Cdk4/6 inhibitors and overall survival: power of first-line trials in metastatic breast cancer
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Palbociclib, ribociclib, and abemaciclib have been investigated in combination with aromatase inhibitors as first-line therapy for metastatic hormone receptor-positive breast cancer
(PALOMA-2, MONALEESA-2 and MONALEESA-7, MONARCH-3 trials, respectively); pivotal trials led to absolute median progression-free survival (PFS) gain of about 15 months. We aimed to estimate,
for each trial, the statistical power to demonstrate a significant gain in overall survival (OS). Power was calculated with Freedman’s formula. Given the allocation ratio and the number of
events, power was computed as a function of hazard ratio. We focused on four specific hazard ratio values (0.94, 0.89, 0.81, and 0.77), which are estimated to correspond to absolute 3, 6,
12, and 15 months gain in OS, respectively. For these calculations, the type I error rate was stated at 5% with a two-sided test, and we assumed that the risk of death was constant over
time. PALOMA-2 and MONALEESA trials have an almost similar power despite different allocation ratios, while MONARCH-3 has a more limited power. Overall, the power of the four trials to
demonstrate a statistically significant improvement in OS is less than 70% if the prolongation in median OS is ≤12 months, whatever the OS data maturity. This analysis shows that OS results
are jeopardized by limited powers, and a meta-analysis might be required to demonstrate OS benefit. Conversely, if a significant OS improvement is observed in some but not at all trials,
this discrepancy might be more attributable to chance than to a truly different drug efficacy. SIMILAR CONTENT BEING VIEWED BY OTHERS COMPARATIVE OVERALL SURVIVAL OF CDK4/6 INHIBITORS IN
COMBINATION WITH ENDOCRINE THERAPY IN ADVANCED BREAST CANCER Article Open access 07 February 2024 ABEMACICLIB AS INITIAL THERAPY FOR ADVANCED BREAST CANCER: MONARCH 3 UPDATED RESULTS IN
PROGNOSTIC SUBGROUPS Article Open access 22 June 2021 REAL-WORLD EFFECTIVENESS OF CDK 4/6 INHIBITORS IN ESTROGEN-POSITIVE METASTATIC BREAST CANCER Article Open access 20 June 2024
INTRODUCTION Endocrine therapies are the cornerstone of hormone receptor-positive (HR+) HER2-negative (HER2−) breast cancer treatment at both early and metastatic stages. Endocrine therapies
for metastatic breast cancer (MBC) have remained largely unchanged for the past two decades, and include tamoxifen, aromatase inhibitors (AI), and fulvestrant.1 In 2012, results of
BOLERO-2, a randomized placebo-controlled phase 3 conducted in patients with HR+ HER2− MBC progressing under first-line nonsteroidal AI, have been reported.2 This trial compared the efficacy
of a steroidal AI (exemestane) to that of a combination of exemestane and everolimus, a mTOR inhibitor. Patients in the everolimus-exemestane arm had a significantly longer PFS, with a
