Gradient tungsten-doped bi3tinbo9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Bi3TiNbO9, a layered ferroelectric photocatalyst, exhibits great potential for overall water splitting through efficient intralayer separation of photogenerated carriers motivated
by a depolarization field along the in-plane _a_-axis. However, the poor interlayer transport of carriers along the out-of-plane _c_-axis, caused by the significant potential barrier between
layers, leads to a high probability of carrier recombination and consequently results in low photocatalytic activity. Here, we have developed an efficient photocatalyst consisting of
Bi3TiNbO9 nanosheets with a gradient tungsten (W) doping along the _c_-axis. This results in the generation of an additional electric field along the _c_-axis and simultaneously enhances the
magnitude of depolarization field within the layers along the _a_-axis due to strengthened structural distortion. The combination of the built-in field along the _c_-axis and polarization
along the _a_-axis can effectively facilitate the anisotropic migration of photogenerated electrons and holes to the basal {001} surface and lateral {110} surface of the nanosheets,
respectively, enabling desirable spatial separation of carriers. Hence, the W-doped Bi3TiNbO9 ferroelectric photocatalyst with Rh/Cr2O3 cocatalyst achieves an efficient and durable overall
water splitting feature, thereby providing an effective pathway for designing excellent layered ferroelectric photocatalysts. SIMILAR CONTENT BEING VIEWED BY OTHERS BIPOLAR CHARGE COLLECTING
STRUCTURE ENABLES OVERALL WATER SPLITTING ON FERROELECTRIC PHOTOCATALYSTS Article Open access 22 July 2022 UNVEILING CHARGE UTILIZATION MECHANISMS IN FERROELECTRIC FOR WATER SPLITTING
Article Open access 11 February 2025 CHARGE REDISTRIBUTION OF A SPATIALLY DIFFERENTIATED FERROELECTRIC BI4TI3O12 SINGLE CRYSTAL FOR PHOTOCATALYTIC OVERALL WATER SPLITTING Article Open access
04 June 2024 INTRODUCTION Converting solar energy to hydrogen by means of photocatalysis is one of the promising ways to solve energy and environmental issues1,2,3,4,5. Although numerous
photocatalysts have been searched and designed for overall water splitting in the past decades, the conversion efficiency of solar energy to hydrogen is still low due to the strong
recombination of photogenerated carriers. Recently, ferroelectric materials (e.g., PbTiO3, BiFeO3, Na0.5Bi0.5TiO3, and Bi3TiNbO9) with built-in electric field that could facilitate
photogenerated carrier separation have attracted wide attention in the field of photocatalysis6,7,8,9. Among them, Bi3TiNbO9 is an Aurivillius-type layered ferroelectric photocatalyst, which
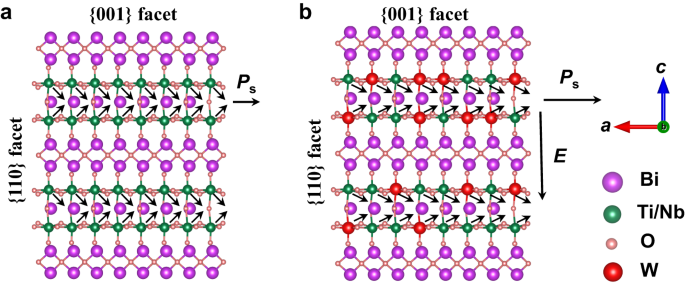
possesses a structural distortion induced depolarization field along the in-plane _a_-axis arising from the dipole interaction between bismuth in the (Bi2O2)2+ layer and oxygen in the
(BiTiNbO7)2- perovskite layer (Fig. 1a)10,11. Previous studies of Bi3TiNbO9 mainly focused on the effect of depolarization field on the photogenerated carrier separation and defect
engineering for surface active site modification. These synthesized Bi3TiNbO9 based photocatalysts have demonstrated good potential in photocatalytic water reduction or oxidation with the
assistance of sacrificial reagents and CO2 reduction12,13,14. In our latest work, Bi3TiNbO9 has revealed efficient and stable photocatalytic overall water splitting by selective exposure of
a robust (BiTiNbO7)2- perovskite layer for effective transfer of electron to the cocatalyst, affirming the great promise of Bi3TiNbO9 as a photocatalyst for solar energy to hydrogen
conversion15. The (Bi2O2)2+ and (BiTiNbO7)2- layers in Bi3TiNbO9 stack along the _c_-axis direction, resulting in a tendency to expose the {001} facet as the basal surface and the formation
of a nanosheet morphology. The photogenerated carriers exhibit an anisotropic migration property in the Bi3TiNbO9 nanosheet photocatalysts, where the photogenerated electrons usually reach
the basal {001} facet across the interlayer, but the photogenerated holes prefer to reach the lateral {110} facet within the intralayer13,16. Due to the large interlayer barrier, the
photogenerated electrons migrate slowly along the _c_-axis, which increases the recombination probability of the photogenerated carriers17. Although reducing the thickness of the layered
material along the _c_-axis to obtain an ultrathin structure18 or selective exposure of different layers can shorten the migration distance of photogenerated electrons from the bulk to
surface15, to some extent, the nature of poor interlayer charge transport remains unchanged. Therefore, the problem of large interlayer charge transport barrier represents an intrinsic
bottleneck in designing efficient layered Bi3TiNbO9 photocatalysts for overall water splitting. Here, we introduced an additional built-in electric field, perpendicular to the depolarization
field in Bi3TiNbO9 nanosheets, by gradient tungsten (W) element doping induced energy band structure adjustment between surface and bulk to break such a bottleneck. Typically, donor dopants
can effectively increase the number of free electrons, thus raising the corresponding Fermi level of semiconductors19,20,21,22,23. The concentration of this donor dopant W6+ shows a
gradient decrease from surface to bulk along the _c_-axis (Fig. 1b), giving rise to a doping-depth-dependent variation in the gap between the Fermi level and the conduction band minimum
(CBM) from surface to bulk and thus a formation of additional built-in electric field along the _c_-axis. This introduced electric field could be used for conquering the potential barrier
between the layers of (Bi2O2)2+ and (BiTiNbO7)2- for the photogenerated electrons, motivating the photogenerated electrons to the basal {001} facet. Moreover, the W dopant could strengthen
the structural distortion, resulting in an enhanced depolarization field along the _a_-axis for the anisotropic flow of the photogenerated carriers. Combined with selective exposure of the
robust (BiTiNbO7)2- perovskite layer, W6+ doped Bi3TiNbO9 exhibits efficient and stable photocatalytic overall water splitting, which is 10.4 times higher than that of the original
Bi3TiNbO9. Our work systematically studied the regulation of the crystal structure and electronic structure arising from element doping in Bi3TiNbO9, providing a reference for the
application of Aurivillius compound in photocatalytic overall water splitting. RESULTS PHOTOCATALYSTS CHARACTERIZATION W6+ ion doped Bi3TiNbO9 samples were prepared by a conventional flux
method24. Due to the layered structure and the influence of anions in molten salt, all the Bi3TiNbO9-_x_ W (_x_ = 0, 1%, 3%, 5%, 7%, 12% in molar ratio) samples exhibit a typical nanosheet
morphology with lateral width of _ca_. 1 μm and thickness of _ca_. 50 nm (Supplementary Fig. 1), indicating a trivial effect of W doping on the morphology. The inference of the crystal
structure was confirmed by the X-ray diffraction (XRD) characterization. As shown in Supplementary Fig. 2a, the diffraction patterns of all samples are basically the same as the standard
card (PDF#39-0233, space group: A21am) of Bi3TiNbO9. Note that there is a gradual variation in the relative intensity of the diffraction peaks at 14 and 24°, which are indexed as the (001)
and (110) planes of Bi3TiNbO9, respectively. As analyzed in Supplementary Fig. 2b, the ratio of (001)/(110) shows a volcanic curve with the increase of W6+ ion doping amount. Raman spectra
were obtained with 532 nm wavelength excitation to detect the bonding situation and the structure of Bi3TiNbO9-_x_ W (_x_ = 0, 1%, 3%, 5%, 7%, 12%) nanosheets (Supplementary Fig. 3). The
_E_g modes involving the stretching mode of diagonal oxygen ions on the _ab_ plane of the BO6 octahedron split into _B_2g at 533 cm-1 and _B_3g at 573 cm-1, which can be rationalized by the
orthogonal distortion of Bi3TiNbO925,26,27. The relative intensity of the two peaks increases with the W doping concentration in a certain range, which is in accord with the trend of ratio
of (001)/(110) in XRD, pointing out that W6+ ions are doped into the perovskite layers, thus contributing to structural distortion changes. The element composition and chemical states of the
Bi3TiNbO9-_x_ W (_x_ = 0, 5%) were determined by X-ray photoelectron spectroscopy (XPS) characterization. As shown in the Nb 3_d_ spectrum of the Bi3TiNbO9-5%W (Fig. 2a), there are two
additional peaks at 209.79 and 207.02 eV as compared with Bi3TiNbO9, of which the Nb 3_d_ peak positions are in good agreement with those of Nb2O5 (Supplementary Fig. 4f). The chemical
states of Bi 4 _f_, Ti 2_p_, and O 1 _s_ of Bi3TiNbO9-W are nearly the same as those of Bi3TiNbO9 (Supplementary Fig. 4). These results reveal that W6+ ions occupy the Nb5+ ion sites in the
perovskite structure, which is also supported by theoretical results in the following section of Fig. 3. Moreover, there are some impurity peaks that can be assigned to KNbWO6 (PDF #25-0667)
shown in the XRD diffraction pattern of Bi3TiNbO9-25% W with heavy doping (Supplementary Fig. 5), also affirming the substitution of Nb5+ by W6+. X-ray absorption spectroscopy (XAS) of the
W _L_3-edge was performed to probe the local coordination environment. The second derivative of X-ray absorption near-edge structure (XANES) spectrum in Supplementary Fig. 6 demonstrates two
peaks with an energy difference of about 4.3 eV due to the split 5_d_ states caused by the ligand field. And the splitting gap is in agreement with the value of octahedral wolframates
(3.0–5.6 eV)28,29. By further analyzing the Fourier transformed data of the extended fine structure region (EXAFS, Fig. 2b), the fitting curve reveals a W-O bond distance of 1.80 ± 0.01 Å
and a coordination number of W atoms of 5.0 ± 0.7, which indicates the unsaturated coordination environment of W atoms in these Bi3TiNbO9-W nanosheets. The distribution of W elements in
Bi3TiNbO9-W nanosheets was confirmed by XPS depth profiles and elemental mapping analysis. As shown in Fig. 2c, the atomic ratio of W/(Ti+Nb) decreases gradually from 22.6% to 13.3% with
increasing the etching depth by argon ions up to around 20 nm, proving the gradient doping of W. In addition, the combined XPS and ICP-OES investigations reveal that the W/(Ti+Nb) ratio on
the surface is much higher than that in the bulk for all W-doped samples (Supplementary Fig. 7). Notably, the difference between the measured surface and bulk W/(Ti+Nb) ratio increases
rapidly with W-doped concentrations of higher than 5%. These results suggest that the diffusion of doping atoms in the material is limited and the saturation doping concentration is about
3.5%. The spatial distribution of W was further examined by energy-dispersive X-ray spectroscopy (EDS). Viewing from Supplementary Fig. 8, the elements Bi, Ti, Nb, O, and W show a
homogeneous distribution, demonstrating the homogenous doping of the W element in the basal {001} facet of the Bi3TiNbO9-W nanosheets. To further study the W distribution along the _c_-axis,
the two stacked Bi3TiNbO9-W nanosheets were cut by focused ion beam, and their cross-section region was inspected by high-angle annular dark-field scanning transmission electron microscopy
(HAADF-STEM, Supplementary Fig. 9). The layered structure of Bi3TiNbO9-W nanosheets can be clearly identified in Fig. 2d, in which the double-layer Bi atom is the (Bi2O2)2+ layer and the
single-layer Bi atom is the (BiTiNbO7)2- layer, respectively. The microstructure at the interface between two nanosheets suggests that the outermost layer of Bi3TiNbO9-W is the (Bi2O2)2+
layer. The corresponding EDS mapping images show a gradient W distribution feature in a single Bi3TiNbO9-W nanosheet, with a maximal W content on the surface (Fig. 2e), further indicating
the gradient doping of W along _c_-axis. Density functional theory (DFT) calculations explored the effect of W doping on the electronic structure of Bi3TiNbO9. Based on the analysis of the
ion valence and radius (Ti4+: 0.605 Å, Nb5+: 0.64 Å, W6+: 0.60 Å), W atom may replace both the Ti and the Nb atoms in Bi3TiNbO9-W. Therefore, we have firstly calculated the formation
energies of W-doped Bi3TiNbO9 (2 × 2 × 1) with one Ti or one Nb atom replaced by one W atom, aiming to identify the specific doping site for the W atom in Bi3TiNbO9. As a result, the
formation energies are calculated to be 4.00 and 2.59 eV for W replacing Ti and Nb, respectively. This indicates an energetical preference for W dopants to occupy the Nb sites and is in good
agreement with the XPS spectra (Fig. 2a) and XRD patterns (Supplementary Fig. 5). Therefore, we only consider cases of W replacing Nb for electronic structure calculations of Bi3TiNbO9-W.
