Identification of genes associated with cortical malformation using a transposon-mediated somatic mutagenesis screen in mice
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Mutations in genes involved in the production, migration, or differentiation of cortical neurons often lead to malformations of cortical development (MCDs). However, many genetic mutations
involved in MCD pathogenesis remain unidentified. Here we developed a genetic screening paradigm based on transposon-mediated somatic mutagenesis by in utero electroporation and the
inability of mutant neuronal precursors to migrate to the cortex and identified 33 candidate MCD genes. Consistent with the screen, several genes have already been implicated in neural
development and disorders. Functional disruption of the candidate genes by RNAi or CRISPR/Cas9 causes altered neuronal distributions that resemble human cortical dysplasia. To verify
potential clinical relevance of these candidate genes, we analyzed somatic mutations in brain tissue from patients with focal cortical dysplasia and found that mutations are enriched in
these candidate genes. These results demonstrate that this approach is able to identify potential mouse genes involved in cortical development and MCD pathogenesis.
Development of the mammalian cerebral cortex is a complex dynamic process that can be broken down into a number of partially overlapping stages during gestation. The neuroepithelial cells
(NEPs) first form the pseudostratified neural tube and subsequently transform into radial glia cells (RGCs) as cortical neurogenesis starts1,2. Cortical neurons generated directly or
indirectly from RGCs in the ventricular zone (VZ) then migrate along radial fibers to form the highly organized cortical layers3,4,5. The timing and dynamics of these cellular processes
require precise genetic regulations; any perturbations may lead to cortical malformations6,7,8. In human, malformations of cortical development (MCDs) often result in pediatric neurological
dysfunctions presented by epilepsy, intellectual disability, developmental delay, and even autism9.
To date, the genetic causes of a number of MCDs have been identified, including microcephaly (e.g., MCPH1, ASPM, CPAP, CDK5RAP2, and STIL), lissencephaly (e.g., LIS1, DCX, ARX, and TUBA1A),
double cortex (e.g., DCX), periventricular nodular heterotopia (e.g., ARFGEF2), and tuberous sclerosis (e.g., TSC1 and TSC2)10,11,12. Recently, deep whole-exome sequencing (WES) has
uncovered somatic mutations of genes involved in the mTOR pathway to cause focal cortical dysplasia (FCD)13,14,15,16, a form of MCDs characterized by localized cortical malformation17.
However, many other genes potentially involved in cortical development and MCD pathogenesis remain unidentified15.
To identify new genes potentially involved in cortical development and the pathogenesis of MCDs, we took advantage of forward genetic screening by somatic mutagenesis during brain
development. Previously, transposons have been utilized for mutagenesis attributed to their ability to randomly insert into the genome and cause mutations. Particularly, piggyBac (PB), a
transposon originally found in the cabbage looper moth Trichoplusia ni, shows high mobility when introduced into mammalian cells18. In addition, the insertion sites in the genome are
relatively easy to identify—compared to radiation and chemical mutagenesis. Thus PB is an effective way of forward genetic screens in a range of model organisms19,20,21.
Here we developed an in vivo genetic screen paradigm that utilizes in utero electroporation of PB transposon into mouse embryos to induce insertional mutations in RGCs. Using this method, we
identified dozens of candidate genes potentially involved in cortical development. Combining with bioinformatics analysis, RNA interference (RNAi) and CRISPR/Cas9 technologies, we were able
to verify their potential roles in the development of the cerebral cortex. To explore the clinical relevance of these candidate genes, we analyzed the somatic mutations present in brain
tissue from human FCD patients and found that somatic mutations coincide with many of these candidate genes. These findings demonstrate that our method is able to identify new genes that are
involved in cortical development and associated with neurodevelopmental disorders.
To identify potential genes involved in cortical development, we established a genetic screening paradigm by combining transposon mutagenesis and in utero electroporation (Fig. 1). The
transposable element PB with its corresponding transposase were delivered into neural stem cells (i.e., RGCs) in the developing neocortex by in utero electroporation at embryonic day 14.5
(E14.5), at which time cortical neurogenesis was most active22. Under the activity of transposase, the transposon could potentially cause insertional mutations in the genomes of neural stem
cells and, subsequently, their progenies (Fig. 1a). When the insertion occurs within a gene, it likely results in disrupted or hypomorphic alleles due to the large size (~11 kb) of the
inserted PB. As the normal progeny cells differentiate and migrate to the cortex, cells carrying mutations that cause defects in neuronal development and/or migration would stay in the VZ
and SVZ after birth. This altered cell distribution allowed us to isolate defective cells and identify potential genes important for this process (Fig. 1b).
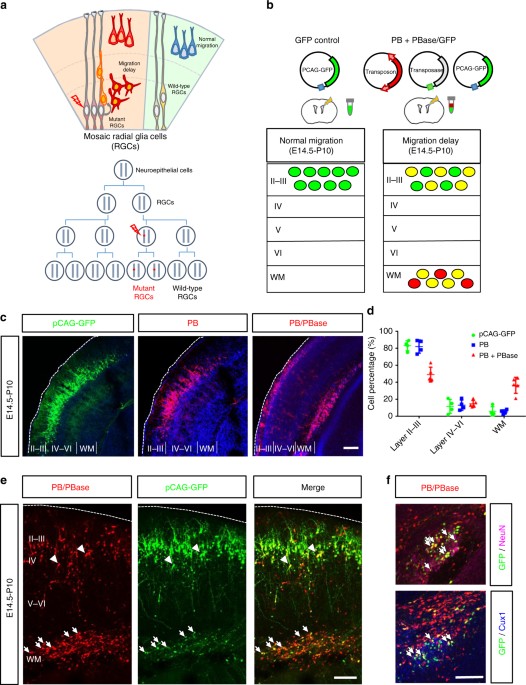
Altered neuronal distribution during cortical development by piggyBac transposon mutagenesis. a Top: a schematic diagram of effects of somatic mutations in cortical development. While normal
GRCs produce neurons that migrate normally to the cortex, RGCs that carry detrimental mutations may cause migration delay of their progeny. Bottom: early somatic mutation events lead to a
large number of mutant cells carrying the same mutation through clonal expansion. b A schematic diagram of the PB-induced neuronal migration delay in the developing mouse cortex. The brains
were electroporated with GFP (green) alone or with PB and PBase (red) at E14.5 and the distribution of cells was assessed at P10. c In mouse brains electroporated with pCAG-GFP (green, left
panel) or PB (red, center panel) at E14.5, the labeled cells were primarily found in layer 2/3 of the cerebral cortex at P10. In contrast, an additional ectopic layer of cells was found
beneath the cortex in brains electroporated with PB and PBase (right panel), this combination allowing the insertion of PB into the genome. Bar = 200 μm. d Cell distributions in different
brain regions of the cerebral cortex. Control pCAG-GFP: n = 4 animals; PB: n = 4 animals; PB + PBase: n = 6 animals. *: p