hazard ratio (HR) = 0.43, 95% CI [0.35; 0.54].2 In that second-line setting, despite a 4.6-month prolongation in median PFS, adding everolimus to exemestane did not confer a statistically
significant improvement in the overall survival (OS): HR = 0.89, 95% CI [0.73; 1.10].3 This negative result increased the concerns about the limited cost-effectiveness of everolimus in that
setting.4,5 More recently, further significant progresses have been reported in HR+ HER2− MBC: four randomized phase 3 trials have reported superior progression-free survivals (PFS) for AI
and cdk4/6 inhibitors combinations compared to AI and placebo as first-line therapy. The PALOMA-2 trial, in which 666 patients have been randomized 2:1 between the AI and palbociclib arm and
the AI and placebo arm, was the first to be reported and demonstrated a PFS HR of 0.58, 95% CI [0.46; 0.72].6 In the MONALEESA-2 trial, 668 patients have been randomized in a 1:1 fashion
between the AI and ribociclib arm and the AI and placebo arm, with a PFS HR of 0.56, 95% CI [0.43; 0.72].7 Superimposable number of included patients and results have been reported with
ribociclib in a second pivotal trial, MONALEESA-7, that was conducted in premenopausal women [8]. Recently, in the MONARCH-3 trial, 493 patients have been randomized in a 2:1 fashion between
the AI and abemaciclib arm and the AI and placebo arm, with a PFS HR of 0.54, 95% CI [0.41;0.72]8 (Table 1). Based on these significant PFS improvements, cdk4/6 inhibitors have been
approved by regulatory agencies for first-line HR+ HER2− MBC and are now being largely used in that setting. However, in the context of a metastatic disease, and not withstanding quality of
life-related endpoints, the ultimate goal of a palliative therapy is to extend OS, while PFS is moderately correlated with OS.9 In all three trials, OS was defined as a secondary endpoint,
and no mature data have been reported so far (20% of deaths were observed in the last MONALEESA-2).10 Per protocol, 278 OS events (41% maturity) and 315 OS events (47% maturity) will trigger
the main OS analysis in PALOMA-2 and MONALEESA-2, respectively. In the MONARCH-3 trial, the main OS analysis is planned as part of a pooled analysis with the MONARCH-2 study; OS analyses of
MONARCH-3 as a single study could be reported as exploratory analyses with no prespecified maturity. As for many trials and despite methodological concerns, it is very likely that unplanned
OS analyses will be reported even after the main analyses have occurred. In this report, we estimated the power of each of the three trials to demonstrate a significant gain in OS according
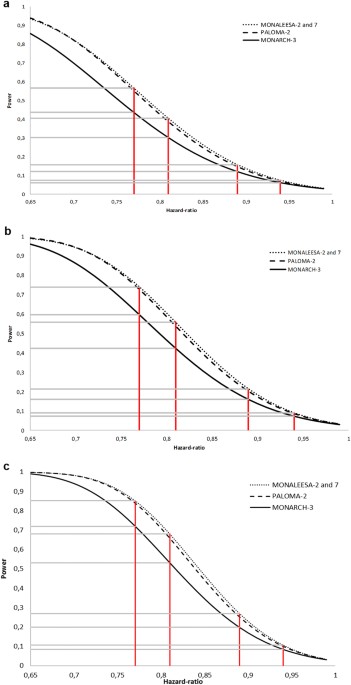
to their intrinsic design (number of patients included, randomization ratio), and the number of events. RESULTS Figure 1 depict, for each OS data maturity and for each of the three phase 3
trial, the power to demonstrate a statistically significant improvement in OS according to different HRs. As expected, the higher the OS maturity, the higher the power is for a given HR.
Unsurprisingly, MONALEESA-2 and -7, which share similar design and number of treated patients, have superimposable powers. We observed that PALOMA-2 and MONALEESA trials have an almost
similar power, MONALEESA trials displaying a marginally increased power. We confirm that the MONARCH-3 trial is less powerful than the three other trials. For each of the four specific HR
values proposed, the power of each trial is displayed on Table 2. Whatever the OS data maturity, if the prolongation in median OS is less than 12 months, the four trials have a power less
than 70%. For a prolongation of 15 months, a power of 80% or more is reached for a death rate of 80%. The power of MONARCH-3 is about 72%. DISCUSSION In the absence of cure, the ultimate
goals of metastatic cancer therapy are to improve duration and/ or quality of survival of the patients. New anticancer drugs should therefore demonstrate a benefit on OS and/or an
improvement of the quality of life. Other clinical endpoints such as PFS may be useful intermediates, but are not surrogates of OS and, in MBC, the level of evidence supporting a surrogacy
of any biomarker,9 including circulating tumor cells count is low.11 Here we showed that MONARCH-3 is less powerful than PALOMA-2, MONALEESA-2, and MONALEESA-7 trials, and that these three
latter trials have an almost similar power; the small difference in power between them is explained by the difference in treatment allocation ratio: 2:1 in the PALOMA-2 trial vs 1:1 in the
MONALEESA trials, the three trials having enrolled a similar number of patients. PALOMA-2 and MONALEESA trials have a <0.70 power to report a statistical difference if the observed
absolute PFS gain (about 15 months, in first line) is translated into a 12-month OS improvement; smaller OS improvements will be more difficult to demonstrate. A power of 80% is observed
only if the OS improvement is 15 months or more. Depending on the final OS HR achieved by cdk4/6 inhibitors, the more limited power of MONARCH-3 may be responsible for a scenario in which a
statistically significant OS gain is observed in PALOMA-2 and MONALEESA trials, but not in MONARCH-3—even if there is no true efficacy difference among the three cdk4/6 inhibitors. Our
analysis illustrate that, OS being the preferred outcome, it will not be satisfactory when median OS is long and differences need to be very large to demonstrate an effect. Such comparisons
are further complicated by the numerous post-progression therapies available in MBC, which will make the two arms more similar. A gain in OS is much more likely to be demonstrated by pooling
individual patient data from different trials. A pooled analysis of MONALEESA-2 and MONALEESA-7 trials will be probably be reported in a near future, as both trials tested the same drug. A
larger meta-analysis including the individual data from all patients including the four trials would have a very high statistical power to demonstrate that cdk4/6 inhibitor do increase OS.
If not made mandatory by regulatory agencies, the feasability of such meta-analysis will rely on the willingness of three competing pharmaceutical companies.to collaborate. Our analyses have
several limitations, as many parameters (post-first line therapies, treatment with cdk4/6 inhibitors at a later stage…) will ultimately influence the OS differences between the two arms of
each trial and between trials. A key parameter will be the OS reached by control arms, which is currently unknown. The PALOMA-2 study hypothesized that a median OS of 34 months in the
control arm of this international trial, while our analyses were based on real life OS data obtained in France in that specific population of AI-sensitive metastatic MBC patients (50
months). In conclusion, power to demonstrate a gain in OS limited in these trials; ultimately, if a significant OS improvement is observed in some but not at all trials, this discrepancy
might be more attributable to chance than to a truly different drug impact on OS. METHODS Power was calculated with Freedman’s formula, which allows us to compute power for studies comparing
survivor functions of two groups by using the log-rank test. Given the allocation ratio and the number of events, power was computed as a function of HR. For these calculations, the type I
error rate was stated at 5% with a two-sided test, and we assumed that the risk of death was constant over time. We particularly focused on four hypothetic prolongations in median OS
following the use of cdk4/6 inhibitors: 3, 6, 12, and 15 months. To translate HR into clinically meaningful data, we hypothesized that the median OS in control arms will be equal to 50
months, as observed in more than 6000 AI-sensitive HR+ HER2− MBC patients treated prior to the cdk4/6 inhibitor era.12 According to the prolongations proposed above, the four HR of interest
are 0.94, 0.89, 0.81, and 0.77, respectively. We calculated the power for two hypotheses of death rates occurring over the follow-up period: 40, 60, and 80%, reflecting the OS data maturity.
The analysis was performed using R software version 3.3.2 [13]. DATA AVAILABILITY STATEMENT All data generated or analyzed during this study are included in this published article.
REFERENCES * Cardoso, F. et al. 3rd ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). _Ann. Oncol_. mdw544 (2016). https://doi.org/10.1093/annonc/mdw544 *
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. _N. Engl. J. Med._ 366, 520–529 (2012). Article PubMed CAS Google Scholar * Piccart, M.
et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2†. _Ann.