As shown in Fig. 3a, Bi3TiNbO9-W exhibits smaller bandgap values (2.35–2.50 eV) compared with that (2.79 eV) of pristine Bi3TiNbO9, due to a contribution from the W 5_d_ orbitals with lower
energy to the CBM (see Fig. 3b). Moreover, the bandgap values of Bi3TiNbO9-W decrease from 2.50 to 2.38, then to 2.35 eV with increasing the W-doping concentration from 3.2 to 6.3, then to
12.5%, respectively. UV-vis absorption spectra of Bi3TiNbO9-_x_ W (_x_ = 0, 1%, 3%, 5%, 7%, 12%) samples in Supplementary Fig. 10 also demonstrated a slight red shift of the absorption edge
with the increase of W doping concentration. As is well known, the core electrons of transition metals normally do not hybridize with the O 2_p_ electrons in metal oxides, giving rise to the
core electron level with very low energy. Such a core electron level does not obviously change by changing the chemical environment and has been widely adopted as a reference for band
alignment30,31. Here, taking the Nb 4 _s_ level as the reference, the band alignment of Bi3TiNbO9 and Bi3TiNbO9-W with different doping concentrations is given in Fig. 3b. As we can see, the
valance band maximum (VBM) of Bi3TiNbO9-W exhibits a slight decrease with increasing the doping concentration. Combined with the gradually reduced bandgap values, a more pronounced decrease
of the conduction band minimum (CBM) can be observed with increasing the doping concentration for Bi3TiNbO9-W, compared with pristine Bi3TiNbO9 (Fig. 3c). Typically, the introduction of W6+
ions in Bi3TiNbO9 could give rise to a shallow donor doping effect, caused by the unsaturated coordination environment of W atoms in these Bi3TiNbO9-W nanosheets as analyzed in Fig. 2b and
can be confirmed by the Mott–Schottky curves shown in Supplementary Fig. 11. The gradient donor W doping could contribute to a gradual decrease in the gap between the Fermi level and the CBM
from the bulk phase to the surface, forming an additional built-in electric field along the _c_-axis, which could provide a driving force for the migration of photoexcited electrons from
bulk to the surface of Bi3TiNbO9-W (Fig. 3d)32. To understand the structural distortion caused by W doping, Rietveld refinement was carried out via the General Structure Analysis System
(GSAS), as shown in Figs. 4a, b. There is a slight shrinkage at the _a_-axis after W doping, while the _b_-axis maintains unchanged properties basically. The corresponding _b_/_a_ value
increases from 1.0055 to 1.0067, implying an enhancement of the polarization along the _a_-axis33,34. The piezoelectric coefficient (_d_33) and dielectric constant (_ε_r) were further used
to investigate the ferroelectric polarization characteristics of the samples. Figures 4c, d shows the piezoelectric responses near the resonant frequency for Bi3TiNbO9 and Bi3TiNbO9-W
samples at different driving voltages, respectively. It can be clearly observed that the piezoelectric resonant peak amplitude (_A_max) increases with the driving voltages. The linear
relationship between \({A}_{\max }/Q\) and _V_ac can be obtained by fitting the harmonic oscillator model, where _Q_ is the quality factor and _V_ac is the driving ac voltage35,36, as shown
in Fig. 4e. The piezoelectric coefficient d33 values are derived to be 1.24 and 2.29 pm/V for Bi3TiNbO9 and Bi3TiNbO9-W, respectively. The remarkable increase in _d_33 indicates that the
ferroelectric polarization intensity (_P_s) of Bi3TiNbO9 is significantly enhanced after W doping37,38. Figure 4f shows the dielectric constant as a function of frequency at room
temperature. Generally, the polarization of dielectric materials is contributed by electronic, ionic, orientation, and space charge polarization39,40. The contribution of space charge
polarization decreases with the increase of frequency, giving rise to an inverse correlation with frequency for the dielectric constant (Fig. 4f). Comparing the dielectric constants of
Bi3TiNbO9 and Bi3TiNbO9-W, it can be inferred that W doping could elicit an increase in the polarizability characteristics of Bi3TiNbO9, in accordance with the shrinkage of the _a_-axis by
the Rietveld refinement (Fig. 4a, b) and the increased piezoelectric coefficient (_d_33) values in Fig. 4e. The difference of photogenerated charge transport behavior between Bi3TiNbO9 and
Bi3TiNbO9-W was comprehensively analyzed. As shown in Fig. 5a, the electrons in Bi3TiNbO9 tend to migrate along the _c_-axis, but might be confined by interlayer barrier between the of
(Bi2O2)2+ and (BiTiNbO7)2- layers, while the holes mostly migrate along the direction of the polarization electric field within the _ab_-plane13,16. The additional built-in electric field
introduced by the gradient doping in Bi3TiNbO9-W could weaken the potential barrier between the layers of (Bi2O2)2+ and (BiTiNbO7)2-, thus promoting the migration of the photogenerated
electrons to the basal {001} surface. Meanwhile, the enhanced depolarization field of Bi3TiNbO9-W along the _a_-axis could improve the transport of the photogenerated holes to the lateral
surface (Fig. 5b). The surface photovoltage (SPV) spectra of Bi3TiNbO9 and Bi3TiNbO9-W were executed to further explore the charge transfer characteristics (Fig. 5c). Owing to the
restriction on the migration of the photogenerated electrons along _c_-axis, the Bi3TiNbO9 exhibits a positive SPV feature in a consistent optical response range (300–400 nm)41,42,43. In
contrast, the negative signals of Bi3TiNbO9-W imply that the electron diffusion to the surface is largely promoted by the modified energy band structures, where the additional build-in
electric field induced by the W gradient doping could facilitate the transport of the photogenerated electrons from bulk to the {001} surface. The difference in the charge transfer processes
is responsible for the higher hydrogen evolution activity and lower oxygen evolution activity of Bi3TiNbO9-W compared with that of Bi3TiNbO9 (Supplementary Fig. 12). The SPV signal on
timescales of microseconds to milliseconds is attributed to the drift (Peak I) and diffusion (Peak II) processes of photogenerated carriers (Fig. 5d)44. Bi3TiNbO9 presents a positive peak II
lasting for 12.3 ms before decaying to zero, while Bi3TiNbO9-W exhibits a negative peak II lasting for 16.7 ms. The extension of the photoresponse time might be ascribed to the enhancement
of the polarization intensity along the _a_-axis direction and the additional electric field along the _c_-axis direction in Bi3TiNbO9-W, which can promote the anisotropic migration and
effective separation of photogenerated charges. The surface catalytic activity of Bi3TiNbO9 and Bi3TiNbO9-W, especially oxygen evolution reaction (OER), was also studied by DFT calculations.
Considering its intrinsic ferroelectricity, there exists a depolarization field along the \([\bar{1}00]\) direction of bulk Bi3TiNbO9 (see Supplementary Fig. 13a). In this case, the
migration of photogenerated holes towards \((\bar{1}\bar{1}0)\) and \(\left(\bar{1}10\right)\) facets can be accelerated by the depolarization field, making the \((\bar{1}\bar{1}0)\) and
\((\bar{1}10)\) facets more active for water oxidation reaction due to the accumulation of holes7,45. Since the \((\bar{1}\bar{1}0)\) facet and the \(\left(\bar{1}10\right)\) facet are
equivalent in crystallography, only the reaction coordinate for OER on the \((\bar{1}\bar{1}0)\) facet of Bi3TiNbO9 and Bi3TiNbO9-W was considered in this work (see Supplementary Fig. 13b).
Supplementary Fig. 13c, d present the calculated free energy profiles of water oxidation on the \((\bar{1}\bar{1}0)\) facets of Bi3TiNbO9 and Bi3TiNbO9-W. As we can see, Bi3TiNbO9 exhibits
an overpotential of 0.78 V for OER at the Nb site, while the overpotential for OER at the W site on the \((\bar{1}\bar{1}0)\) facet of Bi3TiNbO9-W is calculated to be 0.54 V. Basically, a
lower overpotential indicates higher OER activity. However, the potential of photoexcited holes in Bi3TiNbO9 is roughly estimated to be ~2.40 V15, suggesting that the photoexcited holes can
easily overcome the overpotentials for OER on the \((\bar{1}\bar{1}0)\) facet of both Bi3TiNbO9 and Bi3TiNbO9-W. Moreover, as shown in Fig. 3d, the additional built-in electric field along
_c_-axis induced by gradient W doping may impede the migration of photoexcited holes from bulk towards the surface as validated by the SPV signal reversion from positive to negative after W
doping (Fig. 5c, d). In fact, it has been reported that a low concentration of surface-reaching photogenerated holes can extremely limit the photocatalytic oxygen evolution performance46.
Therefore, Bi3TiNbO9-W exhibits an even lower oxygen evolution yield (see Supplementary Fig. 12b) due to the compromise of the minor advantage of reduced OER overpotential and major
disadvantage of less surface-reaching holes by W doping. PHOTOCATALYTIC PERFORMANCE Photocatalytic overall water splitting performance tests were carried out by loading Rh/Cr2O3 cocatalyst
on the surface of Bi3TiNbO9 nanosheets47,48,49. As shown in Fig. 6a, the photocatalytic performance displays a volcanic curve with the W/(Ti+Nb) ratio increasing, which is consistent with
the trend of structural distortion (inferred in Supplementary Fig. 2b, 3). When the ratio of W/(Ti+Nb) reaches 5%, that is, the saturation concentration of doping (Supplementary Fig. 7),
Bi3TiNbO9-W exhibits the optimal photocatalytic overall water splitting activity with the average hydrogen and oxygen evolution rates of 43.99 and 20.66 μmol h-1, respectively, which are
5.55 times higher than those of Bi3TiNbO9. Considering the very close specific surface area (1.2655 versus 1.3352 m2 g-1) of the samples without and with W dopant, one can conclude that the
additional built-in electric field has an essential effect on the photocatalytic overall water splitting activity enhancement. Once the doping amount exceeds the limit value, the formed
impurity phase may be enriched on the surface of the sample, affecting light absorption characteristics and reducing photocatalytic activity. Furthermore, this gradient doping strategy in
Bi3TiNbO9 system shows universality, demonstrating an efficient photocatalytic overall water splitting properties by employing Mo as the dopant as well. However, the activity of Bi3TiNbO9-Mo
is only half of Bi3TiNbO9-W, as shown in Supplementary Fig. 14, which might be mainly attributed to the formation of defect transition levels below the conduction band edge induced by the
Mo dopants. In contrast, W doping does not introduce any defect level in the band gap for the recombination of photogenerated carriers50. Time-dependent photocatalytic overall water
splitting performance tests were used to evaluate the stability of Bi3TiNbO9-W. As shown in Figs. 6b, c, the activities of both Bi3TiNbO9 and Bi3TiNbO9-W decay rapidly during the long-term
test, which can be ascribed to the self-reduction of Bi3+ in the surface (Bi2O2)2+ layers, as revealed in our previous work15. The XRD pattern shows that the diffraction peaks of Bi3TiNbO9
and Bi3TiNbO9-W remain unchanged after the photocatalytic overall water splitting reaction (Supplementary Fig. 15), indicating that the samples have a highly stable crystal structure.
Adjusting the surface structure of Bi3TiNbO9 by acid etching and selectively exposing the robust perovskite layer (denoted as PL) have been successfully demonstrated to be an effective way
to inhibit photocorrosion. We adopted such a strategy to remove the (Bi2O2)2+ layer on the surface of Bi3TiNbO9-W (the resulting sample is named as Bi3TiNbO9-W-PL). The robust surface states
of the obtained Bi3TiNbO9-W-PL catalysts can well balance the transfer of the photogenerated electrons to the perovskite layer-terminated surface and the photogenerated holes to lateral
surface, corresponding to a weak and negative SPV signal demonstrated in Supplementary Fig. 16 Therefore, the Bi3TiNbO9-W-PL exhibits an efficient and stable activity (106.71 μmol h-1 for H2
and 47.94 μmol h-1 for O2) for overall water splitting in a 10 h long-term test (Fig. 6d), which is 10.4 times higher than that of pristine Bi3TiNbO9. The apparent quantum yield (AQY) of
Bi3TiNbO9-W-PL was calculated to be 0.57% at 365 nm, being higher than the reported value for ferroelectric photocatalytic materials claimed (Supplementary Fig. 17, 18 and Supplementary
Tables 1, 2). DISCUSSION In summary, we have successfully synthesized a series of W-doped Bi3TiNbO9 (Bi3TiNbO9-W) nanosheets. The concentration of this donor W dopant shows a gradient
decrease from the surface to the bulk along the _c_-axis, which exhibits favorable characteristics for photocatalytic water splitting. One is that the gradient distribution of the W dopant
can introduce an additional built-in electric field from the surface to the bulk to conquer the potential barrier between the layers of (Bi2O2)2+ and (BiTiNbO7)2- along the _c_-axis for the
migration of the photogenerated electrons. The other is that ion substitution in the perovskite layer could strengthen the structural distortion of Bi3TiNbO9, which can enhance the
depolarization field along the _a_-axis. As a result, the effective anisotropic migration and separation of the photogenerated carriers is fulfilled. Combined with the selective exposure of
the robust (BiTiNbO7)2- perovskite layer, our synthesized Bi3TiNbO9-W-PL catalyst shows an efficient and stable photocatalytic overall water splitting, which is 10.4 times higher than that
of the pristine Bi3TiNbO9. This gradient doping strategy introduced in this work might provide guidance for the design of highly efficient and stable Aurivillius-type layered photocatalysts
for overall water splitting. METHODS PREPARATION OF W-DOPED BI3TINBO9 NANOSHEETS Bi2O3 (0.699 g, Sinopharm (China)), anatase TiO2 (0.08 g, Aldrich, 99%), Nb2O5 (0.133 g, Aladdin, 99.9%),
NaCl-KCl (8 g, Sinopharm (China), a molar ratio of 1:1 and an eutectic temperature of 657 °C), and an appropriate amount of WO3 (Sinopharm (China)) were mixed by an agate mortar for 30 min,
and then transferred into an alumina crucible and calcined at 800 oC for 2 h. The obtained product was washed with hot deionized water to completely remove the flux reagents and dried in
air, and denoted as Bi3TiNbO9-_x_ W (_x_ = 0, 1%, 3%, 5%, 7%, 12% in molar ratio). The synthesis process of Bi3TiNbO9-Mo is the same as that of Bi3TiNbO9-W, except that MoO3 instead of WO3
is used. PREPARATION OF BI3TINBO9-W-PL NANOSHEETS 250 mg of the Bi3TiNbO9-W sample was dispersed into 125 mL 0.32 mol L-1 hydrochloric acid solution and continuously stirred for 5 h. The
sample is then separated by filtration and washed with a large amount of deionized water until neutral. COCATALYST DEPOSITION 150 mg of the sample was dispersed in 100 mL of aqueous methanol
solution (10 vol%). Photoreduction of rhodium chloride (0.9 mg, RhCl3, Alfa, 99.9%) and potassium chromate (0.9 mg, K2CrO4, Sigma-Aldrich, 99%) was carried out in steps under vacuum. The
product was washed thoroughly with distilled water and dried. SAMPLE CHARACTERIZATION The Scanning Electron Microscope (SEM, Nova NanoSEM 430) was used to examine the morphology of the
samples. X-ray diffraction patterns of the samples were collected on a Rigaku D/max 2400 spectrometer using Cu _K_a X-rays of wavelength 1.54056 Å. The Raman spectra were collected on a
Lab-Ram HR 800 spectrometer with an excitation wavelength of 532 nm. The UV-vis diffusion reflection spectra were obtained using a spectrophotometer (Jasco V-770) equipped with an
integrating sphere in diffusion reflection mode. X-ray photoelectron spectroscopy (XPS) measurement was conducted on Escab-250 (Thermofisher Scientific, America) with a monochromatic Al _K_α
X-ray source. All binding energies were referenced to the C 1 _s_ peak (284.6 eV) that arises from adventitious carbon. XPS depth profile analyses were performed using an Ar+ ion gun to
etch stepwise layers at a rate of ~0.1 nm s-1. Elemental analysis was carried out by a means of inductively coupled plasma atomic emission spectroscopy (ICP-OES). The piezoresponse force
microscopy (PFM) of the powder samples were performed by a Scanning Probe Microscopy (SPM, Bruker Dimension Icon) system operated in Vertical PFM mode with Pt/Ir-coated Si cantilever tips.
The dielectric measurements were performed using the two-probe impedance method with an Impedance Analyzer (TH2828A) over the frequency range from 100 Hz to 2 MHz. Cross-sectional HAADF-STEM
images were acquired using an aberration-corrected STEM (Titan Themis 60–300 X-FEG microscope (FEI)) with double aberration (Cs) correctors from CEOS and a monochromator operating at 300
kV. PHOTOCATALYTIC ACTIVITY TESTS Photocatalytic water splitting tests were conducted in an automatic testing system (Beijing Perfectlight Technology Co., Ltd., Labsolar-6A) with a 250 mL
reaction container and a set temperature of 10 °C. The specific operation process is as follows: The sample loaded with cocatalyst (50 mg) was dispersed into 100 mL of pure water. After the
air in the system was completely removed, a 300 W xenon lamp (Beijing Perfectlight Technology Co., Ltd., PLS-SXE300D) at a wavelength of _λ_ ≥ 300 nm with an intensity of 349 mW cm−2 was
used as the light source. The gases generated from the system were analyzed at given time intervals by gas chromatography (Agilent Technologies, 6890 N). SURFACE PHOTOVOLTAGE (SPV)
MEASUREMENT The system included a lock-in amplifier (SR830, Stanford Research Systems, Inc.) with a light chopper (SR540), a 500 W xenon lamp with a monochromator (SBP500, Zolix) as a light
source, and a photovoltaic cell. The monochromatic light chopped with a frequency of 23 Hz is focused on the photovoltaic cell. The photovoltaic signal is amplified by a lock-in amplifier
and transmitted to a computer for recording. TRANSIENT PHOTOVOLTAGE (TPV) MEASUREMENT The system included a Nd: YAG laser source (Q-smart 450 Quantel) providing laser radiation pulse
(wavelength: 355 nm, pulse width: 5 ns and Intensity: 100 µJ), a 500 MHz digital phosphor oscilloscope (TDS 5054, Tektronix) with a preamplifier (5003 Brookdeal Electronics), and a
parallel-plate capacitor-like sample chamber. CALCULATION DETAIL The density functional theory (DFT) calculations were performed by using Vienna Ab-initio Simulation Package (VASP)51. The
interaction between ions and valence electrons was described using projector augmented wave (PAW) potentials52, and the exchange-correlation interaction was described by generalized gradient
approximation (GGA) with the Perdew-Burke-Ernzerhof (PBE) functional53. To overcome the well-known self-interaction error in DFT, the GGA + U method was used to treat the d electrons
(\(U=4{{{{{\rm{eV}}}}}}\) for Ti 3_d_ and Nb 4_d_, \(U=3{{{{{\rm{eV}}}}}}\) for W 5_d_)54. The plane wave energy cutoff was set to be 500 eV. The valence electron configurations of Bi, Ti,
Nb, O, and H atoms are 5_d_106_s_26_p_3, 3_s_23_p_63_d_24_s_2, 4_s_24_p_64_d_45_s_1, 2_s_22_p_4, and 1_s_1, respectively. To describe the W-doped Bi3TiNbO9, a \(2\times 2\times 1\) supercell
containing 16 Ti and Nb atoms was constructed, subsequently, one, two, and four Nb atoms were replaced by W atoms, corresponding to a W-doping concentration of 3.2, 6.3, and 12.5%,
respectively. In order to calculate the Gibbs free energy (Δ_G_) of different intermediates (OH*, O*, OOH*) adsorption on the \((\bar{1}\bar{1}0)\) facet of Bi3TiNbO9, a simplified model
only with the perovskite layer was adopted for the four-electron oxygen evolution reaction (OER), since the perovskite layer was demonstrated to be active for OER55,56. As for the W-doped
case, one surface Nb atom was replaced by a W atom. For this model, except for one five-fold-coordinated Ti, Nb, or W atom on the \((\bar{1}\bar{1}0)\) surface was left to be exposed as the
reaction site for OER, all other unsaturated O and transition metal (Ti, Nb, W) atoms were saturated by H atoms and hydroxyl groups, respectively. The vacuum thickness was set to be 16 Å for
both the _b_ and _c_ directions. The atomic structures were fully optimized with the convergence criteria for residual force and total energy of 0.02 eV Å−1 and 10−6 eV, respectively. The
Gibbs free energy (\(\Delta G\)) was calculated from the DFT simulation by adding the free energy correction according to following equation, $$\Delta G=\Delta
{E}_{{{{{{\rm{DFT}}}}}}}+\Delta {G}_{{{{{{\rm{cor}}}}}}}$$ (1) where the free energy correction (\(\Delta {G}_{{{{{{\rm{cor}}}}}}}\)) for free molecules and adsorbed species was obtained by
using the VASP toolkit56, based on the following definition, $${G}_{{{{{{\rm{cor}}}}}}}={ZPE}+{E}_{{{{{{\rm{vib}}}}}}}+{E}_{{{{{{\rm{tran}}}}}}}+{E}_{{{{{{\rm{rot}}}}}}}+{PV}-{TS}$$ (2)
$${G}_{{{{{{\rm{cor}}}}}}}={ZPE}+{E}_{{{{{{\rm{vib}}}}}}}-{TS}$$ (3) where ZPE is the zero-point energy, \({E}_{{{{{{\rm{vib}}}}}}}\), \({E}_{{{{{{\rm{tran}}}}}}}\), and
\({E}_{{{{{{\rm{rot}}}}}}}\) are thermal corrections to the internal energy from vibrational, translational, and rotational motions of molecules, respective, the PV term converts internal
energy to enthalpy, and the entropy term TS converts enthalpy to Gibbs free energy. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding
author upon request. REFERENCES * Zhou, P. et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. _Nature_ 613, 66–70 (2023). Article ADS CAS PubMed
Google Scholar * Xiao, J., Hisatomi, T. & Domen, K. Narrow-band-gap particulate photocatalysts for one-step-excitation overall water splitting. _Acc. Chem. Res._ 56, 878–888 (2023).
Article CAS PubMed Google Scholar * Li, Z. et al. Blocking the reverse reactions of overall water splitting on a Rh/GaN–ZnO photocatalyst modified with Al2O3. _Nat. Catal._ 6, 80–88
(2023). Article CAS Google Scholar * Wang, Z. et al. Efficiency accreditation and testing protocols for particulate photocatalysts toward solar fuel production. _Joule_ 5, 344–359 (2021).
Article CAS Google Scholar * Suguro, T. et al. A hygroscopic nano-membrane coating achieves efficient vapor-fed photocatalytic water splitting. _Nat. Commun._ 13, 5698 (2022). Article
ADS CAS PubMed PubMed Central Google Scholar * Liu, Y. et al. Internal-field-enhanced charge separation in a single-domain ferroelectric PbTiO3 photocatalyst. _Adv. Mater._ 32, 1906513
(2020). Article CAS Google Scholar * Wan, G. et al. Photocatalytic overall water splitting over PbTiO3 modulated by oxygen vacancy and ferroelectric polarization. _J. Am. Chem. Soc._ 144,
20342–20350 (2022). Article CAS PubMed Google Scholar * He, J. et al. Boosting photocatalytic water oxidation on photocatalysts with ferroelectric single domains. _Adv. Mater._ 35,
2210374 (2023). Article CAS Google Scholar * Liu, Y. et al. Bipolar charge collecting structure enables overall water splitting on ferroelectric photocatalysts. _Nat. Commun._ 13, 4245
(2022). Article ADS CAS PubMed PubMed Central Google Scholar * Frit, B. & Mercurio, J. P. The crystal chemistry and dielectric properties of the Aurivillius family of complex
bismuth oxides with perovskite-like layered structures. _J. Alloy. Compd._ 188, 27–35 (1992). Article CAS Google Scholar * Ismunandar, Kennedy, B. J., Gunawan & Marsongkohadi
Structure of ABi2Nb2O9(A= Sr, Ba): refinement of powder neutron diffraction data. _J. Solid State Chem._ 126, 135–141 (1996). Article ADS CAS Google Scholar * Yu, H. et al. Synergy of
ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction. _Nat. Commun._ 12, 4594 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Yin, X. et al.
Realizing selective water splitting hydrogen/oxygen evolution on ferroelectric Bi3TiNbO9 nanosheets. _Nano Energy_ 49, 489–497 (2018). Article CAS Google Scholar * Jiang, L., Ni, S., Liu,
G. & Xu, X. Photocatalytic hydrogen production over Aurivillius compound Bi3TiNbO9 and its modifications by Cr/Nb co-doping. _Appl. Catal. B_ 217, 342–352 (2017). Article CAS Google
Scholar * Huang, J. et al. Selective exposure of robust perovskite layer of aurivillius-type compounds for stable photocatalytic overall water splitting. _Adv. Sci._ 10, 2302206 (2023).
Article CAS Google Scholar * Yu, H. et al. Coupling ferroelectric polarization and anisotropic charge migration for enhanced CO2 photoreduction. _Appl. Catal. B_ 284, 119709 (2021).
Article CAS Google Scholar * Kang, Y. et al. Selective breaking of hydrogen bonds of layered carbon nitride for visible light photocatalysis. _Adv. Mater._ 28, 6471–6477 (2016). Article
CAS PubMed Google Scholar * Liu, L. et al. Cooperation of oxygen vacancies and 2D ultrathin structure promoting CO2 photoreduction performance of Bi4Ti3O12. _Sci. Bull._ 65, 934–943
(2020). Article CAS Google Scholar * Chen, Z. et al. Non-oxidative coupling of methane: N-type doping of niobium single atoms in TiO2–SiO2 induces electron localization. _Angew. Chem.
-Int. Ed._ 60, 11901–11909 (2021). Article ADS CAS Google Scholar * Xiao, Y. Q. et al. Band structure engineering and defect control of Ta3N5 for efficient photoelectrochemical water
oxidation. _Nat. Catal._ 3, 932–940 (2020). Article CAS Google Scholar * Zhang, Y. et al. Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for
photocatalytic overall water splitting. _Nat. Energy_ 8, 504–514 (2023). Article ADS CAS Google Scholar * Fang, W. et al. Effect of surface self-heterojunction existed in BixY1–xVO4 on
photocatalytic overall water splitting. _ACS Sustain_. _Chem. Eng._ 5, 6578–6584 (2017). ADS CAS Google Scholar * Park, Y., McDonald, K. J. & Choi, K.-S. Progress in bismuth vanadate
photoanodes for use in solar water oxidation. _Chem. Soc. Rev._ 42, 2321–2337 (2013). Article CAS PubMed Google Scholar * Wang, Z. et al. Sequential cocatalyst decoration on BaTaO2N
towards highly-active Z-scheme water splitting. _Nat. Commun._ 12, 1005 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Yuan, J., Nie, R., Chen, Q., Xiao, D. & Zhu,
J. Structural distortion, piezoelectric properties, and electric resistivity of A-site substituted Bi3TiNbO9-based high-temperature piezoceramics. _Mater. Res. Bull._ 115, 70–79 (2019).
Article CAS Google Scholar * Liang, K., Qi, Y. & Lu, C. Temperature-dependent Raman scattering in ferroelectric Bi4−xNdxTi3O12(x = 0, 0.5, 0.85) single crystals. _J. Raman Spectrosc._
40, 2088–2091 (2009). Article ADS CAS Google Scholar * Graves, P. R., Hua, G., Myhra, S. & Thompson, J. G. The Raman modes of the Aurivillius phases: temperature and polarization
dependence. _J. Solid State Chem._ 114, 112–122 (1995). Article ADS CAS Google Scholar * Yamazoe, S., Hitomi, Y., Shishido, T. & Tanaka, T. XAFS study of tungsten L1- and L3-edges:
structural analysis of WO3 species loaded on TiO2 as a catalyst for photo-oxidation of NH3. _J. Phys. Chem. C._ 112, 6869–6879 (2008). Article CAS Google Scholar * Wu, Y. et al. Depressed
transition temperature of WxV1−xO2: mechanistic insights from the X-ray absorption fine structure (XAFS) spectroscopy. _Phys. Chem. Chem. Phys._ 16, 17705–17714 (2014). Article CAS PubMed
Google Scholar * Di Valentin, C., Pacchioni, G. & Selloni, A. Origin of the different photoactivity of N-doped anatase and rutile TiO2. _Phys. Rev. B_ 70, 085116 (2004). Article ADS
Google Scholar * Kraut, E. A., Grant, R. W., Waldrop, J. R. & Kowalczyk, S. P. Precise determination of the valence-band edge in X-ray photoemission spectra: application to
measurement of semiconductor interface potentials. _Phys. Rev. Lett._ 44, 1620–1623 (1980). Article ADS CAS Google Scholar * Yu, Y. et al. Surface engineering for extremely enhanced
charge separation and photocatalytic hydrogen evolution on g-C3N4. _Adv. Mater._ 30, 1705060 (2018). Article Google Scholar * Wolfe, R. W., Newnham, R. E., Smithf, D. K. & Kay, M. I.
Crystal structure of Bi3TiNbO9. _Ferroelectrics_ 3, 1–7 (1972). Article ADS Google Scholar * Shimakawa, Y. et al. Crystal structures and ferroelectric properties of SrBi2Ta2O9 and
Sr0.8Bi2.2Ta2O9. _Appl. Phys. Lett._ 74, 1904–1906 (1999). Article ADS CAS Google Scholar * Garcı́a, R. & Pérez, R. Dynamic atomic force microscopy methods. _Surf. Sci. Rep._ 47,
197–301 (2002). Article ADS Google Scholar * Shah, J. H. et al. Regulation of ferroelectric polarization to achieve efficient charge separation and transfer in particulate RuO2 /BiFeO3
for high photocatalytic water oxidation activity. _Small_ 16, e2003361 (2020). Article PubMed Google Scholar * Vanderbilt, D. & King-Smith, R. D. Electric polarization as a bulk
quantity and its relation to surface charge. _Phys. Rev. B_ 48, 4442–4455 (1993). Article ADS CAS Google Scholar * Duran, C., Trolier-McKinstry, S. & Messing, G. L. Processing and
electrical properties of 0.5Pb(Yb1/2Nb1/2)O3-0.5PbTiO3 ceramics. _J. Electroceram._ 10, 47–55 (2003). Article CAS Google Scholar * Balmuchu, S. P. & Dobbidi, P. The effect of La
doping on multiferroic BiFeO3 ceramic through structural, dielectric, magnetic, ferroelectric, and optical studies. _Phys. B_ 638, 413937 (2022). Article CAS Google Scholar * Divya, N. K.
& Pradyumnan, P. P. Enhancement of photocatalytic activity in Nd doped ZnO with an increase in dielectric constant. _J. Mater. Sci. -Mater. Electron._ 28, 2147–2156 (2016). Article
Google Scholar * Chen, R. et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. _Nature_ 610, 296–301 (2022). Article ADS CAS PubMed Google Scholar * Chen, R.,
Fan, F. & Li, C. Unraveling charge-separation mechanisms in photocatalyst particles by spatially resolved surface photovoltage techniques. _Angew. Chem. -Int. Ed._ 61, e202117567 (2022).
Article ADS CAS Google Scholar * Chen, R., Fan, F., Dittrich, T. & Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface
photovoltage microscopy. _Chem. Soc. Rev._ 47, 8238–8262 (2018). Article CAS PubMed Google Scholar * Zhao, Y. et al. Mechanistic analysis of multiple processes controlling solar-driven
H2O2 synthesis using engineered polymeric carbon nitride. _Nat. Commun._ 12, 3701 (2021). Article ADS CAS PubMed PubMed Central Google Scholar * Liu, G. et al. Selective chemical
epitaxial growth of TiO2 islands on ferroelectric PbTiO3 crystals to boost photocatalytic activity. _Joule_ 2, 1095–1107 (2018). Article CAS Google Scholar * Wang, D., Sheng, T., Chen,
J., Wang, H.-F. & Hu, P. Identifying the key obstacle in photocatalytic oxygen evolution on rutile TiO2. _Nat. Catal._ 1, 291–299 (2018). Article CAS Google Scholar * Nishiyama, H. et
al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. _Nature_ 598, 304–307 (2021). Article ADS CAS PubMed Google Scholar * Xiao, J. D. et al. Enhanced overall
water splitting by a zirconium-doped TaON-based photocatalyst. _Angew. Chem. -Int. Ed._ 61, e202116573 (2022). Article ADS CAS Google Scholar * Wang, Q. et al. Oxysulfide photocatalyst
for visible-light-driven overall water splitting. _Nat. Mater._ 18, 827–832 (2019). Article ADS CAS PubMed Google Scholar * Pakeetood, P. et al. Hybrid-functional study of native
defects and W/Mo-doped in monoclinic-bismuth vanadat. _J. Phys. Chem. C._ 123, 14508–14516 (2019). Article CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative
schemes for ab initio total-energy calculations using a plane-wave basis set. _Phys. Rev. B_ 54, 11169–11186 (1996). Article ADS CAS Google Scholar * Kresse, G. & Joubert, D. From
ultrasoft pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758–1775 (1999). Article ADS CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M.
Generalized gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865–3868 (1996). Article ADS CAS PubMed Google Scholar * Anisimov, V. I., Zaanen, J. & Andersen, O. K. Band
theory and Mott insulators: Hubbard U instead of Stoner I. _Phys. Rev. B_ 44, 943–954 (1991). Article ADS CAS Google Scholar * Retuerto, M. et al. Na-doped ruthenium perovskite
electrocatalysts with improved oxygen evolution activity and durability in acidic media. _Nat. Commun._ 10, 2041 (2019). Article ADS PubMed PubMed Central Google Scholar * Dionigi, F.
et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. _Nat. Commun._ 11, 2522 (2020). Article ADS CAS PubMed PubMed Central
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Key R&D Program of China (no. 2021YFA1500800), National Natural Science Foundation of China
(no. 51825204, 52120105003, 52188101), and CAS Projects for Young Scientists in Basic Research (YSBR-004), International Partnership Program of the Chinese Academy of Sciences
(174321KYSB20200005). G. L. thanks the financial support from the New Cornerstone Science Foundation through the XPLORER PRIZE. AUTHOR INFORMATION Author notes * These authors contributed
equally: Jie Huang, Yuyang Kang, Jianan Liu. AUTHORS AND AFFILIATIONS * Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 72
Wenhua Road, Shenyang, 110016, China Jie Huang, Yuyang Kang, Jianan Liu, Tingting Yao, Jianhang Qiu, Biaohong Huang, Weijin Hu, Yan Liang, Chunlin Chen, Li-Chang Yin, Hui-Ming Cheng &
Gang Liu * School of Materials Science and Engineering, University of Science and Technology of China, 72 Wenhua Road, Shenyang, 110016, China Jie Huang, Jianan Liu, Weijin Hu & Gang Liu
* National Laboratory of Solid State Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing, Jiangsu, 210093, China Peipei Du * College of Chemistry,
Jilin University, Changchun, 130012, China Tengfeng Xie * Nanomaterials Centre, School of Chemical Engineering and Australian Institute for Bioengineering and Nanotechnology, The University
of Queensland, St Lucia, QLD, 4072, Australia Lianzhou Wang * Institute of Technology for Carbon Neutrality, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen,
518055, China Hui-Ming Cheng Authors * Jie Huang View author publications You can also search for this author inPubMed Google Scholar * Yuyang Kang View author publications You can also
search for this author inPubMed Google Scholar * Jianan Liu View author publications You can also search for this author inPubMed Google Scholar * Tingting Yao View author publications You
can also search for this author inPubMed Google Scholar * Jianhang Qiu View author publications You can also search for this author inPubMed Google Scholar * Peipei Du View author
publications You can also search for this author inPubMed Google Scholar * Biaohong Huang View author publications You can also search for this author inPubMed Google Scholar * Weijin Hu
View author publications You can also search for this author inPubMed Google Scholar * Yan Liang View author publications You can also search for this author inPubMed Google Scholar *
Tengfeng Xie View author publications You can also search for this author inPubMed Google Scholar * Chunlin Chen View author publications You can also search for this author inPubMed Google
Scholar * Li-Chang Yin View author publications You can also search for this author inPubMed Google Scholar * Lianzhou Wang View author publications You can also search for this author
inPubMed Google Scholar * Hui-Ming Cheng View author publications You can also search for this author inPubMed Google Scholar * Gang Liu View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS G.L. led the project. Under the guide of G.L., J.H. with assistance of Y.Y. K. designed and performed the experiments. T.T.Y. and C.L.C.
performed the HAADF-STEM tests. P.P.D. fitted EXAFS data. T.F.X. performed the SPV and TPV tests. B.H.H and W.J.H. performed the measurements of piezoelectric coefficients and dielectric
constants. Y.L. conducted FIB sample cutting. J.L. and L.C.Y. conducted theoretical calculations and wrote related contents. J.H. drafted most of the manuscript, and J.H.Q., Y.Y.K., L.C.Y.
and G.L. edited the manuscript. All authors were involved in data analysis and manuscript revision. CORRESPONDING AUTHOR Correspondence to Gang Liu. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Xiaoxiang Xu and the other, anonymous, reviewer(s) for their contribution to
the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps
and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Huang, J., Kang, Y., Liu, J. _et al._ Gradient tungsten-doped Bi3TiNbO9
ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting. _Nat Commun_ 14, 7948 (2023). https://doi.org/10.1038/s41467-023-43837-4 Download
citation * Received: 17 July 2023 * Accepted: 22 November 2023 * Published: 01 December 2023 * DOI: https://doi.org/10.1038/s41467-023-43837-4 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative