At-home wearables and machine learning sensitively capture disease progression in amyotrophic lateral sclerosis
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Amyotrophic lateral sclerosis causes degeneration of motor neurons, resulting in progressive muscle weakness and impairment in motor function. Promising drug development efforts have
accelerated in amyotrophic lateral sclerosis, but are constrained by a lack of objective, sensitive, and accessible outcome measures. Here we investigate the use of wearable sensors, worn on
four limbs at home during natural behavior, to quantify motor function and disease progression in 376 individuals with amyotrophic lateral sclerosis. We use an analysis approach that
automatically detects and characterizes submovements from passively collected accelerometer data and produces a machine-learned severity score for each limb that is independent of clinical
ratings. We show that this approach produces scores that progress faster than the gold standard Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (−0.86 ± 0.70 SD/year versus
−0.73 ± 0.74 SD/year), resulting in smaller clinical trial sample size estimates (N = 76 versus N = 121). This method offers an ecologically valid and scalable measure for potential use in
amyotrophic lateral sclerosis trials and clinical care.
Novel therapeutic modalities are now aimed at proximal disease mechanisms in amyotrophic lateral sclerosis (ALS) and other neurodegenerative diseases1,2. One major barrier to the successful
and efficient development of disease-modifying therapies for neurodegenerative disorders is a lack of objective clinical outcome measures that account for disease heterogeneity and can
sensitively quantify disease progression over the duration of a clinical trial3,4,5. The standard tool for assessing disease severity in ALS clinical trials and clinical care is a
semi-quantitative rating scale (ALS Functional Rating Scale-Revised6,7 or ALSFRS-R) that uses multiple choice questions to evaluate several behavioral functions (e.g., walking, handwriting,
speech, swallowing). The assessment is most often completed by clinicians specializing in ALS7,8, however recent studies have shown high correlation between clinician-performed ALSFRS-R and
at-home, patient-performed ALSFRS-R9. Clinician or patient-performed ALSFRS-R is a useful assessment of global motor function; however, it is subjective, categorical, and is only performed
intermittently over time, which limits its sensitivity for measuring disease change and contributes to the need for relatively large and expensive trials10,11. This is a particular challenge
in rare diseases and results in pressure to include relatively homogenous cohorts with faster rates of disease progression, which restricts participation of some individuals and may not be
representative of the entire ALS population12.
There is a great opportunity to reduce the size and cost of ALS trials, increase the population of individuals who can participate, and accelerate the evaluation of promising therapeutics
through the development of new categories of sensitive quantitative motor outcome measures13,14,15. Quantitative motor outcome measures may be task-based (i.e., measuring behavior during the
performance of a specific task) or task-free, where an individual’s natural behavior is measured passively and continuously at home. There has been recent development of several task-based
approaches to quantify speech and limb function in ALS using scalable technologies at home9,16,17,18 and only a single report of a task-free approach in ALS using a waist-worn
accelerometer19. Task-based measures, however, have some of the same limitations as rating scales in that they are based on a relatively small number of data samples and cannot easily
account for diurnal and day-to-day variability, they rely on the participant’s ability and motivation to perform the task, and they are susceptible to learning and placebo effects.
Task-free assessment approaches which passively and continuously measure natural behavior at home have the potential to overcome these limitations and be transformative by making reliable
and sensitive measures available at scale. Furthermore, they have the potential to produce measures that more closely reflect the day-to-day function of the individual by measuring the
individual’s own selection of behaviors. However, the information obtained by the tool must be interpretable and meaningful to support its use in clinical trials or clinical care.
Here we demonstrate that a submovement-focused analysis of triaxial accelerometer data20,21, recorded from wrist and ankle sensors worn by hundreds of individuals with ALS at home during
natural behavior, produces interpretable and robust measures of motor function and disease progression. We develop a machine learning approach to train a model that is sensitive to disease
change by utilizing the information for how individuals’ sensor-based movement patterns change over time, rather than being constrained by existing clinical assessments such as ALSFRS-R. We
show that the model’s severity estimates and longitudinal trajectories are reliable and consistent with ALSFRS-R, but are more sensitive than the clinical scale for measuring change over
time. Thus, we demonstrate that objective, sensitive, and scalable measures of motor function and disease change can be obtained from passive analysis of everyday behavior using inexpensive
wearable sensors.
We analyzed accelerometer data from wrist and ankle-worn sensors collected as part of the Precision Medicine Program launched by the ALS Therapy Development Institute (ALS TDI) in 2014 (see
Methods). Individuals were asked to wear a sensor on each wrist and ankle continuously for a week each month. Participants also performed a sequence of 5 limb-based exercises on alternating
days, lasting a total of approximately 5 min. Participants were instructed that sensors must be worn during the brief exercises, but to also wear the sensors as much as possible throughout
the week without further specifying periods of wear time. An analysis of accelerometer data collected only during the task-based assessments was previously reported17. Here, we analyze the
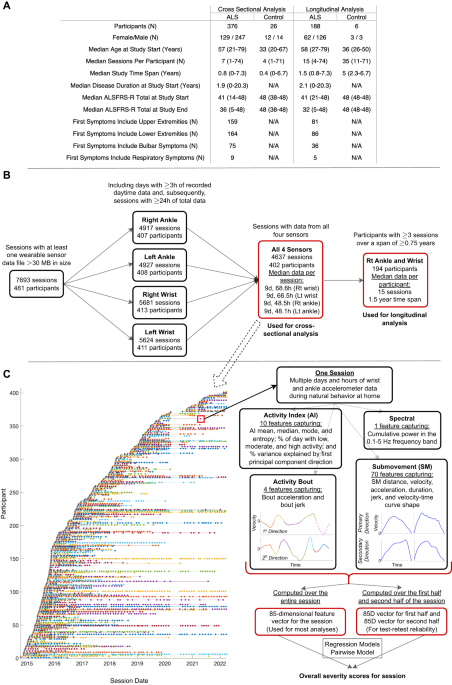
entirety of accelerometer data collected at home as individuals performed their typical daily routine without any constraints. Participant clinical and demographic data are shown in Fig. 1A,
including the median ALSFRS-R at the study start and study end. 95% of participants lived in the United States (41 states represented), 3% lived in Canada, and 1% lived in the United
Kingdom. 93.5% of participants were White, 2% Hispanic, 2% Asian, 1% Black,