Migrating mule deer compensate en route for phenological mismatches
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Billions of animals migrate to track seasonal pulses in resources. Optimally timing migration is a key strategy, yet the ability of animals to compensate for phenological mismatches en route
is largely unknown. Using GPS movement data collected from 72 adult female deer over a 10-year duration, we study a population of mule deer (Odocoileus hemionus) in Wyoming that lack
reliable cues on their desert winter range, causing them to start migration 70 days ahead to 52 days behind the wave of spring green-up. We show that individual deer arrive at their summer
range within an average 6-day window by adjusting movement speed and stopover use. Late migrants move 2.5 times faster and spend 72% less time on stopovers than early migrants, which allows
them to catch the green wave. Our findings suggest that ungulates, and potentially other migratory species, possess cognitive abilities to recognize where they are in space and time relative
to key resources. Such behavioral capacity may allow migratory taxa to maintain foraging benefits amid rapidly changing phenology.
Each year, animals worldwide migrate across vast landscapes and seascapes to exploit seasonal resources, escape severe weather, breed, avoid predation, or derive other benefits1. To maximize
energetic gain during migration, many animals synchronize their movements with ephemeral peaks in resource quality or abundance2,3,4. Resource tracking is a key benefit of migration because
it promotes nutritional gain, survival, and reproductive success5,6. To match their movements with resource phenology, migratory animals often rely on changes in local conditions (i.e.,
proximate cues)2,3. For example, some Neotropical birds use the flowering phenology of honey mesquite (Prosopis glandulosa) to initiate their spring migrations with peaks in arthropod
abundance7. Proximate cues, however, may fail to reflect resource phenology further along a migratory route or distant seasonal range, which can cause animals to become mismatched from peaks
in resource phenology during migration2,3. When individuals lack the behavioral plasticity to alter their movements en route, migratory taxa can suffer reduced demographic performance and
population decline from phenological mismatch8,9,10.
Climate change is altering patterns of resource phenology, while the expanding human footprint can decouple animal migration from key resources11,12. Together, these rapid environmental
changes are expected to cause dramatic, and potentially detrimental, phenological mismatches for migratory taxa3,5,11. For example, birds, ungulates, ursids, fish, and cephalopods are
altering the timing of migration in response to climate change but at different rates than their primary food5,11,13. Adjusting movement en route may be the most rapid and least costly way
for many migratory taxa to behaviorally compensate for phenological mismatches, facilitating their adaptation to a rapidly changing world3,11 (but see14,15). Behavioral compensation is
likely beneficial for migratory animals that only have a short time to capitalize on fleeting resources, because without compensation, dramatic changes in weather, resource phenology, and
reliability of cues would carry substantial fitness costs3,11. Most previous studies that have explored behavioral compensation during migration, however, focus on the ability of animals to
compensate for spatial drift when crosswinds or ocean currents push animals off their migratory trajectory16. Aside from a few examples in migratory birds17,18, the ability of animals to
compensate when they drift temporally rather than spatially off course remains undocumented for most migratory taxa11,19.
Large herbivores, including ungulates, acquire high-quality forage by tracking fleeting waves of emerging plants during migration, which is known as “green wave surfing” (i.e., the Green
Wave Hypothesis20,21). By tracking the wave of green-up across a landscape, migrating ungulates can continually access new plant growth that is highly nutritious and easy to digest21.
Previous research indicates that temperate ungulates often initiate spring migration when green-up begins on winter range, allowing them to track peaks in forage quality during spring
migration22,23,24. Strong behavioral responses to plant phenology often facilitate synchronous departure from winter range among individuals22,23,25. Asynchronous migration may occur,
however, when changes in local environmental conditions are subtle and do not correspond to conditions occurring further along the migration trajectory (i.e., the resource gradient is weak,
noisy or absent at the beginning of migration)25. When conditions change and cues become unreliable, the ability of animals to resynchronize their movements with resource phenology will
likely underpin the foraging benefits of spring migration.
To study behavioral compensation for phenological mismatches en route, we took advantage of a unique system where mule deer (Odocoileus hemionus) wintering in a desert ecosystem initiated
spring migration anywhere from 1–103 days apart. From 2011–2020, we collected GPS movement data from 72 adult female mule deer (>1-yr-old) that spent winter in the Red Desert of
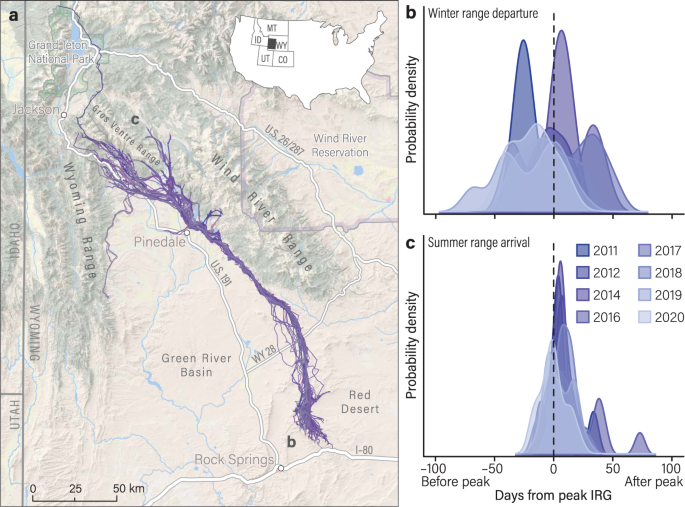
south-central Wyoming, USA and migrated long distances (134–293 km) to high-elevation summer ranges (Fig. 1a). Over the 10-year duration of the study, deer often began migration far ahead or
far behind the green wave (Figs. 2 and 3), allowing us to investigate if and how deer resynchronize their movements with peak green-up en route.
a Each spring, mule deer leave their desert winter range to make a 240-km, one-way, migration in western Wyoming. b The start date of spring migration was standardized to the mean date of
peak IRG on winter ranges for each year of the study (2011–2012, 2014, 2016–2020). The vertical dashed line represents zero days from peak IRG. c The end date of spring migration was
standardized to the mean date of peak IRG on summer ranges for each year. Mule deer started spring migration asynchronously (70 days ahead to 52 days behind peak IRG) but, on average,
arrived on summer range within a narrow window of time (6 days).
Green wave surfing en route was measured as mean days from peak Instantaneous Rate of Green-up (IRG) ± 95% confidence intervals as a function of distance from winter range (solid lines
represent loess regressions). Despite being strongly mismatched ahead or behind the green wave when they began their spring migration, early migrants (n = 47 animal-years; purple),
mid-migrants (n = 58 animal-years; green), and late migrants (n = 47 animal-years; orange) ended spring migration largely synchronized and closer to peak IRG. Early and late migrants
seemingly compensated for being mismatched with the green wave during migration. The horizontal dashed line represents perfect surfing (0 days from peak IRG).
a Despite starting spring migration 30 ± 5 days (x̄ ± 95% CI) ahead peak Instantaneous Rate of Green-up (IRG; dashed horizontal line), early migrants (n = 47 animal-years; purple) ended
their spring migration 4 ± 3 days behind peak IRG. b Mid-migrants (n = 58 animal-years; green) were 2 ± 5 days ahead peak IRG at the start of spring migration but 7 ± 3 days behind peak IRG
at the end of spring migration. c Although late migrants (n = 47 animal-years; orange) started spring migration 20 ± 5 days behind peak IRG, they ended spring migration 11 ± 4 days behind
peak IRG. The boxplots represent the median value of days from peak IRG (horizontal bar). Whiskers extend to the minima (25th percentile – 1.5 * interquartile range) and maxima (75th
percentile + 1.5 * interquartile range).
Long-distance migrants departed winter range asynchronously with some individuals starting spring migration in mid-February (2 months ahead of peak forage quality) and other individuals
starting spring migration in early June (1.5 months behind peak forage quality; Figs. 1b, 2; Supplementary Tables 1, 2). Migration distance and intrinsic factors, including age and
pregnancy, did not explain variation in the start of spring migration (although nutritional condition weakly influenced when deer departed their winter range; Supplementary Fig. 1;
Supplementary Table 3).
We tested for potential environmental cues that could influence an animal’s decision to migrate and found weak responses to plant phenology and temperature (Supplementary Discussion;
Supplementary Table 4). To quantify cue reliability across space, we examined the rate of change in green-up along the migratory route. In 5 of the 8 tracking years where analysis was
possible, there was either a negative rate of change in green-up or no correlation between the date of peak green-up and distance over the first 32 km of the migration (p = 0.08–0.92),
meaning that the green wave was nonexistent or propagated backwards over this segment of the route (Fig. 4a; Supplementary Table 5). Thus, on the desert winter range and during early
portions of the 240-km migration, spring green-up does not move as a wave, making it difficult to track even if local environmental cues were used to initiate migration. Although the
underlying mechanisms that influence the start of spring migration are unclear, mule deer departed winter ranges at markedly different times relative to peak green-up. The unique variation
among individuals in the onset of migration creates the opportunity to evaluate whether deer can perceive their own location in space and time relative to the green wave (e.g., ahead or
behind peak green-up) and behaviorally adjust their movements en route to compensate for initial phenological mismatches.
a Plotting mean date of peak Instantaneous Rate of Green-up (IRG) along the 240-km migration corridor indicates that the wave of green-up did not propagate consecutively across the landscape
for the first 32 km of migration (dotted segments indicate a negative slope of green-up) but propagated as a wave for the remainder of the migration corridor (solid segments indicate a
positive slope of green-up). b The spring of 2017 illustrates the movements of full compensators (blue dots) relative to the weekly propagation of the green wave. Mule deer that were behind
the green wave tended to move more quickly, while those ahead of the wave moved more slowly. c Mule deer migrated 134–293 km over 50 ± 4 (x̄ ± 95% CI) days from a desert sagebrush shrubland
to a montane ecosystem. d The start date of spring migration (i.e., whether deer were early or late) positively influenced the rate of movement by mule deer during spring migration
(predicted coefficients ± 95% CI; GAMM, R2 = 0.56, p