMed PubMed Central Google Scholar * Soeters, P. B., Wolfe, R. R. & Shenkin, A.
Hypoalbuminemia: pathogenesis and clinical significance. _J. Parenter. Enter. Nutr._ 43, 181–193 (2019). Article CAS Google Scholar * Rifkind, J. M., Mohanty, J. G. & Nagababu, E.
The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. _Front. Physiol._ 5, 500 (2014). PubMed Google Scholar * Liu, Y., Liu, R., Mou, Y. & Zhou,
G. Spectroscopic identification of interactions of formaldehyde with bovine serum albumin. _J. Biochem. Mol. Toxicol._ 25, 95–100 (2011). Article PubMed Google Scholar * Hoberman, H. D.
& George, San R. C. Reaction of tobacco smoke aldehydes with human hemoglobin. _J. Biochem. Toxicol._ 3, 105–119 (1988). Article CAS PubMed Google Scholar * Stolzing, A. & Grune,
T. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. _FASEB J._ 18, 743–745 (2004). Article CAS PubMed Google Scholar * Stolzing, A., Widmer, R.,
Jung, T., Voss, P. & Grune, T. Degradation of glycated bovine serum albumin in microglial cells. _Free Radic. Biol. Med._ 40, 1017–1027 (2006). Article CAS PubMed Google Scholar *
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. _Cell_ 75, 843–854 (1993). Article CAS
PubMed Google Scholar * Saliminejad, K., Khorram Khorshid, H. R., Soleymani Fard, S. & Ghaffari, S. H. An overview of microRNAs: biology, functions, therapeutics, and analysis methods.
_J. Cell Physiol._ 234, 5451–5465 (2019). Article CAS PubMed Google Scholar * Lu, T. X. & Rothenberg, M. E. MicroRNA. _J. Allergy Clin. Immunol._ 141, 1202–1207 (2018). Article CAS
PubMed Google Scholar * Anfossi, S., Babayan, A., Pantel, K. & Calin, G. A. Clinical utility of circulating non-coding RNAs—an update. _Nat. Rev. Clin. Oncol._ 15, 541–563 (2018).
Article PubMed Google Scholar * Chen, L. et al. Trends in the development of miRNA bioinformatics tools. _Brief. Bioinform._ 20, 1836–1852 (2019). Article CAS PubMed PubMed Central
Google Scholar * Lewis, B. P., Burge, C. B. & Bartel, D. P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. _Cell_
120, 15–20 (2005). Article CAS PubMed Google Scholar * Tétreault, N. & De Guire, V. miRNAs: their discovery, biogenesis and mechanism of action. _Clin. Biochem._ 46, 842–845 (2013).
Article PubMed Google Scholar * Basak, I., Patil, K. S., Alves, G., Larsen, J. P. & Møller, S. G. microRNAs as neuroregulators, biomarkers and therapeutic agents in neurodegenerative
diseases. _Cell Mol. Life Sci._ 73, 811–827 (2016). Article CAS PubMed Google Scholar * Danborg, P. B., Simonsen, A. H., Waldemar, G. & Heegaard, N. H. The potential of microRNAs as
biofluid markers of neurodegenerative diseases–a systematic review. _Biomarkers_ 19, 259–268 (2014). Article CAS PubMed Google Scholar * Cho, H. J. et al. MicroRNA-205 regulates the
expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. _Hum. Mol. Genet._ 22, 608–620 (2013). Article CAS PubMed Google Scholar * Lu, S. Y. et al. miR-218-2
regulates cognitive functions in the hippocampus through complement component 3-dependent modulation of synaptic vesicle release. _Proc. Natl. Acad. Sci. USA_ 118,
https://doi.org/10.1073/pnas.2021770118 (2021). * Li, Y. et al. MicroRNA-26a-3p rescues depression-like behaviors in male rats via preventing hippocampal neuronal anomalies. _J. Clin.
Investig._ 131, https://doi.org/10.1172/jci148853 (2021). * Salta, E., Sierksma, A., Vanden Eynden, E. & De Strooper, B. miR-132 loss de-represses ITPKB and aggravates amyloid and TAU
pathology in Alzheimer’s brain. _EMBO Mol. Med._ 8, 1005–1018 (2016). Article CAS PubMed PubMed Central Google Scholar * Salman, M. M. et al. Transcriptome analysis suggests a role for
the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. _Eur. J. Neurosci._ 46, 2121–2132 (2017). Article PubMed Google Scholar
* Denker, B. M., Smith, B. L., Kuhajda, F. P. & Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and
renal tubules. _J. Biol. Chem._ 263, 15634–15642 (1988). Article CAS PubMed Google Scholar * Badaut, J., Fukuda, A. M., Jullienne, A. & Petry, K. G. Aquaporin and brain diseases.
_Biochim. Biophys. Acta_ 1840, 1554–1565 (2014). Article CAS PubMed Google Scholar * MacAulay, N. & Zeuthen, T. Water transport between CNS compartments: contributions of aquaporins
and cotransporters. _Neuroscience_ 168, 941–956 (2010). Article CAS PubMed Google Scholar * Silva, I., Silva, J., Ferreira, R. & Trigo, D. Glymphatic system, AQP4, and their
implications in Alzheimer’s disease. _Neurol Res. Pract._ 3, 5 (2021). * Pirici, I. et al. Inhibition of aquaporin-4 improves the outcome of ischaemic stroke and modulates brain paravascular
drainage pathways. _Int. J. Mol. Sci._ 19, https://doi.org/10.3390/ijms19010046 (2017). * Takano, T., Oberheim, N., Cotrina, M. L. & Nedergaard, M. Astrocytes and ischemic injury.
_Stroke_ 40, S8–S12 (2009). Article PubMed Google Scholar * Yao, X., Hrabetová, S., Nicholson, C. & Manley, G. T. Aquaporin-4-deficient mice have increased extracellular space without
tortuosity change. _J. Neurosci._ 28, 5460–5464 (2008). Article CAS PubMed PubMed Central Google Scholar * Jullienne, A. et al. Modulating the water channel AQP4 alters miRNA
expression, astrocyte connectivity and water diffusion in the rodent brain. _Sci. Rep._ 8, 4186 (2018). Article PubMed PubMed Central Google Scholar * Wang, Y. et al. MicroRNA-29b is a
therapeutic target in cerebral ischemia associated with aquaporin 4. _J. Cereb. Blood Flow. Metab._ 35, 1977–1984 (2015). Article CAS PubMed PubMed Central Google Scholar * Sepramaniam,
S., Ying, L. K., Armugam, A., Wintour, E. M. & Jeyaseelan, K. MicroRNA-130a represses transcriptional activity of aquaporin 4 M1 promoter. _J. Biol. Chem._ 287, 12006–12015 (2012).
Article CAS PubMed PubMed Central Google Scholar * Zheng, Y. et al. Upregulation of miR-130b protects against cerebral ischemic injury by targeting water channel protein aquaporin 4
(AQP4). _Am. J. Transl. Res._ 9, 3452–3461 (2017). CAS PubMed PubMed Central Google Scholar * Sepramaniam, S. et al. MicroRNA 320a functions as a novel endogenous modulator of aquaporins
1 and 4 as well as a potential therapeutic target in cerebral ischemia. _J. Biol. Chem._ 285, 29223–29230 (2010). Article CAS PubMed PubMed Central Google Scholar * Vandebroek, A.
& Yasui, M. Regulation of AQP4 in the central nervous system. _Int. J. Mol. Sci._ 21, https://doi.org/10.3390/ijms21051603 (2020). * Kobayashi, M. et al. AGO CLIP reveals an activated
network for acute regulation of brain glutamate homeostasis in ischemic stroke. _Cell Rep._ 28, 979–991.e976 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhong, Y. et al.
MicroRNA-29b-3p aggravates 1,2-dichloroethane-induced brain edema by targeting aquaporin 4 in Sprague-Dawley rats and CD-1 mice. _Toxicol. Lett._ 319, 160–167 (2020). Article CAS PubMed
Google Scholar * Zheng, L. et al. Overexpression of MicroRNA-145 ameliorates astrocyte injury by targeting aquaporin 4 in cerebral ischemic stroke. _Biomed. Res. Int._ 2017, 9530951 (2017).
Article PubMed PubMed Central Google Scholar * Chen, Z. et al. microRNA-320a prevent Müller cells from hypoxia injury by targeting aquaporin-4. _J. Cell Biochem._ 121, 4711–4723 (2020).
Article CAS PubMed Google Scholar * Mao, X. W. et al. Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model. _FASEB J._ 34, 15516–15530 (2020). Article
CAS PubMed Google Scholar * Lackner, J. R. & DiZio, P. Human orientation and movement control in weightless and artificial gravity environments. _Exp. Brain Res._ 130, 2–26 (2000).
Article CAS PubMed Google Scholar * Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. _Science_ 268, 1749–1753 (1995). Article CAS PubMed
Google Scholar * Gatti, R. A. et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22-23. _Nature_ 336, 577–580 (1988). Article CAS PubMed Google Scholar * Phan, L. M.
& Rezaeian, A. H. ATM: main features, signaling pathways, and its diverse roles in DNA damage response, tumor suppression, and cancer development. _Genes_ 12,
https://doi.org/10.3390/genes12060845 (2021). * Tan, S. L. W. et al. A class of environmental and endogenous toxins induces BRCA2 haploinsufficiency and genome instability. _Cell_ 169,
1105–1118.e1115 (2017). Article CAS PubMed PubMed Central Google Scholar * Ortega-Atienza, S., Wong, V. C., DeLoughery, Z., Luczak, M. W. & Zhitkovich, A. ATM and KAT5 safeguard
replicating chromatin against formaldehyde damage. _Nucleic Acids Res._ 44, 198–209 (2016). Article CAS PubMed Google Scholar * Ditch, S. & Paull, T. T. The ATM protein kinase and
cellular redox signaling: beyond the DNA damage response. _Trends Biochem. Sci._ 37, 15–22 (2012). Article CAS PubMed Google Scholar * Kuang, X. et al. Activation of AMP-activated
protein kinase in cerebella of Atm-/- mice is attributable to accumulation of reactive oxygen species. _Biochem. Biophys. Res. Commun._ 418, 267–272 (2012). Article CAS PubMed PubMed
Central Google Scholar * Kim, T. S. et al. The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress.
_Dis. Model. Mech._ 3, 752–762 (2010). Article CAS PubMed Google Scholar * Kamsler, A. et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state
of brains from Atm-deficient mice. _Cancer Res._ 61, 1849–1854 (2001). CAS PubMed Google Scholar * Barlow, C. et al. ATM is a cytoplasmic protein in mouse brain required to prevent
lysosomal accumulation. _Proc. Natl. Acad. Sci. USA_ 97, 871–876 (2000). Article CAS PubMed PubMed Central Google Scholar * Dar, I., Biton, S., Shiloh, Y. & Barzilai, A. Analysis of
the ataxia telangiectasia mutated-mediated DNA damage response in murine cerebellar neurons. _J. Neurosci._ 26, 7767–7774 (2006). Article CAS PubMed PubMed Central Google Scholar *
Chang, J. H. et al. MicroRNA-203 modulates the radiation sensitivity of human malignant glioma cells. _Int. J. Radiat. Oncol. Biol. Phys._ 94, 412–420 (2016). Article CAS PubMed Google
Scholar * Abbott, N. J., Patabendige, A. A., Dolman, D. E., Yusof, S. R. & Begley, D. J. Structure and function of the blood-brain barrier. _Neurobiol. Dis._ 37, 13–25 (2010). Article
CAS PubMed Google Scholar * Ho, P. T. B., Clark, I. M. & Le, L. T. T. MicroRNA-based diagnosis and therapy. _Int. J. Mol. Sci._ 23, https://doi.org/10.3390/ijms23137167 (2022).
Download references ACKNOWLEDGEMENTS This work was supported by grants from the State Natural Sciences Foundation Monumental Projects (62394314), National Natural Science Foundation of China
(82071214), Beijing Natural Science Foundation (M21004), Fund of Talent Launch Project of Oujiang Laboratory (OJQDSP2022011), and Fund from Kangning Hospital (SLC202304). AUTHOR INFORMATION
Author notes * These authors contributed equally: Tianhao Mei, Ying Chen. AUTHORS AND AFFILIATIONS * Beijing Geriatric Hospital, Beijing, China Tianhao Mei, Ying Chen & Zhiqian Tong *
Zhejiang Provincial Clinical Research Center for Mental Disorders, The Affiliated Wenzhou Kangning Hospital, School of Mental Health, Wenzhou Medical University, Wenzhou, Zhejiang, China
Tianhao Mei, Ying Chen, Hang Zhao, Xingzhou Lyu, Jing Lin & Zhiqian Tong * Department of Radiology, Peking University Third Hospital, Beijing, China. Key Laboratory of Magnetic Resonance
Imaging Equipment and Technique, Beijing, China Yajuan Gao & Hongbin Han * NMPA key Laboratory for Evaluation of Medical Imaging Equipment and Technique, Beijing, China Yajuan Gao &
Hongbin Han * Institute of Medical Technology, Peking University Health Science Center, Beijing, China Yajuan Gao & Hongbin Han * Shenzhen Bay Laboratory, Shenzhen, China Tianye Niu *
University of Science and Technology of China, Anhui, China Tianye Niu Authors * Tianhao Mei View author publications You can also search for this author inPubMed Google Scholar * Ying Chen
View author publications You can also search for this author inPubMed Google Scholar * Yajuan Gao View author publications You can also search for this author inPubMed Google Scholar * Hang
Zhao View author publications You can also search for this author inPubMed Google Scholar * Xingzhou Lyu View author publications You can also search for this author inPubMed Google Scholar
* Jing Lin View author publications You can also search for this author inPubMed Google Scholar * Tianye Niu View author publications You can also search for this author inPubMed Google
Scholar * Hongbin Han View author publications You can also search for this author inPubMed Google Scholar * Zhiqian Tong View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS T.Y.N., H.B.H. and Z.Q.T. developed the concept of this perspective paper. T.H.M. and Z.Q.T. developed a first structure of the manuscript. H.B.H. took
lead in coordination and writing; Y.C., Y.J.G., H.Z., X.Z.L. Y.J.G. and J.L. wrote specific parts of the manuscript. All authors provided critical feedback and helped shape the concept and
perspectives. All authors revised and approved the submitted version of the manuscript. CORRESPONDING AUTHORS Correspondence to Tianye Niu, Hongbin Han or Zhiqian Tong. ETHICS DECLARATIONS
COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Mei, T., Chen, Y., Gao, Y. _et al._ Formaldehyde initiates memory and motor impairments under weightlessness condition. _npj Microgravity_ 10, 100 (2024).
https://doi.org/10.1038/s41526-024-00441-0 Download citation * Received: 27 October 2023 * Accepted: 21 October 2024 * Published: 28 October 2024 * DOI:
https://doi.org/10.1038/s41526-024-00441-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative