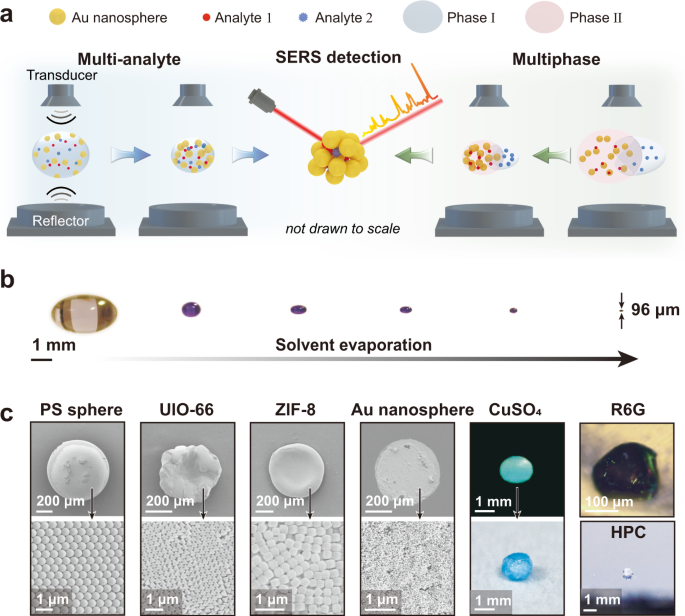
Lossless enrichment of trace analytes in levitating droplets for multiphase and multiplex detection
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Concentrating a trace amount of molecules from liquids, solid objects, or the gas phase and delivering them to a localized area are crucial for almost any trace analyte detection device.
Analytes within a liquid droplet resting on micro/nanostructured surfaces with liquid-repellent coatings can be concentrated during solvent evaporation. However, these coatings suffer from
complex manufacturing procedures, poor versatility, and limited analyte enrichment efficiency. Here, we report on the use of an acoustic levitation platform to losslessly concentrate the
analyte molecules dissolved in any volatile liquid, attached to solid objects, or spread in air. Gold nanoparticles can be simultaneously concentrated with the analytes in different phases,
realizing sensitive, surface-enhanced Raman scattering detection even at attomolar (10−18 mol/L) concentration levels. The acoustic levitation platform-enabled, lossless analyte enrichment
can significantly increase the analytical performance of many conventional microsensing techniques.
Ultrasensitive detection of trace analytes is important in a broad range of fields, ranging from analytical chemistry1 to disease diagnostics2,3,4,5, biomedicine6,7,8,9, environmental
science10,11,12, and national security13,14. In these practical application fields, a trace amount of multiple analytes is either dispersed in water or organic liquids, attached to solid
surfaces, or dispersed within a gaseous mixture3,4,5,6,7,12,13,14,15. Surface-enhanced Raman scattering (SERS) is promising in trace analyte detection because of its high sensitivity,
label-free detection, and miniaturization16,17,18,19,20,21,22,23,24,25. Various SERS substrates integrated with dense and uniformly distributed, ultrasensitive SERS sites (known as “hot
spots” and usually located at 10 times for R6G dispersed in water (Curve II in Fig. 3b). The SERS intensity ratio between R6G and MATT indicated that the R6G molecules dissolved in water had
difficulty entering the preformed Au nanoparticle aggregate in toluene (Fig. 3c). SERS spectra of R6G in water and MATT in toluene with different concentration ratios (50 nM:50 nM, 10 nM:50
nM, 5 nM:50 nM, and 1 nM:50 nM) were measured by introducing Au nanoparticles to the water phase after solvent evaporation using the DLE platform (Fig. 3d). The relationship between the
concentration ratio and the SERS intensity ratio of R6G and MATT is described by log (IR6G/IMATT) = 0.92 log (CR6G/CMATT) + 0.93 with an R2 of 0.998 (Fig. 3e).
a Introducing water into a levitating toluene droplet, or vice versa. Toluene wrapped one end of the ellipsoid water droplet. For multiphase and multiplex SERS detection, introducing Au
nanoparticles to the water phase facilitated the analytes to enter the Au nanoparticle aggregate after solvent evaporation; otherwise, the preaggregated Au nanoparticles in the organic phase
hindered the entrance of the analytes, particularly for the analytes in the water phase (refer to the schematics on the right). b SERS spectra of 50 nM MATT in toluene and 50 nM R6G in
water when Au nanoparticles were dispersed in water (Curve I) and aggregated in toluene (Curve II). c Intensity of the 1647 cm−1 SERS peak of R6G and the 1226 cm−1 SERS peak of MATT and
their intensity ratio in Curves I and II in panel (b). d SERS spectra of MATT and R6G at different concentration ratios. e Relationship between the intensity ratio of the 1647 cm−1 SERS peak
of R6G and the 1226 cm−1 SERS peak of MATT and the concentration ratio between R6G and MATT in different liquids. The error bars were obtained based on at least 10 spectra.
It is frequently required to detect analytes attached to tiny solid objects (e.g., pesticide residue and soil contamination). Multiple pretreatment processes are usually required to separate
analytes from solid objects before detection. The DLE system allowed us to directly analyze the analytes on tiny solid objects by SERS (Fig. 4). Using pesticide residue detection on a slice
of sesame seed as an example, the sesame seed slice was levitated by the acoustic levitator (Fig. 4a). Then, 10 µL of ethanol composed of Au nanospheres was dropped onto the seed slice
(Process I in Fig. 4a, b). The acoustic force helped peel off the pesticide molecules from the seed slice. As the solvent evaporated, the Au nanoparticles and pesticide molecules
simultaneously assembled onto the seed surface (Process II in Fig. 4a, b). Tetramethylthiuram disulfide (TMTD) was selected as a model pesticide because of its wide usage all over the world.
SERS signals of TMTD were observed from the sesame contaminated by TMTD at a concentration of 7.6 pg/cm2 without any pretreatment procedures using the DLE platform (Fig. 4c). Sensitive SERS
detection of the TMTD ethanol solutions at a concentration as low as 100 pM was realized using the DLE platform (Fig. 4d). We further demonstrated the application of the DLE platform in the
SERS detection of environmental pollutants and antibiotics. Decachlorobiphenyl 209 (PCB 209) is a polychlorinated biphenyl (PCB) that can cause serious health issues even at extremely low
concentrations53. It is difficult to levitate PCB 209-contamined sand grains with a diameter of 95%), and rhodamine 6G (R6G, >95%) were obtained from Macklin Chemical. Reagent Co., Ltd.
Cetyltrimethylammonium chloride (CTAC, >95%) was obtained from Tokyo Chemical Industry Co. Ltd. Ascorbic acid (AA, 99%) was purchased from Alfa Aesar. Adenine (>99%) and adenosine (>99%)
were purchased from Sinopharm Chemical Reagent Co., Ltd. 4-Methoxy-α-toluenethiol (MATT, 99%) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. All the reagents were used
as received. Milli-Q water (>18.2 MΩ·cm at 25 °C) was utilized in all experiments.
Single-crystal Au nanospheres with different sizes were synthesized by a seed-mediated growth method56. The first step is fabrication of the CTAB-capped Au clusters. HAuCl4 (0.5 mM, 5 mL)
and CTAB (200 mM, 5 mL) were ultrasonically and thoroughly mixed. Then, fresh NaBH4 (1 mM, 0.6 mL) aqueous solutions were rapidly added to the above mixture. The solution immediately turned
brown. After magnetic stirring for 2 min, the solution was kept undisturbed at 27 °C for 3 h. CTAC (0.2 M, 6 mL), AA (0.1 M, 4.5 mL) and 150 μL of CTAB-capped Au cluster were mixed in a 30
mL glass vial. HAuCl4 solutions (0.5 mM, 6 mL) were then added to the vial by one-shot injection. The mixture was stirred at 27 °C for 15 min. The prepared Au seeds were collected by
centrifugation at 28,600 × g for 20 min and washed with water one time. The Au seeds were dispersed in 3 mL of CTAC (0.02 M) for further growth. Aqueous solutions of CTAC (0.1 M, 40 mL), AA
(0.01 M, 2.2 mL), and 200 μL of Au seeds was mixed in a conical flask. Then, HAuCl4 (0.5 mM, 40 mL) aqueous solutions were introduced to the flask by a syringe pump at an injection rate of
40 mL/h. The solution was kept at 27 °C for 10 min after the injection finished. The prepared Au nanoparticles were collected by centrifugation at 9500 × g for 15 min and washed with water
one time.
A single-axis acoustic levitator consisting of an acoustic emitter and a reflector was employed (SonaRh-1, Shengdu Ltd., China). The frequency of the acoustic emitter was 20.7 kHz. The
operating power was 200 W. Two ceramic heating lamps (~100 W) were utilized to accelerate the droplet evaporation process.
During the droplet levitating process, the sound intensity was slightly controlled by adjusting the distance between the acoustic emitter and the reflector52. To levitate an ethanol droplet,
we increased the reflector-emitter distance to decrease the sound intensity. Then, an ethanol droplet hanging on a pipette tip was exposed to the acoustic field. We slowly decreased the
distance to pull the droplet to leave the pipette tip, obtaining the levitating ethanol droplet. The water droplet was unlikely to explode with suitable sound intensities, and the
reflector-emitter distance remained unchanged during the water droplet loading process.
The levitation force per unit volume of an ellipsoid droplet is larger than that on a spherical droplet with the same volume. The aspect ratio of the axes of the levitating ellipsoid droplet
should remain at ~2. If the ratio is larger than 2, the droplet tends to explode. If the ratio is smaller than 2, the droplet tends to escape from the acoustic field (drops down for large
droplets and ejects for tiny droplets). The ellipsoid droplet gradually turns quasi-spherical during evaporation. Slightly reducing the distance between the acoustic emitter and the
reflector by ~1 mm for our apparatus could squeeze the spherical droplet back to the ellipsoid shape. Air flows should be avoided for levitating very tiny droplets (e.g.,