Oncol. Off. J. Eur. Soc. Med. Oncol._ 25, 2357–2362 (2014). Article CAS Google Scholar * Niraula, S., Ocana, A. & Amir, E. One step forward, two steps back: the story of everolimus in
advanced breast cancer. _Breast Edinb. Scotl._ 24, 529–531 (2015). Article Google Scholar * Diaby, V. et al. Cost-effectiveness analysis of everolimus plus exemestane versus exemestane
alone for treatment of hormone receptor positive metastatic breast cancer. _Breast Cancer Res. Treat._ 147, 433–441 (2014). Article PubMed PubMed Central Google Scholar * Finn, R. S. et
al. Palbociclib and letrozole in advanced breast cancer. _N. Engl. J. Med._ 375, 1925–1936 (2016). Article PubMed CAS Google Scholar * Hortobagyi, G. N. et al. Ribociclib as first-line
therapy for HR-positive, advanced breast cancer. _N. Engl. J. Med._ 375, 1738–1748 (2016). Article PubMed CAS Google Scholar * Goetz, M. P. et al. MONARCH 3: abemaciclib as initial
therapy for advanced breast cancer. _J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol._ 35, 3638–3646 (2017). Article Google Scholar * Fiteni, F. & Bonnetain, F. Surrogate end points for
overall survival in breast cancer trials: a review. _Breast_ 29, 44–48 (2016). Article PubMed Google Scholar * Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase 3 trial
of first-line ribociclib+letrozole in hormone receptor-positive (HR+), HER2-negative (HER2–), advanced breast cancer (ABC). _J. Clin. Oncol._ 35, 1038–1038 (2017). Article Google Scholar *
Cabel, L. et al. Circulating tumor cells: clinical validity and utility. _Int. J. Clin. Oncol._ 22, 421–430 (2017). Article PubMed Google Scholar * Le Saux, O. et al. Assessment of
multiple endocrine therapies for metastatic breast cancer in a multicenter national observational study. _J. Clin. Oncol._ 35, 1052–1052 (2017). Article Google Scholar Download references
ACKNOWLEDGEMENTS This study was funded by Institut Curie SIRIC 2 (grant INCa-DGOS-Inserm_12554). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biometry, Institut Curie, PSL
Research University, Saint Cloud, France Marie-Laure Tanguy, Fréderique Berger & Alexia Savignoni * Department of Medical Oncology, Institut Curie, PSL Research University, Paris, France
Luc Cabel, Jean-Yves Pierga & Francois-Clement Bidard * UVSQ, Paris Saclay University, Saint Quentin en Yvelines, Paris, France Luc Cabel & Francois-Clement Bidard * Paris Descartes
University, Paris, France Jean-Yves Pierga Authors * Marie-Laure Tanguy View author publications You can also search for this author inPubMed Google Scholar * Luc Cabel View author
publications You can also search for this author inPubMed Google Scholar * Fréderique Berger View author publications You can also search for this author inPubMed Google Scholar * Jean-Yves
Pierga View author publications You can also search for this author inPubMed Google Scholar * Alexia Savignoni View author publications You can also search for this author inPubMed Google
Scholar * Francois-Clement Bidard View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors researched, collated, and wrote this paper.
CORRESPONDING AUTHOR Correspondence to Francois-Clement Bidard. ETHICS DECLARATIONS COMPETING INTERESTS F.-C. Bidard is part of advisory boards and received research grants (unrelated to
this study) from Pfizer, Novartis, and Lilly. Other authors have no competing interest. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tanguy, ML., Cabel, L., Berger, F. _et al._ Cdk4/6 inhibitors and overall survival:
power of first-line trials in metastatic breast cancer. _npj Breast Cancer_ 4, 14 (2018). https://doi.org/10.1038/s41523-018-0068-4 Download citation * Received: 28 January 2018 * Revised:
03 June 2018 * Accepted: 04 June 2018 * Published: 26 June 2018 * DOI: https://doi.org/10.1038/s41523-018-0068-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative