Phenotypically complex living materials containing engineered cyanobacteria
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The field of engineered living materials lies at the intersection of materials science and synthetic biology with the aim of developing materials that can sense and respond to the
environment. In this study, we use 3D printing to fabricate a cyanobacterial biocomposite material capable of producing multiple functional outputs in response to an external chemical
stimulus and demonstrate the advantages of utilizing additive manufacturing techniques in controlling the shape of the fabricated photosynthetic material. As an initial proof-of-concept, a
synthetic riboswitch is used to regulate the expression of a yellow fluorescent protein reporter in _Synechococcus elongatus_ PCC 7942 within a hydrogel matrix. Subsequently, a strain of _S.
elongatus_ is engineered to produce an oxidative laccase enzyme; when printed within a hydrogel matrix the responsive biomaterial can decolorize a common textile dye pollutant, indigo
carmine, potentially serving as a tool in environmental bioremediation. Finally, cells are engineered for inducible cell death to eliminate their presence once their activity is no longer
required, which is an important function for biocontainment and minimizing environmental impact. By integrating genetically engineered stimuli-responsive cyanobacteria in volumetric
3D-printed designs, we demonstrate programmable photosynthetic biocomposite materials capable of producing functional outputs including, but not limited to, bioremediation. SIMILAR CONTENT
BEING VIEWED BY OTHERS 3D-PRINTED HIERARCHICAL PILLAR ARRAY ELECTRODES FOR HIGH-PERFORMANCE SEMI-ARTIFICIAL PHOTOSYNTHESIS Article 07 March 2022 ALGAL CELL BIONICS AS A STEP TOWARDS
PHOTOSYNTHESIS-INDEPENDENT HYDROGEN PRODUCTION Article Open access 04 April 2023 TRANSCRIPTIONAL REGULATION OF LIVING MATERIALS VIA EXTRACELLULAR ELECTRON TRANSFER Article 23 May 2024
INTRODUCTION Synthetic stimuli-responsive polymeric materials have been fabricated to sense and respond to different environmental conditions such as chemicals, pH, light, and temperature.
Such polymers have been utilized for applications including therapeutics1, drug delivery2, biomedical devices3, biosensors4,5, electronics6,7, and soft robotics8. However, in most cases, the
ability of these materials to respond with high specificity and produce the desired output is limited by the nature of the available stimuli and is restricted by the specifics of a
chemically driven response. The development of purely synthetic materials is therefore limited by the coupling of available external cues as well as the extent and nature of the material’s
response. Unlike traditional materials systems, biological systems innately respond and adapt to their surrounding environment and can generate bioproducts with a wide range of functions.
The field of synthetic biology aims to exploit these natural systems by engineering them to perform high-value user-defined functions for the production of biofuels, proteins, fertilizers,
bioplastics, and catalysts9,10,11,12,13. Recent progress in synthetic biology enables the design and manipulation of cellular signaling pathways for regulating gene expression for the
constitutive or induced (in response to an environmental stimulus) production of a desired biosynthetic output. The emerging field of engineered living materials (ELMs) aims to design
programmable materials14,15,16,17,18,19,20,21. Compared to synthetic stimuli-responsive materials, ELMs use genetically engineered biological components integrated into a composite material
to produce functional outputs in response to environmental cues. Recent ELMs have utilized a diversity of microorganisms including bacteria, yeast, fungi, and algae. Some notable examples
include the fabrication of skin patches for wound healing22, sweat-responsive biohybrid fabrics23, a biodegradable aqua plastic24, the incorporation of chloroplasts for self-repairing
hydrogels25, and photosynthetic O2 generators for enhancing mammalian cell viability26. While the potential of ELMs is vast, there are significant challenges in their development including
the necessity for genetically stable engineered strains, the formulation of materials to support the growth and viability of engineered cells over long durations of time, and programming of
cells to be stimuli-responsive at the material interface. Thus, an appropriate pairing of biological components and polymeric materials is critical to developing any stimuli-responsive ELM.
With the aim of developing innovative sustainable materials and overcoming the limitations of traditional stimuli-responsive materials, we incorporate photosynthetic cyanobacterial cells
within a polymer composite material. Cyanobacteria are appealing candidates for inclusion in ELMs due to their viability in low-cost media, with the added benefit that the carbon source for
these phototrophs, CO2, is freely available. Moreover, the abundance of genetic engineering tools available for several cyanobacterial species allows for modular systems of plasmid design
and gene expression controlled by external cues. Engineered plasmids can be self-replicating or designed for the integration of heterologous genes into the chromosome. Over the last decade,
a number of tools have been developed and used for genetic modification in cyanobacteria and applied for heterologous expression of high-value products and regulatory circuits27,28,29.
Notably, the development of various cyanobacteria-compatible promoters, ribosomal binding sites (RBSs), reporters, modular vectors, and markerless selection systems have contributed greatly
to the evolution of cyanobacterial synthetic biology30,31. Of relevance to this study is the use of a riboswitch as a tool for regulating gene expression. Riboswitches are aptameric
sequences in the mRNA that regulate gene expression in response to ligand binding and can be used to alter rates of protein production. Previous studies have demonstrated the utility of the
theophylline-responsive riboswitch-F in the cyanobacterium _Synechococcus elongatus_ PCC 7942 (_S. elongatus_), which, when supplemented with the small molecule theophylline, undergoes a
conformational change exposing the RBS and allows the mRNA to bind to a ribosomal subunit, resulting in the translation of a target protein32. In the stimuli-responsive material presented in
this study, the translation of proteins from heterologous genes of interest is regulated in the cyanobacteria-polymer composite using a chemically responsive synthetic riboswitch.
Bioremediation is an area where ELMs containing genetically modified organisms are likely to provide substantial benefit33,34. Previous work has demonstrated that cyanobacteria are capable
of producing both native and recombinant enzymes with relevance to bioremediation35,36,37. In this work, we aim to develop stimuli-responsive ELMs that decontaminate chemical pollutants by
regulating the production of a laccase enzyme capable of degradation. Furthermore, we engineer cyanobacteria to have a ‘kill switch’ and show the induction of lytic cell death upon
riboswitch activation, limiting biofouling in the case of cells leaching from the ELM. The ELM presented in this study responds to chemical stimuli with phenotypically complex outputs beyond
that which could be achieved in a fully synthetic system. RESULTS FABRICATION OF ELMS WITH _S. ELONGATUS_ AND ALGINATE HYDROGELS Several ELMs were fabricated that contain engineered strains
of _S. elongatus_ expressing heterologous proteins, and the material properties, cell viability, and biological activity of the composite materials were investigated. ELMs were constructed
to contain _S. elongatus_ strains engineered to express a variety of proteins under the regulation of a theophylline-responsive riboswitch to demonstrate stimuli-responsive photosynthetic
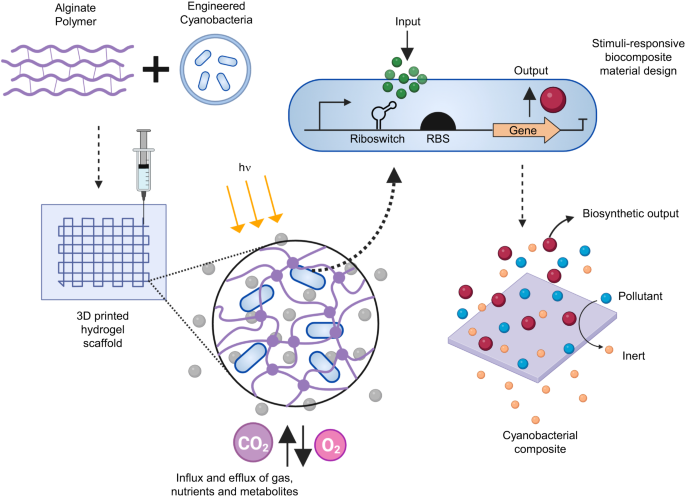
biomaterials. Figure 1 illustrates a schematic overview of the fabrication of these ELMs that combines genetically engineered cyanobacterial cells with an alginate polymer to create a
3D-printed hydrogel composite material; the gene of interest in the cells is activated by the presence of an inducer molecule (input) producing a specific protein of interest (output). 3D
PRINTING OF _S. ELONGATUS_ CELLS To create complex geometric cellular scaffolds, we fabricated cell-laden polymeric gels using direct-ink-writing (DIW) additive manufacturing techniques.
Along with the transparency and porosity of the hydrogel material, another critical advantage of using a gel-based ink is the ability to protect the cells from mechanical shear stress during
the extrusion process. This strategy minimizes damage to cell membranes, enhancing cell viability in the printed pattern. Identifying a biocompatible and printable polymer is critical when
encapsulating microorganisms in any 3D-printed matrix. Initial optimization experiments were conducted to identify a suitable material for encapsulating cyanobacterial cells that would
sustain the growth and viability of the cells at ambient temperatures and light levels. Several natural and synthetic polymers capable of forming hydrogels were tested and our chosen
candidate was an alginate hydrogel. The transparency of the hydrogel allows for light penetration, while the presence of relatively large pores within the matrix facilitates diffusion of gas
and nutrients38; these properties and a capacity for water retention make alginate an excellent candidate for photosynthetic ELMs. Alginate is a natural polysaccharide from seaweed composed
of β-D-mannuronic acid and α-L-guluronic acid that is physically cross-linked using divalent cations39. The optimal condition for alginate to be cross-linked and printable at room
temperature was determined to be a mixture of 4% w/v alginate solution with a 50 mM CaSO4 slurry suspension in a 2:1 ratio, resulting in a final 2.67% w/v alginate solution in 16.67 mM
CaSO4. A crucial step in DIW printing is obtaining a material with suitable viscosity such that the printed structure can retain its shape post-printing40. This property was achieved by
adding a solution of 100 mM CaCl2 to the hydrogel scaffold post-printing for 15 min to strengthen and stabilize the gel structure. The ink formulation was stable enough to retain its shape
for various patterns, consisting of 5–17 layers of bioink (Supplementary Data 1). For hydrogels embedded with cyanobacterial cells, the printed patterns were incubated under light in BG-11
medium post-printing to promote growth. To achieve an optimal gel formulation allowing for both 3D bioprinting and viability of the biological component, we utilized the same two-stage
crosslinking strategy to prepare our gel-based bioink, producing a pattern with high structural integrity. MECHANICAL PROPERTIES OF HYDROGELS Rheological measurements were carried out to
test the mechanical properties of the 3D-printed hydrogel with and without wild-type (WT) _S. elongatus_ cells. The viscoelastic moduli with increasing oscillatory strain in both the
unloaded gel (printed alginate hydrogel without cells) and the bioink (cell-laden gel) exhibited similar storage modulus values at lower oscillatory strain (Supplementary Fig. 1a).
Additionally, we observed a shift in crossover frequencies between the unloaded gel and the bioink samples (Supplementary Fig. 1a); the viscosity of the bioink was higher than the virgin
alginate inks (Supplementary Fig. 1b). The gels demonstrated a shear-thinning behavior, a key requirement for DIW-based printing. While highly viscous inks are favorable for increasing print
resolution, too high of viscosity can result in clogging of the print needle and reduced cell viability. In this study, needle clogging was observed when the alginate concentrations were
greater than ~3% w/v, highlighting the necessity of a balance between printability, hydrogel integrity, and cell growth and viability. Furthermore, storage modulus (G′), loss modulus (G′′),
and viscosity are influenced by factors including alginate concentration, molecular weight, crosslinker concentration (at both stages of printing), degree of crosslinking, and concentration
of cells. Although we were unable to quantify the precise number of cells within the hydrogel matrix at any given time point due to experimental limitations, we observed that the presence of
cells increases the storage modulus of the hydrogel between an oscillation strain range of ~1-10%. GROWTH AND VIABILITY OF _S. ELONGATUS_ CELLS IN ALGINATE HYDROGELS Over seven days of
growth, the biomass of WT _S. elongatus_ cells within various designs of hydrogel constructs became visibly denser (as indicated by dark green coloration); however, designs that had low
surface area to volume ratios, such as a disk design (2 cm in diameter), resulted in a decrease in biomass towards the center of the hydrogel, suggesting a limitation of gas and/or nutrient
exchange (Fig. 2a). For this reason, subsequent experiments were conducted using hydrogels printed in a 29 × 29 mm grid configuration to achieve a higher surface area to volume ratio to
enhance mass transport. While an enhanced surface area to volume ratio was targeted in the study, the thickness, as well as density of printed layers, will also likely affect the growth
behavior of cyanobacterial ELMs. The grid-patterned hydrogel resulted in a greater density of biomass per unit volume hydrogel, highlighting the utility of additive manufacturing to impact
geometry, and hence, the viability of the ELMs. Field emission scanning electron microscopy (FESEM) images of an unloaded gel illustrate the porous nature of the hydrogel scaffold ((Fig. 2b
(i & ii) and Supplementary Fig. 2a, b), with apparent pore sizes ranging from less than 40 μm to over 60 μm. Micrographs of hydrogels printed with bioink illustrate a combination of
individual cells and colonies of cells adhered to the hydrogel surface ((Fig. 2b (iii & iv) and Supplementary Fig. 2c–e). Enlarged FESEM images are provided in Supplementary Fig. 2.
Given an average _S. elongatus_ cell size of approximately 2 μm, and that the pore sizes observed in the biocomposite hydrogel often exceeded 60 μm, the pore diameter is likely not a
limitation to the growth of cyanobacteria within the matrix. To visualize the proportion of viable to dead cells within the bioprinted matrix, the resultant hydrogels were imaged using
confocal microscopy for chlorophyll autofluorescence and cell death using SYTOX Blue, a blue nucleic acid stain that penetrates cells with damaged cell membranes (Fig. 2c and Supplementary
Figs. 3–5). Red fluorescent patches, indicative of healthy colonies of cyanobacteria, were visible throughout the hydrogel over seven days of growth, whereas blue patches indicative of dead
cells were distributed heterogeneously in the hydrogel. Additional brightfield and superimposed images from confocal data of a hydrogel containing WT _S. elongatus_ cells from 0 days and 7
days post-printing are available in Supplementary Fig. 5. An assessment of cell viability from confocal image analyses of a hydrogel containing WT _S. elongatus_ shows 94.9 ± 2.2% and 76.8 ±
8.2% of total cells to be viable on Day 0 and Day 7 after printing, respectively (Supplementary Fig. 6). A representative 3D reconstruction of confocal z-stacked imaging data for the
hydrogel containing WT _S. elongatus_ cells shows the presence of both live and dead cells in a 3D space (Supplementary Fig. 7 and Supplementary Movie 1). PHOTOSYNTHETIC ACTIVITY OF LIVING
HYDROGELS To assess the photosynthetic activity of the living hydrogels, the O2 microenvironment and O2 turnover were measured in light and darkness using O2 microsensors. O2 evolution was
detected from an antibiotic-resistant _S. elongatus_ strain WT(SpRSmRGmR), which is used as a control for engineered strains described in later sections (Supplementary Data 4), embedded in
hydrogels (Supplementary Fig. 8a). O2 microsensors revealed an actively photosynthesizing and hyperoxic microenvironment on the surface of the living hydrogels (Supplementary Fig. 8b). For
the hydrogel containing WT(SpRSmRGmR) cells, mean O2 concentration adjacent to the hydrogel surface was 346 ± 4.9 μM SE; (Tukey post hoc _p_ = < 0.01) at an incident irradiance of 80 µmol
photons m−2 s−1. Net photosynthesis (Fig. 2d) observed for the WT(SpRSmRGmR) strain was 0.031 nmol O2 cm−2 s−1 (Tukey post hoc, _p_ < 0.01). No oxygen evolution was detected from the
unloaded hydrogel; however, slight respiratory activity was detected indicative of the presence of low levels of opportunistic bacteria. During darkness hydrogels encapsulating the
WT(SpRSmRGmR) strain showed limited respiratory activity and O2 concentrations were close to ambient seawater values (Supplementary Fig. 8e). Measurements of variable chlorophyll fluorimetry
were performed to determine the photosynthetic efficiency of the living hydrogels over a range of irradiance regimes. For the WT(SpRSmRGmR) _S. elongatus_ strain, photosynthesis was
saturated between 200 and 250 µmol photons m−2 s−1 with an irradiance at onset of saturation (_E_k = _P_max/_α_) of ~85 µmol photons m−2 s−1, indicative of low-light adaptation
(Supplementary Fig. 8d). Electron transport above 250 µmol photons m−2 s−1 was fully inhibited (Supplementary Fig. 8f). While hydrogels containing _S. elongatus_ could grow from low initial
cell densities under low to moderate light, growth was arrested in samples grown under irradiances greater than 300 μmol photon m−2 s−1. Photosynthesis vs irradiance curves support
saturation of photosynthesis at similar irradiance levels for these low-light adapted hydrogels (Supplementary Fig. 8d). Because solar radiation levels on the earth’s surface can reach
approximately 2000 μmol photons m−2 s−1, freshly printed biocomposites will likely suffer in natural high-light environments. However, aquatic environments such as lakes and rivers often
contain large concentrations of colored dissolved organic matter that rapidly attenuate light with depth41, providing a hospitable environment for the ELMs presented here. REGULATION OF YFP
EXPRESSION IN HYDROGELS To investigate whether cells grown within the hydrogel could respond to an external chemical stimulus, _S. elongatus_ was transformed with plasmids pAM4909, pAM5027,
and pAM5057 to yield strains YFP+ (constitutive expression YFP positive control), YFP− (negative control), and RiboF-YFP+ (riboswitch-regulated YFP expression), respectively (Supplementary
Data 2, 4). A schematic of the RiboF-YFP+ genetic circuit is illustrated in Fig. 3a; the engineered _S. elongatus_ strain responds to the chemical inducer theophylline from the surrounding
environment by a conformational change in mRNA bearing Riboswitch-F, which regulates translation of the YFP reporter protein. The YFP+ construct contains a constitutive _con_II promoter
driving the expression of YFP. After treatment with 1 mM theophylline in dimethyl sulfoxide (DMSO) or a 1% DMSO vehicle control for 24 hours, YFP expression was determined qualitatively
within the hydrogel using fluorescence microscopy (Fig. 3b). A larger version of Fig. 3b is available as Supplementary Fig. 9a–c. No YFP fluorescence was observed from hydrogels containing
the YFP− strain. Constitutive YFP fluorescence was observed from hydrogels containing the YFP+ strain in a medium containing either 1 mM theophylline or 1% DMSO. For hydrogels containing the
RiboF-YFP+ strain, growth in 1 mM theophylline produced similar fluorescence levels to hydrogels containing the YFP+ control strain, while no YFP fluorescence was detected in hydrogels
supplemented with 1% DMSO. These data demonstrate that the cells embedded within the hydrogel can respond to an external chemical stimulus and be engineered for tight regulation of
expression. Experiments conducted with WT strains did not yield any YFP fluorescence from hydrogels when imaged (Supplementary Fig. 10a). To assess whether viable engineered cells may have
leaked from the hydrogel into the surrounding medium, media from 5-day-old hydrogels containing the YFP−, YFP+, RiboF-YFP+, or WT strains were incubated with 1 mM theophylline or 1% DMSO for
24 hours and YFP fluorescence was quantified with a fluorescence plate reader. An ~16.8-fold increase in fluorescence was detected in supernatants from the RiboF-YFP+ samples induced with
theophylline compared to the uninduced sample (Fig. 3c). No significant difference was observed between induced and uninduced samples of supernatants from all other hydrogels (Fig. 3c and
Supplementary Fig. 10b). The presence of viable cells in the surrounding media may be attributed to a low level of diffusion of cells via mechanical agitation in an aqueous solution
throughout the hydrogel and at the hydrogel-medium interface, resulting in a slow release of cells. CONSTRUCTION OF CONSTITUTIVE LACCASE-EXPRESSING AND INDUCIBLE CELL DEATH STRAINS To create
living hydrogels suitable for bioremediation, a strain capable of constitutively producing a laccase enzyme was constructed with an additional feature of inducible cell death. The CotA
laccase from _Bacillus subtilis_ was chosen, as extensive work characterizing the copper-dependent enzyme has demonstrated its capacity for oxidizing synthetic dyes, and it has been
previously expressed in cyanobacteria35,42,43,44. A neutral site 2 (NS2) chromosome-integration plasmid (pAM5825) was constructed that encodes a laccase enzyme (CotA) expressed from the
constitutive _con_II promoter (Supplementary Data 2, 4), and the construct was inserted into the _S. elongatus_ chromosome. In response to an observed basal level of cell leakage from the
hydrogels over time, the system was engineered for inducible cell death as a mechanism to minimize the potential for contamination and biofouling by the ELM. The overexpression of the native
_S. elongatus_ gene _Synpcc7942_0766_ results in the excision of a prophage from the _S. elongatus_ genome, causing cellular lysis. In addition to pAM5825, a neutral site 1 (NS1)
chromosome-integration plasmid (pAM5829; Supplementary Data 2) containing a _con_II promoter–theophylline-responsive riboswitch upstream of a copy of the _S. elongatus_ gene
_Synpcc7942_0766_ was constructed and inserted into the _S. elongatus_ chromosome at NS1, creating strain Laccase+-riboF-Lysis+, and printed in hydrogels in tandem with a corresponding
control strain Laccase−-riboF-Lysis−. Figure 4a illustrates a schematic of the Laccase+-riboF-Lysis+ genetic circuit. Concentrations of O2 at the hydrogel surface and oxygen evolution and
respiration rates of the hydrogels embedded with the Laccase+-riboF-Lysis+ were similar to hydrogels embedded with the WT(SpRSmRGmR) strain (Supplementary Fig. 8a–e; see supplementary
information note for details), and genotypic characterization and expression of the 52-kDa CotA enzyme were confirmed (Supplementary Fig. 12a, b). ABTS-LACCASE ACTIVITY TEST FOR 3D-PRINTED
LACCASE+-RIBOF-LYSIS+ AND CONTROL LACCASE−-RIBOF-LYSIS− PATTERNS Laccase activity was tested against ABTS both within the ELM hydrogel and from the surrounding solution. The hydrogels were
first separated from their surrounding BG-11 media and submerged in a reaction buffer mixture with 2 mM ABTS. Following 1 hour of incubation, a set of hydrogels containing
Laccase−-riboF-Lysis− and Laccase+-riboF-Lysis+ saturated with ABTS solution were removed, placed on the surface of sterile agar LB plates, and left to incubate for 4 days (Fig. 4b). Over
the 4-day period, a purple coloration developed in the Laccase+-riboF-Lysis+ hydrogel indicative of the oxidation of ABTS, while the hydrogel containing the Laccase−-riboF-Lysis− strain
remained the natural green hue produced by cyanobacterial pigments that was observed in all patterns at time 0. To quantify the laccase activity of the ELMs in the surrounding solution, a
set of hydrogels containing Laccase−-riboF-Lysis-, Laccase+-riboF-Lysis+, and unloaded gels were submerged in a buffer solution containing ABTS and incubated for 4 days with daily sampling
for quantification of the oxidation of ABTS in the reaction buffer (Fig. 4c). After 24 hours, 71.5 ± 13.6 nmol/ml of ABTS was oxidized by the Laccase+-riboF-Lysis+ hydrogel. A small but
significant level of activity against ABTS was observed in the Laccase−-riboF-Lysis− relative to the control unloaded gel. Over the course of 4 days of incubation, the level of oxidized ABTS
from the hydrogel embedded with Laccase+-riboF-Lysis+ increased to 172.0 ± 21.3 nmol/mL, which was significantly greater than either the Laccase−-riboF-Lysis--containing or unloaded gels
(_P_ value < 0.01). The oxidation of the ABTS substrate to its cationic radical form was also evident from the dark green coloration observed in the hydrogels bearing strain
Laccase+-riboF-Lysis+ over time (Supplementary Fig. 13). To assess whether the CotA enzyme escapes from the hydrogel, either by means of secretion or cell lysis within the gel, the laccase
activity of the supernatant of media surrounding hydrogels was measured from samples taken five days post-printing (Supplementary Fig. 14). The activity observed from the supernatant
surrounding the Laccase+-riboF-Lysis+ containing hydrogels was significantly greater than either the Laccase−-riboF-Lysis− containing or unloaded gels. However, a low level of ABTS oxidation
was observed in supernatants from hydrogels embedded with Laccase−-riboF-Lysis− relative to unloaded hydrogels at each timepoint, indicating that the secretion or lysis products of the
control Laccase−-riboF-Lysis− strain also confer some level of oxidative activity against the substrate ABTS, albeit a reduced level relative to the hydrogels embedded with
Laccase+-riboF-Lysis+. Additionally, a strain in which the expression of laccase is regulated by Riboswitch-F (RiboF-Laccase+) was constructed, printed within hydrogels, and tested for
laccase activity with and without the addition of theophylline (see Supplementary Fig. 11 and Supplementary Note). INDIGO CARMINE OXIDATION ACTIVITY FOR 3D-PRINTED LACCASE+-RIBOF-LYSIS+ AND
CONTROL LACCASE−-RIBOF-LYSIS− PATTERNS To assess whether hydrogels containing the engineered strains of Laccase+-riboF-Lysis+ can serve as a tool for bioremediation, the hydrogels were
assayed for their ability to decolorize a common textile dye pollutant, indigo carmine, a capability previously demonstrated for a heterologously-expressed laccase35. Five-day-old hydrogels
containing either Laccase+-riboF-Lysis+, Laccase−-riboF-Lysis-, or an unloaded-control were submerged in a BG-11 solution containing 0.1 mg/ml of indigo carmine. The decolorization of indigo
carmine was subsequently monitored over the course of 10 days and quantified by proxy of absorption at 612 nm, the absorption maximum of indigo carmine in the visible spectrum (Fig. 4d, e).
The hydrogel samples embedded with Laccase+-riboF-Lysis+ had an average decolorization of 72.8 ± 6.8%, while the Laccase−-riboF-Lysis− and unloaded hydrogel had decolorization averages of
3.8 ± 1.0% and 18.1 ± 2.0%, respectively. The percent dye decolorization in hydrogels containing Laccase−-riboF-Lysis− and unloaded hydrogels is due to the adsorption of indigo carmine by
the alginate polymer matrix, as has been previously reported for other substrates45,46, and is observable as the blue hue of unloaded hydrogels removed from the solution (Supplementary Fig.
15). The hydrogel embedded with Laccase+-riboF-Lysis+ successfully decolorized ~70% of the indigo carmine over the course of 10 days with the added benefit of being physically removable from
the system without requiring energy-intensive steps to clarify a liquid culture, such as centrifugation. The unloaded hydrogel also produced a significant level of apparent indigo carmine
decolorization due to the absorption of indigo carmine by the hydrogel matrix itself. Interestingly, the unloaded hydrogel consistently had a greater level of decolorization due to
non-specific adsorption of the dye molecule than did hydrogels containing the Laccase−-riboF-Lysis− control strain. INDUCIBLE CELL DEATH OF CYANOBACTERIA When designing ELMs, consideration
of the unintended consequences of contamination from the biological component into the surrounding environment should be taken into consideration. The _Synpcc7942_0766_ gene, when
overexpressed, results in the excision of a defective prophage from the _S. elongatus_ genome47, resulting in cellular lysis (Fig. 5a). Hydrogels containing Laccase+-riboF-Lysis+ or
Laccase−-riboF-Lysis− (control) strains were transferred to fresh medium after 5 days of initial growth and incubated for an additional 12 days in fresh growth media supplemented with either
1 mM theophylline in DMSO, 1% DMSO, or medium only (Fig. 5b). After 12 days of growth, visual inspection indicated that accumulation of biomass in the surrounding medium was absent in the
flask containing the Laccase+-riboF-Lysis+ strain supplemented with 1 mM theophylline. The timescale of response to induction of cell death in the surrounding medium was assessed by
supplementing fresh hydrogel supernatants containing cells of either Laccase+-riboF-Lysis+ or Laccase−-riboF-Lysis− strains with either 1 mM theophylline or 1% DMSO and monitoring growth of
the cultures for four days (Fig. 5c). Within 48 hours, ectopic induction of Synpcc7942_0766 in the Laccase+-riboF-Lysis+ strain supplemented with 1 mM theophylline resulted in a decrease in
OD750, initially visible as clumps of senescent cell matter that continued to break down to produce a pale hazy medium, indicating death of the culture (Fig. 5c, d). Cultures of
Laccase+-riboF-Lysis+ supplemented with 1% DMSO and Laccase−-riboF-Lysis− supplemented with either 1 mM theophylline or 1% DMSO continued to grow at similar rates to one another,
demonstrating the utility of engineered inducible cell death in an ELM as a mechanism of targeted reduction of biological contamination by the material. Riboswitches are increasingly being
used for applied bioengineering due to the simplicity of ligand-aptamer interactions13,48,49. The use of riboswitches to regulate protein expression within hydrogels was successful for YFP,
laccase, and the lysis-inducing protein and should function well in future biocomposites that contain alternative cyanobacterial or algal strains. We attribute the success of regulation by
riboswitches to the diffusion of the small molecule theophylline throughout the porous alginate hydrogel. DISCUSSION We optimized the composition of an alginate-based hydrogel for 3D
printing and viability of the cyanobacterium _S. elongatus_, and engineered several strains of the cyanobacterium to be stimulus-responsive and produce functional outputs while embedded
within the hydrogel. Previously, hydrogels constructed with cyanobacteria as a biological component36,50,51 have been limited to wild-type strains. In this study, we leveraged genetic
toolkits for synthetic biology to produce photosynthetic ELMs with functional outputs and tailored regulatory circuits. The use of riboswitches to regulate gene expression within the living
material yielded stimulus-responsive materials with the capacity for dye decolorization and inducible cell death to prevent cellular contamination in the environment. While the
photosynthetic ELMs designed in this study have functional outputs suitable for bioremediation, numerous improvements could make the material more appropriate for use outside of the
laboratory setting. Notably, improvements in enzymatic activity per unit volume of hydrogel will be necessary to move from the laboratory scale to large-scale applications, such as
bioremediation in lakes or water treatment plants. One strategy for the improvement of activity from the material could be the modification of protein products to be tagged for secretion
from the cell resulting in higher concentrations in solution and allowing for the production of bioproducts that can be toxic to the cells at high intracellular concentrations. Knowledge of
mechanisms and systems for the secretion of larger recombinant proteins in cyanobacteria such as _S. elongatus_ PCC 7942 will greatly benefit the output potential of cyanobacterium-based
ELMs. Given the complexity of the composite material system, we were unsuccessful in identifying the localization of the CotA protein in the ELM. There are several potential scenarios under
which catalysis could occur, including intracellular catalysis in which the substrate and/or mediator molecules cross into the cell and the product is exported or diffuses out of the cell,
extracellular catalysis in which the laccase is exported from the cell, extracellular catalysis by a stable laccase following cell lysis, or likely a combination of the above. Structurally,
factors such as the ratio of the β-D-mannuronic (M) and _α_-L-guluronic (G) acid (M/G) in the alginate, type of crosslinking (e.g., chemical or ionic), and/or the concentration of divalent
ions significantly affects the structural integrity and the degree of swelling of the hydrogel network. The presence of the divalent ions in the surrounding environment plays a crucial role
in the crosslinking process and may result in the weakening of the gel if Ca2+ ions are substituted52,53. Careful consideration should be paid to the influence of materials containing
biological components on the natural environment, such as biofouling, and subsequent effects on ecological systems. The ELMs presented here address this issue by incorporating a mechanism
for inducible cell death by the addition of the small molecule theophylline. While theophylline is a natural product present in small quantities in tea and cocoa, it is not native to aquatic
systems and its use in the natural environment could pose a risk of unintended chemo-ecological consequences. Alternative regulatory circuits with more compatible inductive signals could be
engineered for use in the natural environment. For example, spectrally responsive photoreceptors or temperature-sensitive signaling transduction pathways could allow for diurnal or seasonal
cell death of biological contamination. The ELM presented here serves as a proof-of-concept for the engineering and incorporation of cyanobacteria into a polymeric matrix to produce
stimuli-responsive materials with functional outputs of real-world utility. Utilizing cyanobacteria as the biological component, these ELMs fundamentally depend on only light, CO2 as a
carbon source, and minimal nutrients for survival. While the biocomposites demonstrated here have been designed for use in bioremediation, the functional outputs of cyanobacterial ELMs could
be engineered to be broadly impactful. METHODS CULTIVATION OF CYANOBACTERIA Wild-type control and genetically engineered _S. elongatus_ PCC 7942 cyanobacterial cells were cultivated in
BG-11 medium54 in conical flasks and grown at 30 °C while shaking at 120 rpm under continuous illumination of 75 μmol photons m−2 s−1. Irradiance measurements were conducted with a 4π
quantum light meter (model QSL-100, Biospherical Instruments). BG-11 was supplemented with 2 μg/ml spectinomycin, 2 μg/ml streptomycin, and/or 2 μg/ml gentamycin for selection as required.
Unless otherwise noted, cells were grown for 5–6 days from an initial OD750 of 0.05 to the mid-log phase (OD750 = 0.5–0.6) prior to the preparation of bioinks. PREPARATION OF ALGINATE INK
AND BIOINK Alginate hydrogels were prepared using a mixture of sodium alginate with CaSO4 and CaCl2 as crosslinking agents at optimized concentrations. In brief, a 4% w/v sodium alginate
solution was prepared by dissolving sodium alginate powder (Sigma-Aldrich) in autoclaved deionized water. The solution was continuously stirred overnight at room temperature, achieving a
clear, viscous solution. Stock solutions of CaSO4 and CaCl2 were prepared in autoclaved deionized water at concentrations of 50 mM and 100 mM, respectively. All solutions were stored at 4 °C
until use. A hydrogel ink solution was prepared for printing by mixing the alginate solution and CaSO4 in a 2:1 ratio. The slurry suspension of 50 mM CaSO4 was mixed thoroughly (25–30
times) with alginate solution using an in-house fabricated 3-way syringe mixer and then transferred to a 3 cc syringe for 3D printing. The ink in the 3 cc syringe was placed within a 50 mL
falcon tube and centrifuged at 2200 × _g_ for 10 min to remove any air bubbles prior to printing. Bioinks were prepared with cells from cultures at an OD750 of 0.5–0.6. The cells were
collected by centrifugation at 3500 × _g_ for 5 min. The pellet was mixed in the plotting paste using the 3-way syringe mixer (Supplementary Fig. 16). All glassware used in the process was
pre-sterilized by autoclaving and all accessories required for cell mixing and 3D printing were pre-sterilized by UV sterilization. Both the ink and the bioink were prepared fresh prior to
each experiment. 3D PRINTING OF ALGINATE INK AND BIOINK CaSO4 was used to partially crosslink the alginate chains to make the gel suitable for direct-ink-writing (DIW) printing. Various
combinations of alginate solutions (0.5–5% w/v) and CaSO4 (25-100 mM) concentrations were tested at different pressures (50–350 kPa) using steel blunt end syringe needles (22, 27, and
32-gauge sizes) to optimize conditions for printability. The homogeneous mixture that gave the most stable structure at a given needle size and pressure was used for printing the bioink. In
brief, the ink with or without cyanobacterial cells was loaded in a 3 cc printing syringe barrel, equipped with a 27-gauge needle. The ink was 3-D printed with a pre-designed geometry using
the Inkredible+ Bioprinter (Cellink, USA). The printing parameters were as follows: pressure ~200 kPa, infill density 50%, fill pattern rectilinear, and speed 80 mm/sec. All prints were
produced in ambient conditions at room temperature. The printed geometries were designed using CAD software and converted to Gcode using the Cellink Heartware v2.4.1. software. Ink without
_S. elongatus_ cells was used as a cell-free control hydrogel in all experiments. Post printing, the multilayered 3D-printed structures were immersed in 100 mM CaCl2 for 15 min to allow the
patterns to be fully cross-linked and then incubated in 47 mm petri dishes containing 5 ml BG-11 medium supplemented with antibiotics. Unless otherwise mentioned, all the scaffolds with
either WT or engineered strains were kept in an incubator at an irradiance level of 20 μmol photons m−2 s−1 and 28 °C for initial growth prior to experimentation. RHEOLOGICAL
CHARACTERIZATION AND VISCOSITY MEASUREMENTS Viscoelastic moduli and viscosity measurements of the alginate ink with and without the inclusion of WT cells were carried out using a Discovery
HR-30 Hybrid Rheometer (TA Instruments). Data was acquired using the TRIOS Software. Both measurements were conducted using a 20 mm parallel plate geometry in a Peltier plate setup. For
amplitude sweep measurements, angular frequency was 10 rad/sec and strain ranged from 0.01% to 1000.0%. For viscosity, flow sweep was measured at a continuous shear rate of 0.01 s−1 to
1000.0 s−1. All characterizations were conducted at a temperature of 25 °C. GROWTH OPTIMIZATION OF THE 3D-PRINTED WT _S. ELONGATUS_ The growth of WT _S. elongatus_ cells was monitored and
optimized in different geometries of 3D-printed patterns over time. Specifically, cells encapsulated in three different printed structures (disk, honeycomb, and grid_A) were incubated in
BG-11 medium with constant illumination for 7 days and were visually monitored for color density over time. Images were taken at regular time intervals. CELL VIABILITY OF 3D-PRINTED WT _S.
ELONGATUS_ Cell viability of WT _S. elongatus_ within the 3D-printed patterns was assessed using confocal microscopy. Live/dead cell assays were performed by imaging chlorophyll
autofluorescence and SYTOX Blue (SB; Thermo Fisher Scientific) stain fluorescence. 3D-printed grid_A patterns were cut to yield two interconnected hollow squares under sterile conditions at
regular time intervals and incubated in 5 μL of a 1 mM SB stock solution in 1 mL of BG-11 medium for 5 min in the dark. The effective concentration of the stain was 5 μM. After incubation,
the samples were washed three times with BG-11, and images were recorded and analyzed on a Leica Sp8 confocal microscope with lightning deconvolution and white light laser, using the LAS X
software. The channel for SB detection was set at excitation 405 nm, and the emission was collected at 450–510 nm. The channel for chlorophyll autofluorescence was set at excitation 488 nm
and the emission was collected at 685–720 nm. Samples from different time points were imaged to monitor cell viability. Independent regions of the hydrogel were used for imaging of each
timepoint to minimize contamination. For cell viability analysis three confocal images from each timepoint from independent samples (_n_ = 3) were taken. Raw data were processed in image
processing software Fiji55 using the JACoP56 colocalization plugin. The fraction of overlapping channel intensities was calculated using Mander’s Coefficients (using a threshold value of 28
for each channel). The live/dead cell percentage was calculated based on the obtained value. Z-stack image acquisition was performed for the construction of 3D images. A total of 69 confocal
images were taken from the hydrogel containing WT _S. elongatus_ with _x_ and _y_ axes physical lengths of 289.21 µm and a _z_ axis physical length of 51.69 µm. 3D images were reconstructed
in Imaris Viewer v9.9.0. FIELD EMISSION SCANNING ELECTRON MICROSCOPY Electron microscopy images of multiple regions of freeze-dried hydrogel samples, printed with and without WT cells, were
taken on an FEI Apreo FESEM (Thermo Scientific). Sample subsections were cut and stored at −80 °C for 24 hours. The samples were subsequently lyophilized to remove residual water, and the
dried samples were prepared for imaging. The samples were adhered to SEM sample stubs using double-sided carbon tape and were coated uniformly with gold particles for 60 msec. Images were
taken with an ETD detector at 10-20 kV, using the xT microscope control v13.9.1. O2 MICROSENSOR MEASUREMENTS Clark-type O2 microsensors (tip size 25–50 µm, Unisense, Aarhus, Denmark) were
used to measure the O2 microenvironment and O2 turnover of the living hydrogels in light and in darkness. Microsensor measurements were performed as described previously57,58. Briefly, O2
sensors were mounted on a motorized micromanipulator (MU1, PyroScience GmbH, Germany) that was attached to an optical table. Cell-loaded and unloaded hydrogels were placed in a custom-made
acrylic system that provided slow laminar flow at a rate of approximately 1 cm/s. O2 microsensor measurements were performed at the cross-section between the printed horizontal and vertical
areas. Preliminary O2 mapping suggested that these are the areas of highest photosynthetic activity. Microsensor profiles were generated by carefully positioning the sensor at the surface of
the hydrogel with the aid of a digital microscope (Dino-Lite, US) and O2 values were logged using Unisense software (Sensor Trace Suite (Logger v3.3)). O2 profiles were measured from the
hydrogel surface through the diffusive boundary layer into the overlying water column in steps of 50–100 µm using the motorized micromanipulator that was operated by the manufacturer’s
software (Pyroscience software (Profix v4.6)). Net photosynthesis was determined at an incident downwelling irradiance of 80 μmol photons m−2 s−1 as provided by a fiber optic halogen light
source (ACE, Schott GmbH). Dark respiration was calculated as the diffusive O2 flux in darkness. The diffusive O2 flux was calculated using Fick’s first law of diffusion as described
previously57 using a diffusion coefficient of DO2 = 2.2186 × 10−5. The index of light adaptation (i.e., the irradiance at onset of saturation) _E_k was calculated as _E_k = _P_max/_α_; where
_P_max = maximal photosynthesis rate and _α_ = the initial slope of the light curve indicating light use efficiency. Data was plotted using OriginPro v2021. YFP MONITORING BY FLUORESCENCE
MICROSCOPY FROM CELL-LADEN HYDROGEL YFP fluorescence from the cells within the hydrogel matrix was qualitatively measured by capturing images of hydrogel patterns on a Nikon Eclipse Ni
fluorescence microscope equipped with a Nikon DS Qi2 camera at 20X or 40X magnification. 3D-printed cell-laden grids were incubated in media for 5 days, removed, and fresh 3 ml media
supplemented with either 1 mM theophylline or a 1% DMSO vehicle control was added to the gel. After 24 hours, the patterns were removed from the solutions and a subsection of each gel was
cut for imaging. Brightfield, chlorophyll autofluorescence, and YFP fluorescence images were captured for each sample containing the different YFP strains. A TRITC channel was used to
monitor in vivo chlorophyll autofluorescence from cyanobacteria. Images were analyzed using the NIS-Elements Viewer v5.21.00. YFP MONITORING BY FLUORESCENCE MEASUREMENTS FROM SURROUNDING
MEDIUM To assess the presence of YFP in the surrounding medium, the 24-hour theophylline/DMSO-supplemented samples (as prepared above) were analyzed using fluorescence measurements. The
surrounding media of the hydrogel was collected and centrifuged at 4500 × _g_ for 5 min and the emission intensity of YFP from the supernatant was measured in a 96-well plate with a Tecan
Infinite M200 plate reader (TECAN). The excitation and emission wavelengths were set for YFP to excitation 490/9 nm and emission 535/20 nm. Measurements were taken from hydrogels containing
3 independent clones of each YFP strain along with the WT cell-loaded sample. SDS-PAGE OF COTA Cultures of WT and Laccase+-riboF-Lysis+ were grown in liquid culture under 75 μmol photons m−2
s−1 with continuous shaking at 30 °C to an OD750 of 0.5. 10 mL of cultures were pelleted by centrifugation at 4500 × _g_ and aspirated. The pellets were then resuspended in 1 mL of cold
lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl) supplemented with 1 mM PMSF and Complete Mini Protease Inhibitor (Roche). The samples were then homogenized in a BeadBlaster 24 R (Benchmark
Scientific) at 4 °C. The crude protein was then clarified by centrifugation at 4 °C for 20 min at 18,000 × _g_, after which the crude protein concentration was determined using a Pierce
Coomassie Plus Protein Assay (Bradford), with bovine serum albumin used as a standard. After boiling the sample with 2× Laemmli Sample Buffer, 30 μg of sample was loaded into a Mini-PROTEAN®
TGX Precast Gel (Bio Rad) and run at 150 V. The gel was then incubated in InstantBlue Coomassie Protein Stain (Abcam) overnight prior to imaging. ABTS ACTIVITY ASSAYS Laccase activity of
either the hydrogel or the surrounding solution was measured using ABTS (Thermo Fisher Scientific). For assays involving the induction of RiboF-Laccase+, an ~83 mm3 subsection of a hydrogel
(two interconnected hollow squares) was added to 1 mL of BG-11 in a 2 mL Eppendorf tube and supplemented with either 1 mM theophylline (Sigma-Aldrich; 100 mM stock dissolved in 100% DMSO) or
1% DMSO as control and allowed to incubate for 3 days under 50 μmol photons m−2 s−1 prior to conducting the ABTS assays. The hydrogel sample was then transferred to a 1 mL reaction buffer
mixture of 100 mM sodium acetate buffer, 3.16 μM CuSO4, pH 5.0, and 2 mM ABTS in a 1.5 ml conical microtube and incubated in darkness at 28 °C. The oxidation of ABTS was determined by
measuring the absorbance of the reaction mixture at 420 nm59 after 24 hours. A blank reading at 420 nm of the reaction mixture without a hydrogel was subtracted from all samples. For ABTS
assays within the hydrogels with Laccase+-riboF-Lysis+ cells, an ~83 mm3 subsection of the hydrogel was submerged in 1 ml of reaction buffer mixture (as mentioned above) and incubated for 1
hour. The hydrogel ELMs were then removed from the solution and placed on sterile agar LB plates and incubated for 4 days in a dark environment at 28 °C. Photographs were taken at regular
time intervals. For laccase activity in the surrounding solution, similar subsections of the hydrogels were submerged in 1 ml of the reaction buffer (as mentioned above) containing 2 mM ABTS
and incubated for 4 days in darkness at 28 °C. The absorbance of the reaction mixture was measured at 420 nm at regular time intervals, relative to the absorbance at 0 h. A blank reading at
420 nm consisting of the reaction mixture without a hydrogel was subtracted from all samples. Additionally, for the Laccase+-riboF-Lysis+ hydrogel set, supernatant laccase activity was
measured. 1 mL of the medium surrounding a 5-day grown hydrogel ELM was centrifuged for 5 minutes at 4500 × _g_. 500 μL of the supernatant was combined with 460 μL 200 mM sodium acetate
buffer, pH 5.0, and 40 μL of a 50 mM stock of ABTS for a final concentration of 100 mM sodium acetate buffer, pH 5.0, and 2 mM ABTS. The samples were incubated in darkness at 28 °C, and
oxidation of ABTS was measured at regular time intervals, relative to the absorbance at 0 h. A blank reading at 420 nm consisting of the BG-11 in the reaction mixture was subtracted from all
supernatant samples. For all the assay measurements, 200 μL of samples were taken in a 96-well plate and measured with a Tecan Infinite M200 plate reader (TECAN). INDIGO CARMINE
DECOLORIZATION ASSAYS The decolorization of indigo carmine by hydrogels was determined by suspending an 83 mm3 subsection of a hydrogel (two interconnected hollow squares) in a 500 μL
solution of BG-11 media with 0.1 mM indigo carmine (Sigma-Aldrich). Indigo carmine concentrations were determined spectrophotometrically by generating a standard curve for absorbance at 612
nm for 200 μL samples in a 96-well plate with a Tecan Infinite M200 plate reader. The indigo carmine hydrogel mixture was incubated in darkness, and the absorbance of 200 μL samples was
measured at 612 nm at time 0, and intermittently till 10 days of incubation at 28 °C. The percentage of dye decolorization after 10 days was determined relative to absorbance at 612 nm at
time 0 for a given sample. INDUCIBLE CELL DEATH BY PHAGE-GENE LYSIS WITHIN 3D-PRINTED HYDROGEL Small grid_B hydrogel patterns with strains Laccase−-riboF-Lysis− and Laccase+-riboF-Lysis+
were printed and incubated for 5 days of growth. After 5 days, the constructs were transferred to flasks containing 5 ml of either BG-11 medium, 1 mM theophylline (prepared from a 100 mM
stock dissolved in 100% DMSO), or 1% DMSO as a control. The flasks were kept under 75 μmol photons m−2 s−1 with continuous shaking at 30 °C. Images of the experimental samples were captured
on day 0 and day 12. INDUCIBLE CELL DEATH BY PHAGE-GENE LYSIS IN LIQUID CULTURE Strains Laccase−-riboF-Lysis− and Laccase+-riboF-Lysis+ were grown in liquid culture under 75 μmol photons m−2
s−1 with continuous shaking at 30 °C to an initial OD750 of 0.2–0.3, prior to being transferred to six-well plates in 8 ml aliquots. Samples were then induced with either 1 mM theophylline
(prepared from a 100 mM stock dissolved in 100% DMSO) or 1% DMSO as a control. The OD750 of the cultures was measured daily for four days following induction. CONSTRUCTION OF PLASMIDS AND
ENGINEERED STRAINS To create plasmid pAM5823, pAM4950 was digested with SwaI (NEB) to release the _ccdB_ toxic gene, and the linearized plasmid was re-ligated with a Quick Ligase Kit (NEB)
following manufacturer instructions. Plasmid pAM5825 was created by digesting pAM4950 with SwaI and performing a Gibson Assembly (NEB) between the linearized product and a _cotA_ gBlocks
Gene Fragment synthesized by Integrated DNA Technologies (IDT) containing a PconII promoter and RBS upstream of the codon-optimized _cotA_ sequence. For Gibson Assembly, the _cotA_ gBlocks
gene fragment contained 20 base pair ends of homology with the linearized pAM4950 product and a _con_II promoter and ribosomal binding site sequence derived from pAM4909 upstream of the
_cotA_ gene. The _cotA_ sequence was derived from a reverse-translation of the protein sequence of CotA from _Bacillus subtilis_ (accession number “WP_003243170.1”) and was codon optimized
for _S. elongatus_. pAM5826 was created by digesting pAM5051 with EcoRI (NEB), and performing a Gibson Assembly between the linearized product and the PCR product of pAM5825 with primer pair
cotA_5051-F/cotA_5051-R. pAM5829 was created by digesting pAM4940 with SwaI and performing a Gibson Assembly of the linearized product and the PCR product of _S. elongatus_ gDNA and the
primer pair 0766_riboF_pAM4940-F/0766_riboF_pAM4940-R targeting the amplification of _Synpcc7942_0766_. The sequence of all plasmids constructed in this study was verified by Sanger
sequencing. Complete lists of all plasmids, their respective sequence, and primers used in this study are included in Supplementary Data 2, and 3 respectively. Plasmids pAM4909, pAM5027, and
pAM5057 were transferred into _S. elongatus_ by biparental conjugation on selective media60. Transformation of _S. elongatus_ with all other plasmids was achieved using standard
protocols61. All _S. elongatus_ transformations were performed using WT strain AMC06. _S. elongatus_ transformations resulting in strains harboring replicating plasmids were performed using
strain AMC2663 from the Golden lab collection, in which RSF1010-based plasmids can replicate. Genotyping of _S. elongatus_ strains expected to contain integration into a chromosome-neutral
site (not including YFP+, YFP−, and Ribo-YFP+ which bear self-replicating extrachromosomal plasmids) was performed using colony PCR with Q5 DNA Polymerase (NEB), using primer pair
NS1_Screen-F/NS1_Screen-R for transformations in neutral site 1 (NS1), and NS2_Screen-F/NS2_Screen-R for transformation in neutral site 2 (NS2). A complete list of strains used in this study
is included in Supplementary Data 4. STATISTICS AND REPRODUCIBILITY Data are presented as the mean ± standard deviation unless noted otherwise. _P_ values were calculated using a two-tailed
Student’s _t_ test. The definition of significance was set a priori to _p_ < 0.05. Within figures, asterisks represent: *_P_ < 0.05; **_P_ < 0.01; ***_P_ < 0.001; ****_P_ <
0.0001. No statistical method was used to predetermine sample size. Sample size of _n_ = 3 independently transformed clones for all assays was chosen according to convention and due to the
high reproducibility of results. No data were excluded from analysis. REPORTING SUMMARY Further information on research design is available in the Nature Portfolio Reporting Summary linked
to this article. DATA AVAILABILITY All data supporting this study are included in this article, Supplementary Information, Supplementary Data, and Source Data files. Source data are provided
with this paper. REFERENCES * Wei, H., Cui, J., Lin, K., Xie, J. & Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. _Bone Res._
10, 1–19 (2022). CAS Google Scholar * Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. _Nat. Mater._ 12, 991–1003 (2013). ADS CAS PubMed
Google Scholar * Jeong, B. & Gutowska, A. Lessons from nature: stimuli-responsive polymers and their biomedical applications. _Trends Biotechnol._ 20, 305–311 (2002). CAS PubMed
Google Scholar * Liu, J. Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors. _Soft Matter_ 7, 6757–6767 (2011). ADS CAS Google Scholar * Endo, T.,
Ikeda, R., Yanagida, Y. & Hatsuzawa, T. Stimuli-responsive hydrogel–silver nanoparticles composite for development of localized surface plasmon resonance-based optical biosensor. _Anal.
Chim. Acta_ 611, 205–211 (2008). CAS PubMed Google Scholar * Lee, G., Choi, Y. S., Yoon, H.-J. & Rogers, J. A. Advances in physicochemically stimuli-responsive materials for on-demand
transient electronic systems. _Matter_ 3, 1031–1052 (2020). Google Scholar * Zarek, M. et al. 3D printing of shape memory polymers for flexible electronic devices. _Adv. Mater._ 28,
4449–4454 (2016). CAS PubMed Google Scholar * Shen, Z., Chen, F., Zhu, X., Yong, K.-T. & Gu, G. Stimuli-responsive functional materials for soft robotics. _J. Mater. Chem. B_ 8,
8972–8991 (2020). CAS Google Scholar * Smanski, M. J. et al. Synthetic biology to access and expand nature’s chemical diversity. _Nat. Rev. Microbiol._ 14, 135–149 (2016). CAS PubMed
PubMed Central Google Scholar * Liu, W. & Stewart, C. N. Plant synthetic biology. _Trends Plant Sci._ 20, 309–317 (2015). CAS PubMed Google Scholar * Slomovic, S., Pardee, K. &
Collins, J. J. Synthetic biology devices for in vitro and in vivo diagnostics. _Proc. Natl Acad. Sci._ 112, 14429–14435 (2015). ADS CAS PubMed PubMed Central Google Scholar * Khalil, A.
S. & Collins, J. J. Synthetic biology: applications come of age. _Nat. Rev. Genet._ 11, 367–379 (2010). CAS PubMed PubMed Central Google Scholar * Brooks, S. M. & Alper, H. S.
Applications, challenges, and needs for employing synthetic biology beyond the lab. _Nat. Commun._ 12, 1390 (2021). ADS CAS PubMed PubMed Central Google Scholar * Tang, T.-C. et al.
Materials design by synthetic biology. _Nat. Rev. Mater._ 6, 332–350 (2021). ADS CAS Google Scholar * Rivera-Tarazona, L. K., Campbell, Z. T. & Ware, T. H. Stimuli-responsive
engineered living materials. _Soft Matter_ 17, 785–809 (2021). ADS CAS PubMed Google Scholar * Naik R.R. & Singamaneni, S. Introduction: bioinspired and biomimetic materials. _Chem.
Rev_. 117, 12581–12583 (2017). * Czapar, A. E., Tiu, B. D. B., Veliz, F. A., Pokorski, J. K. & Steinmetz, N. F. Slow-release formulation of cowpea mosaic virus for in situ vaccine
delivery to treat ovarian cancer. _Adv. Sci._ 5, 1700991 (2018). Google Scholar * Lee, P. W. et al. Biodegradable viral nanoparticle/polymer implants prepared via melt-processing. _ACS
Nano_ 11, 8777–8789 (2017). CAS PubMed PubMed Central Google Scholar * Rodrigo-Navarro, A., Sankaran, S., Dalby, M. J., del Campo, A. & Salmeron-Sanchez, M. Engineered living
biomaterials. _Nat. Rev. Mater._ 6, 1175–1190 (2021). ADS Google Scholar * Molinari, S., Tesoriero, R. F. & Ajo-Franklin, C. M. Bottom-up approaches to engineered living materials:
challenges and future directions. _Matter_ 4, 3095–3120 (2021). CAS Google Scholar * Nguyen, P. Q., Courchesne, N.-M. D., Duraj-Thatte, A., Praveschotinunt, P. & Joshi, N. S.
Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. _Adv. Mater._ 30, 1704847 (2018). Google Scholar * González, L.
M., Mukhitov, N. & Voigt, C. A. Resilient living materials built by printing bacterial spores. _Nat. Chem. Biol._ 16, 126–133 (2020). PubMed Google Scholar * Wang, W. et al.
Harnessing the hygroscopic and biofluorescent behaviors of genetically tractable microbial cells to design biohybrid wearables. _Sci. Adv._ 3, e1601984 (2017). ADS PubMed PubMed Central
Google Scholar * Duraj-Thatte, A. M. et al. Water-processable, biodegradable and coatable aquaplastic from engineered biofilms. _Nat. Chem. Biol._ 17, 732–738 (2021). CAS PubMed PubMed
Central Google Scholar * Kwak, S.-Y. et al. Polymethacrylamide and carbon composites that grow, strengthen, and self-repair using ambient carbon dioxide fixation. _Adv. Mater._ 30,
e1804037 (2018). PubMed Google Scholar * Maharjan, S. et al. Symbiotic photosynthetic oxygenation within 3d-bioprinted vascularized tissues. _Matter_ 4, 217–240 (2021). CAS PubMed Google
Scholar * Ducat, D. C., Way, J. C. & Silver, P. A. Engineering cyanobacteria to generate high-value products. _Trends Biotechnol._ 29, 95–103 (2011). CAS PubMed Google Scholar *
Oliver, N. J. et al. Cyanobacterial metabolic engineering for biofuel and chemical production. _Curr. Opin. Chem. Biol._ 35, 43–50 (2016). CAS PubMed Google Scholar * Taton, A., Ma, A.
T., Ota, M., Golden, S. S. & Golden, J. W. NOT gate genetic circuits to control gene expression in cyanobacteria. _ACS Synth. Biol._ 6, 2175–2182 (2017). CAS PubMed PubMed Central
Google Scholar * Santos-Merino, M., Singh, A. K. & Ducat, D. C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. _Front. Bioeng. Biotechnol._ 7, 33
(2019). * Till, P., Toepel, J., Bühler, B., Mach, R. L. & Mach-Aigner, A. R. Regulatory systems for gene expression control in cyanobacteria. _Appl. Microbiol. Biotechnol._ 104,
1977–1991 (2020). CAS PubMed PubMed Central Google Scholar * Ma, A. T., Schmidt, C. M. & Golden, J. W. Regulation of gene expression in diverse cyanobacterial species by using
theophylline-responsive riboswitches. _Appl. Environ. Microbiol._ 80, 6704–6713 (2014). ADS PubMed PubMed Central Google Scholar * Tay, P. K. R., Nguyen, P. Q. & Joshi, N. S. A
synthetic circuit for mercury bioremediation using self-assembling functional amyloids. _ACS Synth. Biol._ 6, 1841–1850 (2017). PubMed Google Scholar * Mohsin, M. Z. et al. Advances in
engineered _Bacillus subtilis_ biofilms and spores, and their applications in bioremediation, biocatalysis, and biomaterials. _Synth. Syst. Biotechnol._ 6, 180–191 (2021). CAS PubMed
PubMed Central Google Scholar * Liang, Y. et al. Textile dye decolorizing Synechococcus PCC7942 Engineered With CotA Laccase. _Front. Bioeng. Biotechnol._ 6, 95 (2018). * Han, S. et al.
Bioremediation of malachite green by cyanobacterium Synechococcus elongatus PCC 7942 engineered with a triphenylmethane reductase gene. _Appl. Microbiol. Biotechnol._ 104, 3193–3204 (2020).
CAS PubMed Google Scholar * Chakdar, H., Thapa, S., Srivastava, A. & Shukla, P. Genomic and proteomic insights into the heavy metal bioremediation by cyanobacteria. _J. Hazard Mater._
424, 127609 (2022). CAS PubMed Google Scholar * Augst, A. D., Kong, H. J. & Mooney, D. J. Alginate hydrogels as biomaterials. _Macromol. Biosci._ 6, 623–633 (2006). CAS PubMed
Google Scholar * Webber, R. E. & Shull, K. R. Strain dependence of the viscoelastic properties of alginate hydrogels. _Macromolecules_ 37, 6153–6160 (2004). ADS CAS Google Scholar *
Li, L., Lin, Q., Tang, M., Duncan, A. J. E. & Ke, C. Advanced polymer designs for direct-ink-write 3d printing. _Chemistry_ 25, 10768–10781 (2019). CAS PubMed Google Scholar * Thrane,
J.-E., Hessen, D. O. & Andersen, T. The absorption of light in lakes: negative impact of dissolved organic carbon on primary productivity. _Ecosystems_ 17, 1040–1052 (2014). CAS Google
Scholar * Durão, P. et al. Copper incorporation into recombinant CotA laccase from _Bacillus subtilis_: characterization of fully copper loaded enzymes. _J. Biol. Inorg. Chem._ 13, 183–193
(2008). PubMed Google Scholar * Hullo, M.-F., Moszer, I., Danchin, A. & Martin-Verstraete, I. CotA of _Bacillus subtilis_ is a copper-dependent laccase. _J. Bacteriol._ 183, 426–5430
(2001). * Martins, L. O. et al. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the _Bacillus subtilis_ endospore
coat. _J. Biol. Chem._ 277, 18849–18859 (2002). CAS PubMed Google Scholar * Chan, E.-S., Yim, Z.-H., Phan, S.-H., Mansa, R. F. & Ravindra, P. Encapsulation of herbal aqueous extract
through absorption with ca-alginate hydrogel beads. _Food Bioprod. Process._ 88, 195–201 (2010). CAS Google Scholar * Zhang, M. & Zhao, X. Alginate hydrogel dressings for advanced
wound management. _Int. J. Biol. Macromol._ 162, 1414–1428 (2020). CAS PubMed Google Scholar * Adomako, M. et al. Comparative Genomics of Synechococcus elongatus explains the phenotypic
diversity of the strains. _mBio_ 13, e0086222 (2022). * Weigand, J. E. & Suess, B. Aptamers and riboswitches: perspectives in biotechnology. _Appl. Microbiol. Biotechnol._ 85, 229–236
(2009). CAS PubMed Google Scholar * Verhounig, A., Karcher, D. & Bock, R. Inducible gene expression from the plastid genome by a synthetic riboswitch. _Proc. Natl Acad. Sci._ 107,
6204–6209 (2010). ADS CAS PubMed PubMed Central Google Scholar * Sawa, M. et al. Electricity generation from digitally printed cyanobacteria. _Nat. Commun._ 8, 1327 (2017). ADS PubMed
PubMed Central Google Scholar * Joshi, S., Cook, E. & Mannoor, M. S. Bacterial Nanobionics via 3D Printing. _Nano Lett._ 18, 7448–7456 (2018). ADS CAS PubMed Google Scholar *
Bialik-Wąs, K., Królicka, E. & Malina, D. Impact of the type of crosslinking agents on the properties of modified sodium alginate/poly(vinyl Alcohol) hydrogels. _Molecules_ 26, 2381
(2021). PubMed PubMed Central Google Scholar * Costa, M. J. et al. Physicochemical properties of alginate-based films: effect of ionic crosslinking and mannuronic and guluronic acid
ratio. _Food Hydrocoll._ 81, 442–448 (2018). CAS Google Scholar * Allen, M. M. Simple conditions for growth of unicellular blue-green algae on plates1, 2. _J. Phycol._ 4, 1–4 (1968). CAS
PubMed Google Scholar * Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). CAS PubMed Google Scholar * Bolte, S. &
Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. _J. Microsc._ 224, 213–232 (2006). MathSciNet CAS PubMed Google Scholar * Wangpraseurt, D.
et al. Bionic 3D printed corals. _Nat. Commun._ 11, 1748 (2020). ADS CAS PubMed PubMed Central Google Scholar * Wangpraseurt, D. et al. Bioprinted living coral microenvironments
mimicking coral‐algal symbiosis. _Adv. Funct. Mater._ 32, 2202273 (2022). * Childs, R. E. & Bardsley, W. G. The steady-state kinetics of peroxidase with
2,2’-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. _Biochem. J._ 145, 93–103 (1975). CAS PubMed PubMed Central Google Scholar * Elhai, J., Vepritskiy, A., Muro-Pastor,
A. M., Flores, E. & Wolk, C. P. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. _J. Bacteriol._ 179, 1998–2005 (1997). CAS
PubMed PubMed Central Google Scholar * Clerico, E. M., Ditty, J. L. & Golden, S. S. Specialized techniques for site-directed mutagenesis in cyanobacteria. _Methods Mol. Biol._ 362,
155–171 (2007). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Jack Reddan for assistance with the construction of pAM5825, Marie Adomako for the construction of
pAM5610, Bryan Bishé and Arnaud Taton for assistance with the conjugation of strains YFP+, YFP−, and RiboF-YFP+, Ryan Simkovsky for consultation regarding the design of strain
Laccase+-riboF-Lysis+, Nathan Soulier for assistance with the genotyping of strains RiboF-YFP+ and Laccase+-riboF-Lysis+, and David Wirth for assistance with the creation of the 3-way
syringe mixer. This work was primarily sponsored by the UC San Diego Materials Research Science and Engineering Center (UCSD MRSEC), supported by the National Science Foundation Grant
DMR-2011924. The authors acknowledge the use of facilities and instrumentation supported by the National Science Foundation through the UCSD MRSEC DMR-2011924, the Department of
NanoEngineering Materials Research Center (NE-MRC), the UCSD School of Medicine Microscopy core facility funded by NINDS Grant P30 NS047101, and the Gordon and Betty Moore Foundation Aquatic
Symbiosis Model Systems (Grant 9325 to D.W. and S.C.). The use of microscopy core equipment was supported by Jennifer Santini and Marcella Erb. Figs. 1 and 5a were created with
BioRender.com. AUTHOR INFORMATION Author notes * These authors contributed equally: Debika Datta, Elliot L. Weiss. AUTHORS AND AFFILIATIONS * Department of Nanoengineering, University of
California San Diego, La Jolla, CA, USA Debika Datta, Daniel Wangpraseurt, Erica Hild, Shaochen Chen & Jonathan K. Pokorski * Scripps Institution of Oceanography, University of
California San Diego, La Jolla, CA, USA Elliot L. Weiss & Daniel Wangpraseurt * Department of Molecular Biology, University of California San Diego, La Jolla, CA, USA Elliot L. Weiss,
James W. Golden & Susan S. Golden * Center for Nano-ImmunoEngineering and Institute for Materials Discovery and Design, University of California San Diego, La Jolla, CA, USA Jonathan K.
Pokorski Authors * Debika Datta View author publications You can also search for this author inPubMed Google Scholar * Elliot L. Weiss View author publications You can also search for this
author inPubMed Google Scholar * Daniel Wangpraseurt View author publications You can also search for this author inPubMed Google Scholar * Erica Hild View author publications You can also
search for this author inPubMed Google Scholar * Shaochen Chen View author publications You can also search for this author inPubMed Google Scholar * James W. Golden View author publications
You can also search for this author inPubMed Google Scholar * Susan S. Golden View author publications You can also search for this author inPubMed Google Scholar * Jonathan K. Pokorski
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS D.D. and E.L.W. equally contributed to the writing of the manuscript and are co-first
authors. D.D. formulated and optimized the bioink, designed and performed 3D printing of samples, conducted rheology and viscosity measurements, conducted growth optimization, cell viability
measurements, electron microscopy, and the analysis of data. E.L.W. designed, optimized, and engineered _S. elongatus_ strains, performed SDS PAGE, and analyzed data. D.D. and E.L.W.
optimized and performed YFP fluorescence assay, ABTS activity assay, indigo carmine decolorization assay, and the inducible cell death assay. D.W. performed O2 microsensor measurements,
analyzed data, and assisted in writing the manuscript. E.H. contributed to the design and 3D printing of the samples. D.W., E.H., and S.C. provided feedback and comments on the manuscript.
S.S.G., J.G., and J.K.P. supervised the project and edited the manuscript. CORRESPONDING AUTHORS Correspondence to Susan S. Golden or Jonathan K. Pokorski. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Maurycy Daroch, Tzu-Chieh Tang, and the other, anonymous, reviewer(s)
for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES
DOCUMENT SUPPLEMENTARY DATA 1-4 SUPPLEMENTARY MOVIE 1 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Datta, D., Weiss, E.L., Wangpraseurt, D. _et al._ Phenotypically complex living
materials containing engineered cyanobacteria. _Nat Commun_ 14, 4742 (2023). https://doi.org/10.1038/s41467-023-40265-2 Download citation * Received: 02 March 2023 * Accepted: 20 July 2023 *
Published: 07 August 2023 * DOI: https://doi.org/10.1038/s41467-023-40265-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative