Intracellular trafficking of notch orchestrates temporal dynamics of notch activity in the fly brain
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT While Delta non-autonomously activates Notch in neighboring cells, it autonomously inactivates Notch through _cis_-inhibition, the molecular mechanism and biological roles of which
remain elusive. The wave of differentiation in the _Drosophila_ brain, the ‘proneural wave’, is an excellent model for studying Notch signaling in vivo. Here, we show that strong
nonlinearity in _cis_-inhibition reproduces the second peak of Notch activity behind the proneural wave in silico. Based on this, we demonstrate that Delta expression induces a quick
degradation of Notch in late endosomes and the formation of the twin peaks of Notch activity in vivo. Indeed, the amount of Notch is upregulated and the twin peaks are fused forming a single
peak when the function of Delta or late endosomes is compromised. Additionally, we show that the second Notch peak behind the wavefront controls neurogenesis. Thus, intracellular
trafficking of Notch orchestrates the temporal dynamics of Notch activity and the temporal patterning of neurogenesis. SIMILAR CONTENT BEING VIEWED BY OTHERS PUCKERED AND JNK SIGNALING IN
PIONEER NEURONS COORDINATES THE MOTOR ACTIVITY OF THE _DROSOPHILA_ EMBRYO Article Open access 11 December 2023 CELL-TYPE-SPECIFIC CHROMATIN OCCUPANCY BY THE PIONEER FACTOR ZELDA DRIVES KEY
DEVELOPMENTAL TRANSITIONS IN _DROSOPHILA_ Article Open access 09 December 2021 DETERMINING ZEBRAFISH DORSAL ORGANIZER SIZE BY A NEGATIVE FEEDBACK LOOP BETWEEN CANONICAL/NON-CANONICAL WNTS
AND TLR4/NFΚB Article Open access 08 November 2023 INTRODUCTION Notch (N) signaling plays diverse roles in many biological processes1. N-mediated lateral inhibition is reiteratively used to
select a small number of differentiated cells from a large number of undifferentiated cells in a spatially and temporally regulated manner2. We previously demonstrated that N-mediated
lateral inhibition regulates the speed of proneural wave progression when combined with epidermal growth factor (EGF)-mediated reaction diffusion3,4. A membrane-bound ligand, Delta (Dl),
plays major roles in N-mediated lateral inhibition5. It non-autonomously activates N in adjacent cells through a process “_trans_-activation.” Upon binding with Dl, the intracellular domain
of N is cleaved to produce the N intracellular domain (NICD), which forms a complex with a DNA-binding transcription regulator, Suppressor of Hairless (Su(H)), and regulates target gene
transcription6,7,8. On the other hand, N is autonomously inactivated by Dl expressed in the same cell through a process “_cis_-inhibition,” whose molecular mechanism and biological
significance remain largely elusive9,10,11,12. The direct interaction between Dl and N seems to trigger _cis_-inhibition by inhibiting N prior to or following its transport to the plasma
membrane13. There are two possible mechanisms of _cis_-inhibition. First, the _cis_-interaction of the ligand and receptor may shut off the transport of N from the endoplasmic reticulum (ER)
to the plasma membrane14. Second, the _cis_-interaction may trigger the catalytic process that results in N degradation. For example, the Dl–N complex may be internalized from the plasma
membrane to cause N degradation. Protein degradation in late endosomes has been shown to play important roles in activating and inactivating N signaling during
_trans_-activation15,16,17,18,19,20,21,22,23,24. However, the potential roles of intracellular trafficking of Dl and N in _cis_-inhibition remain largely unknown. On the surface of the
developing fly brain, the wave of differentiation, “proneural wave,” propagates along the two-dimensional sheet of neuroepithelial cells (NEs), which sequentially differentiate into
neuroblasts (NBs), the neural stem-like cells. In the previous study, we formulated a mathematical model of the proneural wave, which includes N activity (_N_), Dl expression (_D_), EGF
signal activity (_E_), and the state of NB differentiation (_A_). _A_ is related to the expression levels of Achaete-Scute Complex proteins (AS-C). The model successfully reproduces the
complex behaviors of the proneural wave in various genetic backgrounds25,26. N is activated along the wavefront, forming an activity peak that negatively regulates the wave propagation27,28.
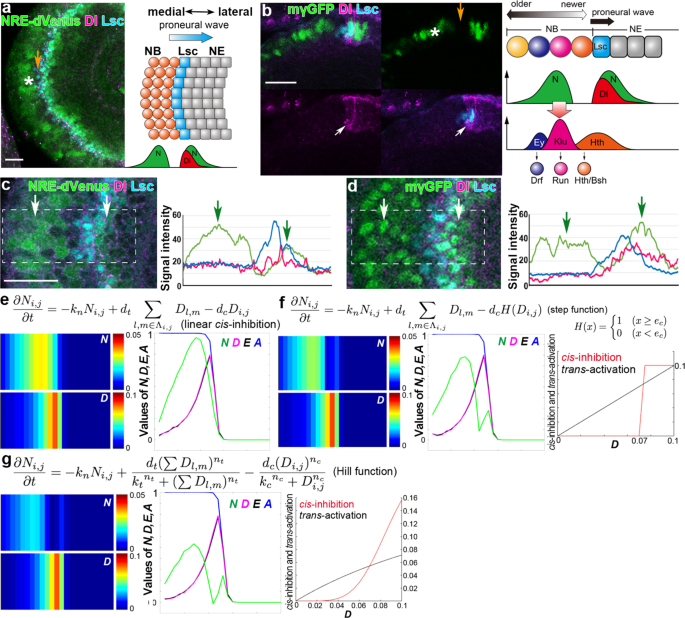
However, N is activated again behind the proneural wave, showing twin peaks of N activity in vivo3,29 (Fig. 1a–d). If the location of Dl-expressing cells does not change, the combination of
_trans_-activation and _cis_-inhibition would robustly form the twin activity peaks of Notch11. However, Dl expression propagates as the proneural wave progresses. The mechanism that forms
the twin peaks of N activity and the biological significance of the second N activity peak have not been addressed thus far. Behind the proneural wave, NBs start producing diverse types of
neurons. The production of neural diversity is controlled by the transition of temporal transcription factors sequentially expressed in NBs. Homothorax (Hth), Klumpfuss (Klu), Eyeless (Ey),
Sloppy paired (Slp), Dichaete (D), and Tailless (Tll) are known to act as the temporal transcription factors in the developing medulla (Fig. 1b)30,31. While Hth expression is already
upregulated in NEs prior to NB differentiation, the expression of the other temporal factors is upregulated behind the proneural wave. Thus, the proneural wave could be the initial trigger
of the temporal transcription factor cascade following Klu. Since the second N peak is found in NBs behind the wavefront, N signaling could trigger the transition of temporal transcription
factor expression. In this study, we reproduce the twin peaks of N activity by modifying the previous mathematical model and demonstrate that a strong nonlinearity in _cis_-inhibition
robustly reproduces the twin peaks. As a potential candidate mechanism of the nonlinear behavior of _cis_-inhibition, we assume that Dl may transport N to late endosomes, in which Rab7 and
the ESCRT (endosomal sorting complexes required for transport) complex quickly degrade N, which results in the inactivation of N between the twin peaks. Indeed, partial knockdown of _Dl_ or
inactivation of ESCRT complex causes upregulation of N activity and fusion of the twin peaks, forming a single peak of N activity. These results support the idea that intracellular
trafficking of N triggers _cis_-inhibition, which changes the dynamics of N activity. We further explore the biological significance of the twin peaks of N activity. Interestingly, the
second N activity peak coincides with Klu expression in the medulla NBs. We demonstrate that Klu expression depends on Dl expression along the proneural wavefront, and the formation of the
single N peak by inhibiting ESCRT function results in the abnormal temporal patterning of NBs and neurons. Thus, we demonstrate the molecular mechanism of _cis_-inhibition that orchestrates
the temporal dynamics of N activity and the temporal patterning of neurogenesis. RESULTS NONLINEAR _CIS_-INHIBITION ESTABLISHES THE TWIN PEAKS In our previous mathematical model, the
_cis_-inhibition term was proportional to Dl expression3. When the magnitude of _trans-_activation and _cis_-inhibition is roughly equivalent, N is activated only once around the wavefront.
However, N is activated again behind the wavefront, showing the twin activity peaks (Fig. 1a–d)3,29. We used two independent N activity markers. E(spl)mγ-GFP (mγGFP) shows nuclear signals
that are more prominent in the first peak than in the second peak (Fig. 1d)32. In contrast, NRE-dVenus shows cytoplasmic signals that are more prominent in the second peak than in the other
(Fig. 1c)33. If Dl is continuously expressed in the same cells, the combination of _trans_-activation and _cis_-inhibition would robustly form the twin peaks of Notch activity11. However,
Dl-expressing cells change as the proneural wave progresses. To generate a robust gap between the twin peaks, N must be quickly inactivated just after the first activity peak. Since Dl is
specifically expressed at the wavefront (Fig. 1b), Dl-mediated _cis_-inhibition could be the cause of the inactivation following the first peak and the formation of the twin peaks of N
activity. We asked if the twin peaks could be reproduced by modifying _cis_-inhibition in the mathematical model of the proneural wave. It was demonstrated that the kinetics of
_cis_-inhibition are very fast compared with the gradual effect of _trans-_activation from a series of in vitro experiments11. Thus, we incorporated a step function term for
_cis_-inhibition, with which _cis_-inhibition does not occur when Dl expression is below the threshold (_e_c), but quickly inactivates N when Dl expression exceeds the threshold (Fig. 1f).
The twin peaks pattern of N activity was reproduced without significantly compromising the magnitude of N activity for a wide variety of parameter settings (Fig. 1e, f and Supplementary
Figure 1). However, the step function is very artificial and nonbiological. According to the previous literature11, we incorporated the Hill functions for _trans-_activation and
_cis_-inhibition. The Hill function is commonly used to model a biochemical reaction, in which the Hill’s coefficient (_n_t and _n_c) and activation coefficient (_k_t and _k_c; Fig. 1g)
specifies the kinetics of the reaction. We systematically modified the Hill’s and activation coefficients of _cis_-inhibition to modify its response speed (Fig. 1g and Supplementary Figure
2). The twin peaks pattern of N activity, which is similar to the in vivo pattern (Fig. 1a, c, d), was reproduced when the kinetics of _cis_-inhibition is faster compared with that of
_trans-_activation. DELTA ACTIVATES NOTCH ACTIVITY BEHIND THE WAVEFRONT We asked if the mechanism demonstrated in the above in silico experiments exists in vivo. As already demonstrated, the
N signal is activated in cells adjacent to a clone of cells ectopically expressing Dl, while it is efficiently inactivated in NE cells expressing Dl (Fig. 2a)13,29. Since Dl-expressing
cells also receive _trans_-activation from surrounding Dl-expressing cells, _cis_-inhibition appears to be dominant over _trans-_activation, which is consistent with the idea that the
kinetics of _cis_-inhibition are faster compared with that of _trans-_activation. To address this idea in a loss-of-function condition, we utilized the mathematical model. If the strong
expression level of Dl at the wavefront is the trigger of _cis_-inhibition, a mild reduction in Dl production should cause the fusion of the twin peaks, forming a single N activity peak
(Fig. 2b, c). To test this idea in vivo, we made use of two different _UAS-Dl_ RNA interference (RNAi) strains. When expressed in the retina under the control of _GMR-Gal4_, the mild _Dl_
RNAi reduced the eye width from 0.37 to 0.35 mm, while it was further reduced to 0.31 mm by the strong _Dl_ RNAi (Supplementary Figure 3). We found that the mild _Dl_ RNAi under the control
of _optix-Gal4_, which is expressed in the dorsal and ventral subdomains of the optic lobe (Fig. 2d), causes the single N peak. The twin N activity peaks were fused to become a single peak
(Fig. 2e and Supplementary Figure 4a). When we removed Dl expression by expressing the strong RNAi strain, both of the twin peaks of N activity were eliminated (Fig. 2f and Supplementary
Figure 4b) and the same result was also observed in the _Dl_ mutant clone (Supplementary Figure 4c). Note that Dl is specifically expressed at the proneural wavefront cells (Fig. 1b),
suggesting that this Dl expression is responsible for the twin activation of N signaling in cells adjacent to the proneural wavefront. Thus, Dl expression at the wavefront is responsible for
generating the first and second N activity peaks. The intuitive interpretation of the above results is that the Dl expression at the wavefront activates N signaling in the adjacent NE cells
(first peak) and NBs (second peak). N activity is downregulated at the wavefront between the twin peaks of N activity (Fig. 2g). However, the first N activity peak partially overlaps with
Dl expression in vivo (Fig. 1a–d). This is most likely because the proneural wave progresses in a medial-to-lateral orientation. While N activity establishes the twin peaks, the location of
the Dl-expressing wavefront cells may change and partially overlap with the first N activity. DELTA TRIGGERS NOTCH DEGRADATION AT THE WAVEFRONT The above results suggest that the strong Dl
expression at the wavefront triggers quick inactivation of N signaling. Since the expression level of N is downregulated behind the wavefront, the expression of Dl may trigger N degradation
(Fig. 2h). If this is the case, the expression level of N should be upregulated when the function of Dl is compromised. As expected, we observed striking upregulation of the full-length N
protein, which accumulated along the plasma membrane visualized by DE-cadherin (Ecad), when _Dl_ mutant clones were generated at the wavefront (Fig. 2i: _n_ = 15/25, 2j: _n_ = 14/20). In
contrast, _N_ messenger RNA (mRNA) level was not affected in _Dl_ mutant clones (Supplementary Figure 5). These results suggest that post-translational N degradation upon Dl expression is
the basis of the nonlinear nature of _cis_-inhibition. In our mathematical model, the expression level of full-length N protein is assumed to be constant, while the term −_k_n_N_ represents
passive degradation of N signaling, which is independent of Dl expression. Considering the above experimental results, full-length N should be actively degraded through Dl-dependent
_cis_-inhibition, which is incorporated into the model as active downregulation of N signaling in a Dl-dependent manner (Fig. 1e–g). Our model allows _N_ to be negative (Supplementary
Figures 1 and 2), which might correspond to the presence of a repressor complex of Su(H) and Hairless (H)8. We only use the parameter settings with which _N_ remains nonnegative in the
following study. RAB7 AND RAB4 DIFFERENTIALLY COLOCALIZE WITH DELTA It has been reported that Dl and N expressed in the same cell form a complex14,34. Consistent observations are found
between N and Serrate (Ser), the other transmembrane ligand of N35. The formation of the Dl–N complex in _cis_ may be the cause of the rapid N degradation. It has been reported that protein
degradation in late endosomes regulates N signaling15,16,17,18,19,20,21,22,23,24. However, these studies did not focus on the process of _cis_-inhibition. In order to examine if the
degradation machinery in late endosomes is responsible for _cis_-inhibition at the proneural wavefront, we asked whether N and Dl are transported through the intracellular trafficking
pathways. Rab family small GTPases play diverse roles in intracellular trafficking. We systematically screened the Rab-EYFP library strains in which all of the endogenous Rab family genes
were tagged with EYFP36. Since N is downregulated at the wavefront, we initially compared Dl expression with Rab-EYFP distribution. We found that Rab4 and Rab7 are colocalized with Dl at the
wavefront outside the nucleus and inside the membranous Ecad signals (Fig. 3a–f). These puncta are most likely in the cytoplasm. We confirmed the results by using anti-Rab7 antibody and
found that Dl, Rab4, and Rab7 colocalize in the same puncta (Fig. 3h)37. The proximity ligation assay (PLA) also suggested that Rab7 forms a complex with Dl in vivo (Fig. 3g). Since Rab7 and
Rab4 are known to play key roles in late and recycling endosomes, respectively38,39, we hypothesized that the Dl–N complex is transported to the Rab7-positive late endosomes and that Dl is
recycled to the plasma membrane through the Rab4-positive recycling endosomes. Interestingly, we found that Rab4 signals are found inside the Dl puncta, while Rab7 mainly accumulates on the
surface of the Dl puncta at a higher magnification (Fig. 3i). Indeed, the colocalization index of Dl-Rab4 was greater compared with that of Dl-Rab7 (Fig. 3i, right panel), suggesting that
Rab4-dependent Dl recycling is more dominant than Rab7-dependent Dl degradation. The less prominent colocalization of Dl with Rab7 may explain why Dl is not degraded in late endosomes.
Although N is downregulated at the wavefront, we occasionally observed minor colocalizations of N with Rab7 and Rab4 (Fig. 3j). The colocalization indices of N and Rab7/4 were significantly
lower compared with those of Dl and Rab7/4 (Fig. 3k). Importantly, N colocalized with Rab7 more significantly compared with Rab4, suggesting that Rab7-dependent N degradation is more
dominant than Rab4-dependent N recycling. Similarly, colocalization of Dl-GFP, which recapitulates Dl distribution pattern, and N was occasionally observed (Fig. 3l and Supplementary Figure
5c). These results support the hypothesis that N is mainly degraded in late endosomes at the wavefront in a Dl-dependent manner. ESCRT COMPLEX DOWNREGULATES NOTCH ACTIVITY AT THE WAVEFRONT
When proteins are transported from early to late endosomes (or multivesicular bodies), Rab7 and the ESCRT complex (ESCRT-I–III) regulate protein degradation through the fusion of late
endosomes with lysosomes21,38. Since Dl colocalizes with Rab7 at the wavefront, we asked if the Dl–N complex is transported to late endosomes and N is degraded therein. Many studies have
reported the multiple roles of late endosomes in N signaling. When the function of late endosomes is compromised, the expression of Dl and N is upregulated. As a result, N signaling is
ectopically activated12,15,16,17,18,19,20,22,23,24. On the other hand, it is also known that late endosome function is required for N activation. Indeed, N activity is downregulated when the
function of the ESCRT protein is compromised in some experimental conditions20. Thus, late endosomes appear to be involved in multiple aspects of N signaling. When we knocked down _rab7_ by
expressing the dominant-negative form of _rab7_ or _rab7_ RNAi, N protein level was slightly upregulated, showing punctate signals as visualized by N antibody (Figs. 4a, b, j and 5a, n). N
and Dl colocalized with the Rab7DN puncta (Fig. 4a–d). However, the N activity reporter was not significantly affected when _rab7_ was knocked down (see Fig. 5a). Other late endosomal
components may act redundantly with Rab7. We, therefore, focused on the functions of Vps family proteins that are involved in ESCRT complex function. When we knocked down _vps2_, a member of
ESCRT-III, at the wavefront, N protein level was also upregulated, showing punctate signals (Fig. 4e, f). N and Dl colocalized in the Rab7- and Rab4-positive puncta (Fig. 4e–i). We observed
two distinct outcomes in N activity in the _vps2_ RNAi background. In 40% of the cases (_n_ = 8/20), N activity was specifically upregulated in cells situated between the twin peaks nearby
the wavefront indicated by Lsc expression. The level of full-length N protein was slightly upregulated forming a dotted pattern. At the same time, the twin peaks were fused to form a single
peak (Figs. 4e, g, j and 5d–f, n and Supplementary Figure 6a), which is similar to the results of the partial knockdown of _Dl_ shown above (Fig. 2e and Supplementary Figure 4a). Since the
upregulation of the level of full-length N were not as prominent as those found in _Dl_ mutant clones (Fig. 2i, j), there might be as yet unknown mechanisms that regulate N degradation and
_cis_-inhibition. Importantly, Dl expression level was not significantly affected (Fig. 4g, j and Supplementary Figure 6b), suggesting that N activation and the formation of the single peak
are not caused by changes in Dl expression. Since EGF signaling indirectly influences N activity3,27,40, the above phenotype may be the result of the changes in EGF activity. Since EGF
activity as visualized by PntP1 staining was not significantly affected, the N activation phenotypes discussed above were not caused by indirect effects through EGF signaling (Supplementary
Figure 7). In the other 60% of the cases, Dl and full-length N proteins were widely upregulated (Figs. 4h, j and 5m and Supplementary Figure 6c, d; _n_ = 12/20). Low-level N activity was
observed in a wide area encompassing the wavefront (Fig. 5m and Supplementary Figure 6c), which may be related to the hyper-activation of N signaling and/or suppression of N activity in
_vps2_ mutant cells15,16,17,18,20. Since these phenotypes accompany smaller brain sizes compared with the brains showing the specific N activation phenotype discussed above, we assume that
the mild RNAi effect caused the specific N activation between the twin peaks (Fig. 5d). We repeated the same experiments for the other ESCRT complex genes and found essentially the same
results (Fig. 5g–n and Supplementary Figure 8). Importantly, the specific N activation at the wavefront and the fusion of the twin peaks were reproducible in multiple RNAi conditions and
mutant clones of _vps2_ and _vps22_ (Fig. 5g–p and Supplementary Figure 8). Note that the twin peaks were partially fused, while ectopic N activation was not found in cells apart from the
wavefront in the _vps2_ and _vps22_ mutant clones (Fig. 5o, p; _n_ = 9/15 and 11/16). These results cannot be explained by the previously proposed roles of late endosomes that
nonspecifically and uniformly degrade N and Dl. Thus, late endosomes play specific roles in N degradation that are essential for _cis_-inhibition at the proneural wavefront in addition to
the roles in uniform nonspecific N degradation. DELTA TRANSPORT FROM LATE ENDOSOMES TO RECYCLING ENDOSOMES We hypothesize that the Dl–N complex is transported to late endosomes, where only N
is degraded (Fig. 6o). We also hypothesize that Dl is released from late endosomes prior to its degradation and is recycled to the plasma membrane through recycling endosomes, because Rab4
colocalized with Dl more significantly compared with Rab7 (Fig. 3i). When we knocked down _rab4_ with RNAi, Dl expression was accumulated at the wavefront in a milder condition at 25 °C
(Fig. 6a, b, m) and the colocalization of Dl with Rab7 was significantly increased (Fig. 6b). Interestingly, Dl expression was downregulated in a stronger RNAi condition at 30 °C (Fig. 6c,
m). These results suggest that Dl is retained in Rab7-positive late endosomes and is degraded together with N when the function of recycling endosomes is eliminated. Furthermore,
overexpression of _rab4__DN_ mimicked the effects of _rab4_ RNAi in the milder condition, and aggregated distribution of Dl colocalized with Rab7 in the cytoplasm of cells at the wavefront
(Supplementary Figure 9). Similar results were demonstrated in cells mutant for the components of recycling endosomes in the central brain41. It is known that late endosomes become acidic in
the course of fusion with lysosomes, by which proteins are degraded42. The decrease in pH may trigger Dl dissociation from the Dl–N complex. Rabconnectin (Rbcn) is a family of proteins that
regulate endocytic trafficking by regulating the assembly and activity of vacuolar-ATPase (V-ATPase), which is responsible for the acidification of intracellular compartments43,44. Thus, we
focused on the roles of _Rbcn_ and _vha68-2_, a member of V-ATPase genes, in this process. In _vha68-2_ mutant clones, Dl was aggregated and colocalized with Rab7 (Fig. 6d, e). When
_vha68-2_ was knocked down, signals indicating acidic cellular components were significantly reduced as visualized by Lysotracker (Supplementary Figure 10c). At the same time, N degradation
was blocked, and the level of N protein was upregulated at the wavefront (Fig. 6f–j and Supplementary Figure 10). In this condition, we observed ectopic colocalization of Dl and N in Rab7
and Rab4-positive puncta (Fig. 6h–j), suggesting that acidification of the endocytic pathway triggers Dl dissociation from the Dl–N complex and N degradation. These results are consistent
with the impaired endosomal acidification and accumulation of N in late endosomes and lysosomes in _vha68-2_ mutant clones in eye imaginal disc45. Based on the above results, we hypothesize
that the Dl–N complex in Rab7-positive late endosomes is dissociated upon pH decrease. As a result, N is degraded in lysosomes, while Dl is recycled to the cell membrane through
Rab4-positive recycling endosomes. Since pH increase upon _vha68-2_ RNAi caused Dl colocalization with Rab7 and Rab4 (Fig. 6h, i), Dl–N complex may be localized to either late endosomes or
recycling endosomes when acidification is compromised. If Dl is transported from late to recycling endosomes, Dl should more strongly colocalize with Rab7 when _rab4_ is knocked down
together with _vha68-2_. To test this possibility, we compared colocalization of Dl with Rab7 in _vha68-2_ RNAi and _vha68-2 rab4_ double RNAi backgrounds. Indeed, the colocalization of Dl
with Rab7 was significantly increased by knocking down _rab4_ together with _vha68-2_ (Fig. 6h, k, l, n). These results support the model shown in Fig. 6o. THE SECOND NOTCH ACTIVITY CONTROLS
NEUROGENESIS The first peak of N activity is responsible for regulating the speed of proneural wave propagation3,27. What is the biological function of the second peak? We carefully
compared the pattern of N activity and genes that are specifically expressed in the NBs behind the wavefront. Interestingly, the expression of one of the temporal transcription factors, Klu,
coincides with the second peak of N activity (Fig. 7a, l)31,46. So far, Hth, Klu, Ey, Slp, D, and Tll show sequential expression in the medulla NBs30,31. However, the mutual interactions
between Hth, Klu, and Ey have not been identified thus far. Since Hth is upregulated in NEs prior to the arrival of the proneural wave and is continuously expressed in NEs and NBs (Fig. 7l),
the mechanism that regulates Klu expression remains unclear. The N activity in the second peak may regulate the onset of Klu expression in NBs. We initially examined the effect of the
complete elimination of Dl function by generating _Dl_-null mutant clones in which the proneural wave is accelerated27. Since Klu expression persists for a long time following its induction
at the second N activity peak, the premature NB differentiation would still show a persistent Klu expression behind the accelerated wavefront. However, Klu expression was eliminated in _Dl_
mutant cells except for the cells situated along the boundary between the Dl-positive cells (Fig. 7b, _n_ = 16). The residual Klu expression along the clone boundary may be due to the
non-autonomous effect of N _trans_-activation. In contrast, the expression Hth, Ey, and Slp was not significantly affected in _Dl_ mutant clones (Supplementary Figure 11a–c). These results
suggest that N signaling is indeed necessary for Klu expression in NBs. Since we do not have a technique that specifically inactivates the second N peak, we made use of the partial knockdown
of _Dl_ and _vps2_, resulting in the loss of the gap between the twin peaks and, consequently, the fusion of the twin peaks. Interestingly, Klu was precociously expressed in the newborn NBs
under these conditions without significantly affecting the proneural wave progression (Fig. 7c, d), suggesting that the second N activity peak in NBs indeed triggers the expression of Klu.
Note that Klu expression is not activated in the first peak of N activity in NEs (Fig. 7a, l). Klu expression may require additional genetic factors that are specific to NBs. We previously
demonstrated that Hth expression in the newborn NBs promotes the production of brain-specific homeobox (Bsh)-positive neurons that form the innermost concentric zone in the larval medulla
(Fig. 7e, f, l)47,48. In slightly older NBs, Klu expression triggers the production of Runt (Run)-positive neurons, forming a concentric zone just outside the Bsh-positive neurons31.
Consistent with the precocious Klu expression in NBs, we occasionally observed Run-positive neurons in an area inside the Bsh-positive neurons in _Dl_ and _vps2_ RNAi conditions (Fig. 7g, h,
i). These defects were restricted to the dorsal part of the brain, most likely due to the stronger expression of _optix-Gal4_ in the dorsal brain (Fig. 7e). The Klu expression in the
newborn NBs, which only express Hth in the control background, might cause the production of Run-positive neurons earlier than Bsh-positive neurons, resulting in their abnormal distributions
in the medulla (Fig. 7l). Note that Hth expression is widely found in NE and NB cells, and is not affected by Klu31. Therefore, the expression of Hth and production of Bsh-positive neurons
should not be affected. We have demonstrated that Bsh-positive neurons give rise to Mi1 medulla neurons47,48. Similarly, the results of clonal analysis using _run-Gal4_ demonstrate that
Run-positive neurons differentiate into Mi4 and TmY16 neurons (Fig. 7j, k; _n_ = 60 and 49, respectively). The neuronal-type TmY16 has not been documented based on its projection pattern in
the medulla, lobula, and lobula plate (Dr. Kazunori Shinomiya, personal communication). Thus, the temporal regulation of the N dynamics at the proneural wavefront controls the temporal
pattern of neuronal-type specification through the expression of the temporal transcription factor Klu. DISCUSSION In this study, we incorporated nonlinearity in _cis_-inhibition to the
mathematical model of the proneural wave and reproduced the twin activation peaks of N signaling at the wavefront as observed in vivo. The fast nonlinear dynamics of _cis_-inhibition
compared with those of the gradual kinetics of _trans_-activation is consistent with the results of the in vitro cell culture system11. The differential kinetics of _trans_-activation and
_cis_-inhibition provide the rich dynamics of N activity that enable the formation of the twin activation peaks and the regulation of the temporal patterning of neurogenesis. According to
the previous literatures, the upregulation of Dl expression may induce the clustering of N and Dl14,34,35, which leads to an acute suppression of N signal activity via _cis_-inhibition (Fig.
6o). Consistent with this idea, the partial knockdown of _Dl_ caused an upregulation of N activity between the twin peaks and their fusion, which reflects the failure in _cis_-inhibition.
The expression level of full-length N is temporally downregulated behind the wavefront, which is derepressed in _Dl_ mutant clones (Fig. 2h, i), suggesting that _cis_-inhibition is triggered
by N degradation in response to Dl expression. We essentially focused on the roles of intracellular trafficking in regulating N degradation and _cis_-inhibition (Fig. 6o). RNAi and mutant
clones of ESCRT complex genes caused the fusion of the twin peaks of N activity, which is similar to the result of the partial _Dl_ knockdown. We assume that the ESCRT complex regulates N
degradation in the presence of high levels of Dl expression. Since the expression level of full-length N was only partially upregulated, forming a dotted pattern by knocking down _rab7_ and
ESCRT genes, there might be as yet unknown mechanisms that regulate N degradation (Fig. 5, Supplementary Figure 6). We assumed that the clustering of N and Dl is the trigger of N
degradation. However, Dl is not degraded at the wavefront, as evident from its strong membrane localization, despite its localization in Rab7-positive late endosomes. Since the
colocalization of Dl with Rab4 is more prominent than that with Rab7, recycling of Dl to the plasma membrane may be dominant compared with its degradation (Fig. 3i). Indeed, a mild knockdown
of _rab4_ caused Dl accumulation in Rab7-positive late endosomes, while its severe knockdown induced Dl degradation (Fig. 6a–c). Colocalization of Dl with Rab7 upon blocking acidification
in late endosomes was further enhanced by the simultaneous knockdown of _vha68-2_ and _rab4_ (Fig. 6l, n). These results suggest that Dl is dissociated from the Dl–N complex in late
endosomes, and is recycled to the plasma membrane through recycling endosomes (Fig. 6o). The mathematical models in Fig. 1 do not explicitly consider the degradation of N protein upon its
_cis_-interaction with Dl. We further improved the model by considering full-length N (_F_) and active form of N (_S_). In a wide range of parameter settings, this model reproduces the
formation of the twin peaks of N activity, fast degradation, and gradual recovery of the expression level of full-length N (Supplementary Figure 12). Although Dl function is thought to be
inhibited when Dl and N interact in _cis_11, we did not include this reaction in the model, because we do not have any observation that suggests _cis_-inhibition of Dl in the course of the
proneural wave progression. Furthermore, we demonstrated a role of N signaling in regulating the temporal patterning of neural progenitor cells (NPCs) by focusing on the transition of the
temporal transcription factor Klu, whose expression is upregulated in the medulla NBs31. We showed that Dl remotely regulates Klu expression behind the proneural wave through the second peak
of N activity. Thus, fine temporal regulation of N activity through _cis-_inhibition plays essential roles in the temporal patterning of neurogenesis in the fly brain. In the NPCs of the
developing cerebral cortex, the temporal dynamics of N activity also play important roles in the temporal patterning of neurogenesis and gliogenesis49. In this process, the basic
helix–loop–helix transcription factors show oscillatory expression in NPCs and N signaling appears to perform lateral inhibitory feedback during NPC differentiation. The roles of
_cis_-inhibition in this process remain largely elusive. It will be interesting to see how the molecular mechanisms revealed in the current study are conserved in a wide variety of
developmental processes. METHODS MATHEMATICAL MODELING AND NUMERICAL SIMULATION The differential equations were calculated using the explicit finite difference method with the zero flux
boundary condition as described previously3. The mesh size of the square grid model is equal to the cell size (d_x_ = 2, 25 × 25 cells), and the time step size is 0.01. _Four-variable
model_: The model contains four equations and four variables. _E_ is a composite variable for EGF ligand concentration and EGF signaling. _N_ represents N signal activity. _D_ and _A_
represent expression levels of Dl and AS-C, respectively. _d_e is the diffusion coefficient of EGF. _d_t and _d_c represent magnitudes of the _trans_-activation and _cis_-inhibition,
respectively. _i_, _l_ and _j_, _m_ are integers indicating the location of a cell along the _x_ and _y_ axes, respectively. _l_ and _m_ indicate the location of four cells adjacent to a
cell indicated by _i_ and _j_. The initial conditions for _A_, _E_, and _D_ in the anterior-most cells (_x_ = 1, 2, 3) are _A_ = 0.90, 0.31, and 0.02; _E_ = 0.054, 0.021, and 0.0016; and _D_
= 0.062, 0.021, and 0.0013, respectively, to stabilize the proneural wave progression. The initial condition of _N_ is 0 in all cells. $$\frac{{{\mathrm{d}}E_{i,j}}}{{{\mathrm{d}}t}} =
d_{\mathrm{e}}{{\Delta }}E_{i,j} - k_{\mathrm{e}}E_{i,j} + a_{\mathrm{e}}A_{i,j}\left( {1 - A_{i,j}} \right)$$ (1-1) $$\frac{{{\mathrm{d}}N_{i,j}}}{{{\mathrm{d}}t}} = - k_{\mathrm{n}}N_{i,j}
+ d_{\mathrm{t}}\mathop {\sum}\limits_{l,m \in {{\Lambda }}_{i,j}} {D_{l,m} - d_{\mathrm{c}}D_{i,j}}$$ (1-2) $$\frac{{{\mathrm{d}}D_{i,j}}}{{{\mathrm{d}}t}} = - k_{\mathrm{d}}D_{i,j} +
a_{\mathrm{d}}A_{i,j}\left( {1 - A_{i,j}} \right)$$ (1-3) $$\frac{{{\mathrm{d}}A_{i,j}}}{{{\mathrm{d}}t}} = e_{\mathrm{a}}\left( {1 - A_{i,j}} \right){\mathrm{max}}\left\{ {E_{i,j} -
N_{i,j},0} \right\}$$ (1-4) The diffusion of _E_ was calculated as follows: _d_eΔ_E__i_,_j_ = _d__e_ (_E__i_ + 1,_j_ + _E__i_ − 1,_j_ + _E__i_,_j_ − 1 + _E__i_,_j_ − 1 − 4
_E__i_,_j_)/d_x__2_. _k_e, _k_n, and _k_d are passive degradation rates of EGF, N, and Dl, respectively. _a__e_ and _a_d indicate EGF and Dl regulation by AS-C, respectively. When _A_ = 1,
the cells are fully differentiated as NBs. _e_a reflects the speed of differentiation under the control of EGF and N, and is set to 100. The other parameters are set to 1 unless otherwise
noted. _Linear cis-inhibition_: In the previous model, the _trans_-activation and _cis_-inhibition are linear (1-2). _k_n = 1, _d_t = 0.25, and _d_c = 0.1 (Fig. 1e). In this study, (1-2) is
replaced with the following. _Step function_: (1-2) is replaced with (2-1) and (2-2). _cis_-inhibition does not occur when _D_ is below the threshold (_e_c), but quickly induces N
inactivation when it exceeds the threshold. _k_n = 1, _d_t = 0.25, _d_c = 0.1, and _e_c = 0.07 (Figs. 1f and 2b). Magnitude (_d_c) and threshold (_e_c) of _cis_-inhibition are changed in the
range 0.07–0.15 and 0.04–0.08, respectively (Supplementary Figure 1). $$\frac{{{\mathrm{d}}N_{i,j}}}{{{\mathrm{d}}t}} = - k_{\mathrm{n}}N_{i,j} + d_{\mathrm{t}}\mathop {\sum}\limits_{l,m
\in {{\Lambda }}_{i,j}} {D_{l,m} - d_{\mathrm{c}}H(D_{i,j})}$$ (2-1) $$H(x) = \left\{ {\begin{array}{*{20}{c}} {1\;(x \ge e_c)} \\ {0\;(x < e_c)} \end{array}} \right.$$ (2-2) _Hill
function_: (1-2) is replaced with (3-1). Hill’s coefficient (_n_t and _n_c) and activation coefficient (_k_t and _k_c) specify the kinetics of the reaction. _k_n = 1, _d_t = 0.25, _d_c =
0.1, _n_t = 1, _k_t = 1, _n_c = 5, and _k_c = 0.09 (Figs. 1g and 2c). Hill’s coefficient (_n_c) and activation coefficient (_k_c) of _cis_-inhibition are changed in the range 2–7 and
0.07–0.11, respectively (Supplementary Figure 2). $$\frac{{{\mathrm{d}}N_{i,j}}}{{{\mathrm{d}}t}} = - k_{\mathrm{n}}N_{i,j} + \frac{{d_{\mathrm{t}}\left( {\mathop {\sum }\nolimits_{l,m \in
{{\Lambda }}_{i,j}} D_{l,m}} \right)^{n_{\mathrm{t}}}}}{{k_{\mathrm{t}}^{n_{\mathrm{t}}} + \left( {\mathop {\sum }\nolimits_{l,m \in {{\Lambda }}_{i,j}} D_{l,m}} \right)^{n_{\mathrm{t}}}}} -
\frac{{d_{\mathrm{c}}D_{i,j}^{n_{\mathrm{c}}}}}{{k_{\mathrm{c}}^{n_{\mathrm{c}}} + D_{i,j}^{n_{\mathrm{c}}}}}$$ (3-1) _Five-variable model_: (1-2) is replaced with (4-1) and (4-2). _F_ and
_S_ represent the expression level of full-length N and N signal activity, respectively, whose initial conditions are 1 and 0 in all cells. _F_max, _n_, and _k_t represent maximum expression
level, recovery rate, and spontaneous degradation rate of full-length N, respectively. _d_t and _d_c represent magnitudes of the _trans_-activation and _cis_-inhibition, respectively.
Hill’s coefficient (_n_t and _n_c) and activation coefficient (_K_1 and _K_2) specify the kinetics of the reaction. _k_t represents the degradation rate of full-length N upon
_trans_-activation. _k_f represents passive degradation rate of active form of N. _n_ = 1, _F_max = 1, _k_f = 0.1, _d_c = 5, _n_t = 1, _K_1 = 0.3, _k_t = 0.1, _d_t = 0.25, and _k_s = 2.
Hill’s coefficient (_n_c) and activation coefficient (_K_2) for Hill function in _cis_-inhibition are changed in the range 1–7 and 0.05–0.3, respectively (Supplementary Figure 12).
$$\frac{{{\mathrm{d}}F_{i,j}}}{{{\mathrm{d}}t}} = n\left( {F_{{\mathrm{max}}} - F_{i,j}} \right) - k_{\mathrm{f}}F_{i,j} -
\frac{{d_{\mathrm{c}}F_{i,j}D_{i,j}^{n_{\mathrm{c}}}}}{{K_2^{n_{\mathrm{c}}} + D_{i,j}^{n_{\mathrm{c}}}}} - \frac{{k_{\mathrm{t}}F_{i,j}\left( {\mathop {\sum }\nolimits_{l,m \in {{\Lambda
}}_{i,j}} D_{l,m}} \right)^{n_{\mathrm{t}}}}}{{K_1^{n_{\mathrm{t}}} + \left( {\mathop {\sum }\nolimits_{l,m \in {{\Lambda }}_{i,j}} D_{l,m}} \right)^{n_{\mathrm{t}}}}}$$ (4-1)
$$\frac{{{\mathrm{d}}S_{i,j}}}{{{\mathrm{d}}t}} = \frac{{d_{\mathrm{t}}F_{i,j}\left( {\mathop {\sum }\nolimits_{l,m \in {{\Lambda }}_{i,j}} D_{l,m}}
\right)^{n_{\mathrm{t}}}}}{{K_1^{n_{\mathrm{t}}} + \left( {\mathop {\sum }\nolimits_{l,m \in {{\Lambda }}_{i,j}} D_{l,m}} \right)^{n_{\mathrm{t}}}}} - k_{\mathrm{s}}S_{i,j}$$ (4-2) Two- and
one-dimensional plots in Figs. 1e–g, 2b, c, and Supplementary Figures 1, 2, and 12 are the snapshots when the state of differentiation of the central cell exceeds 0.5 (_A_13,13 > 0.5).
The source codes for the numerical simulations will be deposited to a public repository service. FLY STRAINS Fly strains were maintained on standard _Drosophila_ medium at 25 °C. _rab4_ RNAi
was performed at 25 and 30 °C. The following mutant and transgenic flies were used: _Dl__RevF10_ _FRT82B, UAS-Dl__3-1_, _UAS-Dl RNAi_ (strong: BDSC36784; mild: V52188), _Dl-GFP_
(BDSC59819), _rab7-EYFP_ (BDSC62545L), _rab4-EYFP_ (BDSC62542), _UAS-rab7__DN_ (BDSC9778), _UAS-rab7 RNAi_ (BDSC27051, VDRC40338), _UAS-rab4__DN_ (BDSC9768, BDSC9769), _vps2__pp6_ _FRT82B_,
_vps22__NN31_ _FRT82B_, _UAS-vps2 RNAi_ (BDSC38995), _UAS-vps 22RNAi_ (BDSC38289), _UAS-vps23 RNAi_ (BDSC38306), _UAS-vps25 RNAi_ (BDSC26286), _UAS-vps24 RNAi_ (BDSC38281), _UAS-vps32 RNAi_
(BDSC38305), _UAS-vps20 RNAi_ (BDSC40894), _UAS-vps36 RNAi_ (BDSC38286), _UAS-vps37B RNAi_ (BDSC44010), _UAS-vha68-2 RNAi_ (BDSC34582), _vha68-2__R6_ _FRT40A_ (BDSC39621), _UAS-rbcn3A RNAi_
(BDSC34612), _optix-Gal4_ (NP2631), _Ay-Gal4_50, _UAS-CD8GFP_, _UAS-GFP_, _hs-flp_, _ubi-GFP FRT82B_, and _ubi-RFP FRT82B_. N activity was visualized by _E_(_spl_)_-mγGFP_ (_mγGFP_) and
_NRE-dVenus_32,33. CLONAL ANALYSIS _Dl_ overexpression clones were generated by crossing _hs-flp_; _Ay-Gal_4 _UAS-GFP_ strain with _UAS-Dl__3-1_ and applying 15 min heat shock at 34 °C. _Dl_
mutant clones were generated by crossing _hs-flp_; _ubi-GFP FRT82B strain_ with _Dl__RevF10_ _FRT82B_ and applying 50 min heat shock at 37 °C. _vps_ mutant clones were generated by crossing
_hs-flp_; _mγGFP_; _ubi-RFP FRT82B or hs-flp_; _NRE-dVenus_; _ubi-RFP FRT82B_ with _vps FRT82B_ and applying 50 min heat shock at 37 °C. _run-Gal4_ MARCM clones were generated by crossing
_run-Gal4 UAS-CD8GFP FRT19F_; _FRT40A_ strain with _hs-flp_; _tubP-Gal80 FRT40A_ and applying 15 min heat shock at 34 °C. GENERATION OF _RUN-GAL4_ STRAIN The second exon containing the
translational start site of _run_ was replaced by the fragment containing _attP_ site and _GMR-white_ via the homologous recombination with the homology arms for _run_ (runLp and runRp)51 in
the presence of _FRT19F_ (Kyoto125719). The Gal4 containing fragment was integrated into the _attP_ site in the _run_ locus by co-injecting PhiC31 plasmid and _run-Gal4/pGEattB_, which
contains _attB_ site, the fragment containing the translational start site of _run_ (run5pri) and _GMR-white_. The expression pattern of _run-Gal4_ was verified by expressing _UAS-GFP_ and
co-staining with anti-Run antibody. runLp (2.0 kb), runRp (2.2 kb, and run5pri (153 bp) were amplified using the following PCR primers: runLp5__Not_I (catgcggccgcccaagtatgacacttccgcatc),
runLp3__Kpn_I (catggtaccctttatcgggggtcacttggaa), runRp5__Asc_I (catggcgcgcccgggagccaagaagtaagcaaa), runRp3__Xho_I (catctcgagggccaactgtgataggaagttc), runGal5-_Not_
(catgcggccgcttccaagtgacccccgataaag), and runGal3-_Bam_ (catggatcctgtgttgttggccaccatcgtt). Underlined sequences are homologous to the genomic sequences. HISTOCHEMISTRY Immunohistochemistry
was performed as described below48. Details are available upon request. Brains were dissected in phosphate-buffered saline (PBS), transferred to ice-cold 0.8% formaldehyde/PBS solution, and
fixed in 4% formaldehyde/PBS at room temperature for 30–60 min. The brains were washed in PBT (0.3% Triton X in PBS) and blocked in 5–10% normal serum/PBT solution at room temperature for
30–60 min. Primary antibody reaction was performed in a solution containing primary antibodies and 1% normal serum in PBT at 4 °C overnight. The brains were washed in PBT. Secondary antibody
reaction was performed in a solution containing secondary antibodies and 1% normal serum in PBT at 4 °C overnight. After washing in PBT and PBS, the brains were mounted in VECTASHIELD
(Vector Laboratories). The following primary antibodies were used: guinea pig anti-Lsc (1:1200), mouse anti-Dl (1:20; DSHB C594.9B), mouse anti-N (1:20; DSHB C17.9C6), rat anti-Ecad (1:20;
DSHB DCAD2), rabbit anti-Klu (1:1000; Xiaohang Yang, Singapore), guinea pig anti-Run (1:500; Asian Distribution Center for Segmentation Antibodies, Mishima, Japan), and rabbit anti-Rab7
(1:600; Akira Nakamura, Kumamoto University, Japan). The following secondary antibodies were used: anti-mouse Cy3 (1:200; Jackson ImmunoResearch 715-165-151), anti-mouse Cy5 (1:200; Jackson
ImmunoResearch 715-175-151), anti-guinea pig Alexa647 (1:200; Jackson ImmunoResearch 712-605-150), and anti-rabbit Alexa546 (1:200; Invitrogen A-11035). Confocal images were obtained by
Zeiss LSM880 and processed using ZEN, Fiji, and Adobe Photoshop. Signal intensity was quantified within the indicated rectangle areas by ImageJ. IN SITU HYBRIDIZATION In situ hybridization
was performed as briefly described below. Details are available upon request. Larval brains were dissected in ice-cold PBS, transferred to 4% formaldehyde/PBS solution, and fixed at 4 °C
overnight. The formaldehyde solution was removed, and the brains were washed with PBS and 70% ethanol. Incubation at room temperature for 2–5 min after replacing the solution with Wash
Buffer A (100 μl Stellaris RNA FISH Wash Buffer A, 300 μl nuclease-free water, 50 μl deionized formamide). Replacing the solution with Hybridization Buffer (90 μl Hybridization Buffer mix
with 2 μl Stellaris RNA FISH probe designed for exon 6 of _N_ gene labeled with Quasar 570), the brains were incubated at 37 °C for 8 h in the dark. Incubation at 37 °C for 30 min after
replacing the solution with Wash Buffer A. Replacing the solution with TO-PRO3/PBS, the brains were incubated at 37 °C for 30 min. After washing in PBS, the brains were mounted in 80%
glycerol in PBS. DUOLINK PLA PLA was performed using Duolink (Sigma-Aldrich). The dissected fly brains were fixed in 4% formaldehyde in PBT (0.3% Triton X in PBS). After finishing the Lsc
immunostaining, the brains were incubated with Duolink blocking solution for 60 min at 37 °C and in the Duolink antibody diluent containing mouse anti-Dl and rabbit anti-Rab7 antibodies for
overnight at 4 °C. The brains were washed in Duolink Wash Buffer A three times at room temperature, incubated in a solution containing PLA PLUS anti-mouse and PLA MINUS anti-rabbit probes
for 120 min at 37 °C, washed in Duolink Wash Buffer A, and incubated with Duolink ligation stock for 60 min at 37 °C. Then, the brains were washed in Wash Buffer A and incubated with Duolink
polymerase in Duolink amplification stock for 100 min at 37 °C. Finally, the brains were washed in Wash Buffer B and mounted in Duolink in situ mounting medium with DAPI
(4′,6-diamidino-2-phenylindole). LYSOTRACKER STAINING Fly brains were dissected in cold S2 medium and incubated in PBS containing LysoTrancker Red DND99 (1:1000, Thermo Fisher) for 30 min at
room temperature. The brains were washed in PBS and fixed in 4% formaldehyde in PBS. After washing in PBS, the brains were mounted in VECTASHIELD (Vector Laboratories). STATISTICS AND
REPRODUCIBILITY For quantification and statistical analysis, distinct brain areas or samples were measured and analyzed as indicated below. Two-sided _t_ test was used for the statistical
test. Image intensities were not artificially processed, except as otherwise noted. When statistics were not applicable, experiments were independently repeated at least three times with
similar results. IMAGE QUANTIFICATION Signal intensity was quantified within the indicated rectangle areas using Fiji (Figs. 1c–d, 2e–i, 4, 5, and 6f–n). Coloc 2 function of Fiji was used to
calculate the Pearson’s _R_ value to quantify the colocalization between two different signals (Figs. 3i, k and 6b, o). The _xy_ coordinates of cells were obtained by manually selecting
individual cells expressing Run or Bsh (Fig. 7g, h). The _x_ coordinate of the origin was calculated as the average of cell locations along the _x_-axis. The _y_ coordinate of the origin was
determined so that the standard deviation of _R_, the distance from the origin to cells, is minimized. Δ_θ_, the angle of mutant area from the origin, was determined based on the mutant
area of Bsh-positive cells. The same Δ_θ_ was used to determine the control area to be analyzed at the dorsal–ventral boundary where _optix-Gal4_ is not expressed. Sizes of the intersection
between Run- and Bsh-positive areas in the control and mutant areas were calculated using “convhull,” “polyshape,” and “intersects” functions of MATLAB (Fig. 7i). ETHICAL APPROVAL We have
complied with all relevant ethical regulations for animal testing and research. This study did not require an ethical approval. REPORTING SUMMARY Further information on research design is
available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The authors declare that the data supporting the findings of this study are available within the
paper, or available upon request. Source data are provided with this paper. CODE AVAILABILITY The source codes for the numerical simulations and image quantifications are deposited in a
public repository service (https://github.com/satouma7/TwinPeaksCode). REFERENCES * Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal
integration in development. _Science_ 284, 770–776 (1999). Article ADS CAS PubMed Google Scholar * Simpson, P. Lateral inhibition and the development of the sensory bristles of the
adult peripheral nervous system of Drosophila. _Development_ 109, 509–519 (1990). CAS PubMed Google Scholar * Sato, M., Yasugi, T., Minami, Y., Miura, T. & Nagayama, M. Notch-mediated
lateral inhibition regulates proneural wave propagation when combined with EGF-mediated reaction diffusion. _Proc. Natl Acad. Sci. USA_ 113, E5153–E5162 (2016). Article CAS PubMed PubMed
Central Google Scholar * Tanaka, Y., Yasugi, T., Nagayama, M., Sato, M. & Ei, S. I. JAK/STAT guarantees robust neural stem cell differentiation by shutting off biological noise. _Sci.
Rep._ 8, 12484 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Kunisch, M., Haenlin, M. & Campos-Ortega, J. A. Lateral inhibition mediated by the Drosophila
neurogenic gene delta is enhanced by proneural proteins. _Proc. Natl Acad. Sci. USA_ 91, 10139–10143 (1994). Article ADS CAS PubMed PubMed Central Google Scholar * Bray, S. &
Furriols, M. Notch pathway: making sense of suppressor of hairless. _Curr. Biol._ 11, R217–R221 (2001). Article CAS PubMed Google Scholar * Struhl, G. & Adachi, A. Nuclear access and
action of notch in vivo. _Cell_ 93, 649–660 (1998). Article CAS PubMed Google Scholar * Yuan, Z. et al. Structure and function of the Su(H)-Hairless Repressor complex, the major
antagonist of notch signaling in _Drosophila melanogaster_. _PLoS Biol._ 14, e1002509 (2016). Article PubMed PubMed Central CAS Google Scholar * del Alamo, D. & Schweisguth, F.
Notch signalling: receptor cis-inhibition to achieve directionality. _Curr. Biol._ 19, R683–R684 (2009). Article PubMed CAS Google Scholar * Miller, A. C., Lyons, E. L. & Herman, T.
G. cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. _Curr. Biol._ 19, 1378–1383 (2009). Article CAS PubMed PubMed Central Google Scholar * Sprinzak,
D. et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. _Nature_ 465, 86–90 (2010). Article ADS CAS PubMed PubMed Central Google Scholar *
Berndt, N. et al. Ubiquitylation-independent activation of Notch signalling by Delta. _eLife_ 6, https://doi.org/10.7554/eLife.27346 (2017). * del Alamo, D., Rouault, H. & Schweisguth,
F. Mechanism and significance of cis-inhibition in Notch signalling. _Curr. Biol._ 21, R40–R47 (2011). Article PubMed CAS Google Scholar * Sakamoto, K., Ohara, O., Takagi, M., Takeda, S.
& Katsube, K. Intracellular cell-autonomous association of Notch and its ligands: a novel mechanism of Notch signal modification. _Dev. Biol._ 241, 313–326 (2002). Article CAS PubMed
Google Scholar * Moberg, K. H., Schelble, S., Burdick, S. K. & Hariharan, I. K. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit
non-cell-autonomous overgrowth. _Dev. Cell_ 9, 699–710 (2005). Article CAS PubMed Google Scholar * Thompson, B. J. et al. Tumor suppressor properties of the ESCRT-II complex component
Vps25 in Drosophila. _Dev. Cell_ 9, 711–720 (2005). Article CAS PubMed Google Scholar * Vaccari, T. & Bilder, D. The Drosophila tumor suppressor vps25 prevents nonautonomous
overproliferation by regulating notch trafficking. _Dev. Cell_ 9, 687–698 (2005). Article CAS PubMed Google Scholar * Herz, H. M. et al. vps25 mosaics display non-autonomous cell
survival and overgrowth, and autonomous apoptosis. _Development_ 133, 1871–1880 (2006). Article CAS PubMed Google Scholar * Vaccari, T., Lu, H., Kanwar, R., Fortini, M. E. & Bilder,
D. Endosomal entry regulates Notch receptor activation in _Drosophila melanogaster_. _J. Cell Biol._ 180, 755–762 (2008). Article CAS PubMed PubMed Central Google Scholar * Aoyama, N.
et al. Loss- and gain-of-function analyses of vacuolar protein sorting 2 in Notch signaling of _Drosophila melanogaster_. _Genes Genet. Syst._ 88, 45–57 (2013). Article CAS PubMed Google
Scholar * Fortini, M. E. & Bilder, D. Endocytic regulation of Notch signaling. _Curr. Opin. Genet Dev._ 19, 323–328 (2009). Article CAS PubMed PubMed Central Google Scholar *
Vaccari, T. et al. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. _J. Cell Sci._ 122, 2413–2423 (2009). Article CAS
PubMed PubMed Central Google Scholar * Jaekel, R. & Klein, T. The Drosophila Notch inhibitor and tumor suppressor gene lethal (2) giant discs encodes a conserved regulator of
endosomal trafficking. _Dev. Cell_ 11, 655–669 (2006). Article CAS PubMed Google Scholar * Schneider, M., Troost, T., Grawe, F., Martinez-Arias, A. & Klein, T. Activation of Notch in
lgd mutant cells requires the fusion of late endosomes with the lysosome. _J. Cell Sci._ 126, 645–656 (2013). Article CAS PubMed Google Scholar * Egger, B., Boone, J. Q., Stevens, N.
R., Brand, A. H. & Doe, C. Q. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. _Neural Dev._ 2, 1 (2007). Article PubMed PubMed Central CAS
Google Scholar * Yasugi, T., Umetsu, D., Murakami, S., Sato, M. & Tabata, T. Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively
regulated by JAK/STAT. _Development_ 135, 1471–1480 (2008). Article CAS PubMed Google Scholar * Yasugi, T., Sugie, A., Umetsu, D. & Tabata, T. Coordinated sequential action of EGFR
and Notch signaling pathways regulates proneural wave progression in the Drosophila optic lobe. _Development_ 137, 3193–3203 (2010). Article CAS PubMed Google Scholar * Reddy, B. V.,
Rauskolb, C. & Irvine, K. D. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. _Development_ 137, 2397–2408 (2010).
Article CAS PubMed PubMed Central Google Scholar * Contreras, E. G., Egger, B., Gold, K. S. & Brand, A. H. Dynamic Notch signalling regulates neural stem cell state progression in
the Drosophila optic lobe. _Neural Dev._ 13, 25 (2018). Article CAS PubMed PubMed Central Google Scholar * Li, X. et al. Temporal patterning of Drosophila medulla neuroblasts controls
neural fates. _Nature_ 498, 456–462 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Suzuki, T., Kaido, M., Takayama, R. & Sato, M. A temporal mechanism that produces
neuronal diversity in the Drosophila visual center. _Dev. Biol._ 380, 12–24 (2013). Article CAS PubMed Google Scholar * Almeida, M. S. & Bray, S. J. Regulation of post-embryonic
neuroblasts by Drosophila Grainyhead. _Mech. Dev._ 122, 1282–1293 (2005). Article CAS PubMed Google Scholar * Housden, B. E., Millen, K. & Bray, S. J. Drosophila reporter vectors
compatible with PhiC31 integrase transgenesis techniques and their use to generate new notch reporter fly lines. _G3_ 2, 79–82 (2012). Article CAS PubMed PubMed Central Google Scholar *
Fehon, R. G. et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. _Cell_ 61, 523–534 (1990). Article
CAS PubMed Google Scholar * Glittenberg, M., Pitsouli, C., Garvey, C., Delidakis, C. & Bray, S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and
endocytosis. _EMBO J._ 25, 4697–4706 (2006). Article CAS PubMed PubMed Central Google Scholar * Dunst, S. et al. Endogenously tagged rab proteins: a resource to study membrane
trafficking in Drosophila. _Dev. Cell_ 33, 351–365 (2015). Article CAS PubMed PubMed Central Google Scholar * Tanaka, T. & Nakamura, A. The endocytic pathway acts downstream of
Oskar in Drosophila germ plasm assembly. _Development_ 135, 1107–1117 (2008). Article CAS PubMed Google Scholar * Vanlandingham, P. A. & Ceresa, B. P. Rab7 regulates late endocytic
trafficking downstream of multivesicular body biogenesis and cargo sequestration. _J. Biol. Chem._ 284, 12110–12124 (2009). Article CAS PubMed PubMed Central Google Scholar * Mohrmann,
K., Gerez, L., Oorschot, V., Klumperman, J. & van der Sluijs, P. Rab4 function in membrane recycling from early endosomes depends on a membrane to cytoplasm cycle. _J. Biol. Chem._ 277,
32029–32035 (2002). Article CAS PubMed Google Scholar * Jorg, D. J. et al. The proneural wave in the Drosophila optic lobe is driven by an excitable reaction-diffusion mechanism. _eLife_
8, https://doi.org/10.7554/eLife.40919 (2019). * Li, B. et al. The retromer complex safeguards against neural progenitor-derived tumorigenesis by regulating Notch receptor trafficking.
_eLife_ 7, https://doi.org/10.7554/eLife.38181 (2018). * Couturier, L., Trylinski, M., Mazouni, K., Darnet, L. & Schweisguth, F. A fluorescent tagging approach in Drosophila reveals late
endosomal trafficking of Notch and Sanpodo. _J. Cell Biol._ 207, 351–363 (2014). Article CAS PubMed PubMed Central Google Scholar * Yan, Y., Denef, N. & Schupbach, T. The vacuolar
proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. _Dev. Cell_ 17, 387–402 (2009). Article CAS PubMed PubMed Central Google Scholar *
Wissel, S. et al. Time-resolved transcriptomics in neural stem cells identifies a v-ATPase/Notch regulatory loop. _J. Cell Biol._ 217, 3285–3300 (2018). Article CAS PubMed PubMed Central
Google Scholar * Vaccari, T., Duchi, S., Cortese, K., Tacchetti, C. & Bilder, D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch
receptor. _Development_ 137, 1825–1832 (2010). Article CAS PubMed PubMed Central Google Scholar * Yang, X., Bahri, S., Klein, T. & Chia, W. Klumpfuss, a putative Drosophila zinc
finger transcription factor, acts to differentiate between the identities of two secondary precursor cells within one neuroblast lineage. _Genes Dev._ 11, 1396–1408 (1997). Article CAS
PubMed Google Scholar * Hasegawa, E. et al. Concentric zones, cell migration and neuronal circuits in the Drosophila visual center. _Development_ 138, 983–993 (2011). Article CAS PubMed
Google Scholar * Hasegawa, E., Kaido, M., Takayama, R. & Sato, M. Brain-specific-homeobox is required for the specification of neuronal types in the Drosophila optic lobe. _Dev.
Biol._ 377, 90–99 (2013). Article CAS PubMed Google Scholar * Imayoshi, I. et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. _Science_
342, 1203–1208 (2013). Article ADS CAS PubMed Google Scholar * Ito, K., Awano, W., Suzuki, K., Hiromi, Y. & Yamamoto, D. The Drosophila mushroom body is a quadruple structure of
clonal units each of which contains a virtually identical set of neurones and glial cells. _Development_ 124, 761–771 (1997). CAS PubMed Google Scholar * Huang, J., Zhou, W., Dong, W.,
Watson, A. M. & Hong, Y. From the cover: directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. _Proc. Natl Acad. Sci. USA_ 106, 8284–8289
(2009). Article ADS CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank members of Sato lab for supporting fly work, T. Kawauchi, K. Matsuno for
helpful discussion, S. Bray, A. Nakamura, J. Skeath, Bloomington _Drosophila_ Stock Center, Vienna _Drosophila_ Resource Center, _Drosophila_ Genetic Resource Center, Kyoto, Asian
Distribution Center for Segmentation Antibodies, Mishima, and Developmental Studies Hybridoma Bank for flies and antibodies. This work was supported by CREST from JST (JPMJCR14D3 to M.S.,
S.-I.E. and JPMJCR15D2 to M.N.), Grant-in-Aid for Scientific Research (B), (C), Grant-in-Aid for Scientific Research on Innovative Areas and Grant-in-Aid for Early-Career Scientists from
MEXT (17H03542, 17H05739, 17H05761, and 19H04771 to M.S., 19K06674, 19H04956, and 20H05030 to T.Y., and JP20K14364 to Y.T.), Takeda Science Foundation (to M.S. and T.Y.), Cooperative
Research of “Network Joint Research Center for Materials and Devices” (to M.S.). AUTHOR INFORMATION Author notes * These authors contributed equally: Miaoxing Wang, Xujun Han. AUTHORS AND
AFFILIATIONS * Mathematical Neuroscience Unit, Institute for Frontier Science Initiative, Kanazawa University, Kanazawa, Ishikawa, Japan Miaoxing Wang, Xujun Han, Rie Takayama, Tetsuo Yasugi
& Makoto Sato * Laboratory of Developmental Neurobiology, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Ishikawa, Japan Chuyan Liu & Makoto Sato * Department
of Mathematics, Faculty of Science, Hokkaido University, Sapporo, Hokkaido, Japan Shin-Ichiro Ei * Research Institute for Electronic Science, Research Center of Mathematics for Social
Creativity, Hokkaido University, Sapporo, Hokkaido, Japan Masaharu Nagayama * Department of Complex and Intelligent Systems, School of Systems Information Science, Future University
Hakodate, Hakodate, Hokkaido, Japan Yoshitaro Tanaka Authors * Miaoxing Wang View author publications You can also search for this author inPubMed Google Scholar * Xujun Han View author
publications You can also search for this author inPubMed Google Scholar * Chuyan Liu View author publications You can also search for this author inPubMed Google Scholar * Rie Takayama View
author publications You can also search for this author inPubMed Google Scholar * Tetsuo Yasugi View author publications You can also search for this author inPubMed Google Scholar *
Shin-Ichiro Ei View author publications You can also search for this author inPubMed Google Scholar * Masaharu Nagayama View author publications You can also search for this author inPubMed
Google Scholar * Yoshitaro Tanaka View author publications You can also search for this author inPubMed Google Scholar * Makoto Sato View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS M.W. and M.S. conceived and designed the experiments. X.H., C.L., R.T., T.Y., and M.S. performed experiments. M.W. and M.S. acquired, analyzed,
and interpreted the data. S.-I.E., M.N., Y.T., and M.S. formulated the mathematical models. M.W. and M.S. wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Makoto Sato. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks David Sprinzak, Thomas Vaccari and
the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, M., Han, X.,
Liu, C. _et al._ Intracellular trafficking of Notch orchestrates temporal dynamics of Notch activity in the fly brain. _Nat Commun_ 12, 2083 (2021).
https://doi.org/10.1038/s41467-021-22442-3 Download citation * Received: 17 February 2020 * Accepted: 16 March 2021 * Published: 07 April 2021 * DOI:
https://doi.org/10.1038/s41467-021-22442-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative