Estimated glomerular filtration ratio is a better index than creatinine clearance (cockcroft–gault) for predicting the prevalence of atrial fibrillation in the general japanese population
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Direct oral anti-coagulants (DOACs) have been used in patients with non-valvular atrial fibrillation (AF), and renal function evaluation using the CCr (Cockcroft–Gault) is
recommended as a criterion for the reduction of DOAC. In contrast, estimated glomerular filtration rate (eGFR) is usually used as an index of renal function in daily practice. We determined
the age- and gender-specific prevalence rates of AF and whether CCr or eGFR was associated with the prevalence of AF. Data from the periodic health examinations of 108,951 subjects were
collected. Risk factors for AF were determined based on medical history, physical examinations and blood samples, and AF was diagnosed based on electrocardiography. The prevalence rate of AF
was 0.92% (998/108,951). It was four times higher in men than in women and increased with age. Cardiac disease (odds ratio (OR) = 27.07, confidence interval (CI) 23.39–31.37, _p_ = 0.0001),
male gender (OR = 3.65, CI 3.11–4.30), age > 65 years (OR = 2.52, CI 2.14–2.96), hyperlipidemia (OR = 2.51, CI 1.97–3.20), BMI > 25 kg/m2 (OR = 1.37, CI 1.19–1.58) and hypertension
(OR = 1.14, CI 1.11–1.16) were independently associated with a high risk of AF in the multivariate logistic regression analysis. The odds ratio of having AF was significantly higher in
patients with eGFR ≤ 59 (OR = 2.10, CI 1.21–3.86) than in those with eGFR ≥ 90 but was not associated with CCr after adjustments for age, gender, diabetes mellitus and smoking. The
significance of this difference disappeared after additional adjustment for hypertension. Cardiac disease, gender, age, hyperlipidemia, obesity, hypertension and renal dysfunction were
strong risk factors for AF. The evaluation of renal dysfunction as a morbidity risk factor for AF suggests that eGFR should be used. You have full access to this article via your
institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS DIFFERENT CARDIOVASCULAR RISKS ASSOCIATED WITH ELEVATED CREATININE-BASED EGFR AND CYSTATIN C-BASED EGFR Article Open access
02 May 2024 INVERSE RELATIONSHIP BETWEEN LDL-C/HDL-C RATIO AND ATRIAL FIBRILLATION IN CHRONIC KIDNEY DISEASE PATIENTS Article Open access 31 July 2024 IMPACT AND CONSEQUENCES OF THE ERROR OF
ESTIMATED GFR IN PATIENTS WITH HEART FAILURE Article Open access 28 October 2024 INTRODUCTION Atrial fibrillation (AF) is the most common cardiac arrhythmia, especially in older adults [1].
AF is frequently observed as a comorbid disease in patients with hypertension; it is induced by several risk factors, such as aging, left ventricular hypertrophy and left atrial enlargement
[2]. Aging, hypertension and diabetes mellitus are also associated with chronic kidney disease (CKD), which carries a risk of AF [3] and has a considerable impact on its prognosis [4, 5].
Recently, direct oral anti-coagulants (DOACs) have been used in practical medicine, and impaired renal function may lead to bleeding complications in patients taking DOACs [6, 7]. The index
of renal function is the creatinine clearance (CCr); the Cockcroft–Gault equation was specified in the United States Food and Drug Administration’s approved prescribing information for DOACs
[8]. Although studies were limited to evaluating the relationship between CCr and adverse clinical outcomes in AF patients taking anti-coagulants [9,10,11], the recent sub-analysis of the
Fushimi AF registry demonstrated that the group with a CCr < 30 ml/min had significantly higher stroke, systemic embolization and major bleeding rates than the other groups (30 < CCr
< 50 ml/min, 50 ml/min < CCr) in the cohort and the group of patients who were not taking oral anti-coagulants [12]. In contrast, for the early detection of kidney disease, a simple
index—the estimated glomerular filtration rate (eGFR), which is calculated using creatinine and age—has been used, and the lower eGFR levels have been independently associated with the risks
of death, cardiovascular events and hospitalization [13]. However, to our knowledge, no studies have compared the value of CCr and eGFR for determining the risk of AF onset. Moreover, there
is limited current data regarding the prevalence of AF and the risk factors for AF in a large cohort. Therefore, we determined the age- and gender-specific prevalence rates of AF,
identified the risk factors for AF, and compared eGFR and CCr to determine which was the better index for evaluating renal function in association with the risk of AF onset. METHODS The
subjects had periodic health examinations as community residents and employees of companies and governments from April 2013 to March 2014 in Tochigi Prefecture, Japan. All subjects gave
their written informed consent. The study design was approved by the ethics committee of the Tochigi Public Health Service Association, and data were collected from the database of this
institution. The ethics committee of Dokkyo Medical University also approved study protocol according to the Declaration of Helsinki. Symptoms and medical history, including hypertension,
diabetes mellitus, dyslipidemia, cardiac disease and smoking habits, were collected via a questionnaire. Each item was answered with one of four response categories, as follows: currently
being treated, under follow-up, completed treatment and no history of treatment. The medical history was positive if the participants indicated a history of more than one item. Habitual
smoking was defined as a positive history of smoking within the past 5 years. A physical examination that included blood pressure, electrocardiography, blood sampling (serum creatinine,
fasting glucose, hemoglobin A1c, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, uric acid and hemoglobin), body weight and height was
performed. Blood pressure was measured in the sitting and resting position after a few minutes. Body mass index (BMI) was calculated as weight times the square of height. Hypertension was
defined as current treatment with antihypertensive drugs and/or systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg at the health check-up. Diabetes mellitus was
defined as current treatment with oral hypoglycemic drugs and/or insulin, glucagon-like peptide-1 agonist or fasting blood glucose ≥ 126 mg/dl or/and HbA1c ≥ 6.5%. Dyslipidemia was defined
as current treatment with hypolipidemic drugs or serum LDL cholesterol ≥ 140 mg/dl and/or HDL cholesterol < 40 mg/dl and/or triglyceride ≥ 150 mg/dl. eGFR was calculated by using the
three-variable Japanese equation: eGFR (ml/min/1.73 m2) = 194 × age-0.287 × serum creatinine-1.094 × 0.739 (if female) [14]. Renal function was categorized by eGFR level as ≥90, 60–89 and
≤59 [15]. CCr was also calculated by using the Cockcroft–Gault equation: CCr (ml/min) = ((140–age) × body weight)/(72 × serum creatinine) × 0.85 (if female) [16]. Renal function was also
categorized by CCr level as ≥ 80, 50–79 and <50 [17,18,19]. AF was diagnosed by using the automatic computerized analysis performed by the ECG recorder (FCP-7431, Fukuda Denshi, Tokyo,
Japan) at the time of the health examination. Conventional diagnostic criteria for AF (i.e., an irregular ventricular rhythm of supraventricular origin, no visible P wave and irregular
fluctuation of the baseline) were employed. Almost all the subjects with AF who were diagnosed by the automatic ECG system were followed up by the hospital to confirm the diagnosis.
STATISTICAL ANALYSIS All continuous data are expressed as the mean ± SD. Categorical data are presented as proportions (%). Differences in baseline characteristics between groups were
analyzed using Student’s _t_-test or analysis of variance (ANOVA) for continuous variables. The variables potentially associated with AF risk were analyzed by multivariate logistic
regression analysis. Chi-square test was used for analyzing categorical variables reported as percentages (%). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated in all
the regression analyses. Multivariate regression analysis was used to evaluate the association between eGFR, CCr and the prevalence of AF. First, all associations were adjusted by age and
gender and then by hypertension, diabetes mellitus, cardiac disease and smoking. The relationships between eGFR or CCr and age or BMI were assessed by Pearson’s correlation coefficient
(_r_). Statistical analysis was performed using JMP 10.0 software (SAS Institute, Cary, NC, USA). Statistical significance was accepted at _p_ < 0.05. RESULTS A total of 108,951 subjects
were included in this cross-sectional study. Men and women accounted for 54,645 and 54,306 participants, respectively, which were almost equal proportions. The total prevalence rate of AF
was 0.92% (998/108,951), and the rate was four times higher in men than in women (1.46% vs. 0.37%, _p_ < 0.0001). The prevalence of AF for each 10-year increase in age for men and women
is shown in Table 1. Although the prevalence of AF increased with age, it was higher for men than for women in each age group. Of note, AF was rare in men <60 years old (95/32,407: 0.29%)
and in women <70 years old (62/44,609: 0.14%). The baseline clinical characteristics of subjects with or without AF are shown in Supplementary table 1. Almost one-third of the men and
the half of the women with AF reported experiencing palpitations or feeling an irregular pulse. Hypertension, diabetes mellitus, dyslipidemia and cardiac disease were more frequently
comorbid in subjects with AF than in those without AF in both genders (_p_ < 0.0001 for both); however, there were fewer smokers with AF than without AF in both men (_p_ < 0.0001) and
women (_p_ < 0.05). Cardiac disease (OR = 27.07, CI 23.39–31.37, _p _= 0.0001), male gender (OR = 3.65, CI 3.11–4.30), age >65 years (OR = 2.52, CI 2.14–2.96, _p _= 0.0001),
hyperlipidemia (OR = 2.51, CI 1.97–3.20, _p_ = 0.0001), BMI > 25 kg/m2 (OR = 1.37, CI 1.19–1.58, _p_ = 0.0004) and hypertension (OR = 1.14, CI 1.11–1.16, _p_ = 0.001) were independently
associated with a high risk of AF in the multivariate logistic regression analysis, as shown in Table 2. Furthermore, the logistic regression analysis of the continuous variables revealed
that SBP (OR = 1.07, CI 1.03–1.11, _p _< 0.0001) at the time of the health examination was only positively associated with the presence of AF. The baseline clinical characteristics when
CKD was categorized by the eGFR level as ≥90, 60–89 and <59 are shown in Table 3. A total of 85,414 subjects for whom serum creatinine data were available could be analyzed, and a total
of 884 subjects with AF were identified (1.04%). As eGFR decreased, the prevalence of AF, as well as hypertension, dyslipidemia, cardiac disease, SBP increased, additionally, eGFR decreased
with increasing age; however, the body weights of the groups were similar, as shown in Table 3. If the OR of having AF at an eGFR ≥ 90 was calculated as 1, there were significantly high
risks at eGFRs of 60–89 (OR = 1.53, CI 1.14–2.12) and eGFR ≤ 59 (OR = 2.61, CI 1.95–3.75) after adjustment for age and gender. After additional adjustment for diabetes mellitus and smoking,
there was still a significantly high risk at eGFR ≤ 59 (OR = 2.10, CI 1.21–3.86), as shown in Table 4. However, this significance disappeared after additional adjustment for hypertension.
Thus, hypertension was a strong covariate of the risk of having AF in subjects with eGFR ≤ 59. In contrast, the baseline clinical characteristics when the renal function was categorized by
the CCr level (≥80, 50–79, <50) are shown in Table 5. A total 84,931 subjects could be analyzed, and 861 subjects with AF were identified (1.01%). With decreasing CCr, the prevalence of
AF, as well as hypertension, dyslipidemia, cardiac disease and SBP increased, additionally, CCr decreased with increasing age. Of note, body weights decreased with decreasing CCr, as shown
in Table 5. If the OR of having AF at CCr ≥ 80 was calculated as 1, the ORs did not differ among the three groups after adjustment for age and gender (CCr 50–79: OR = 0.97, CI 0.82–1.15, CCr
< 50: OR = 0.91, CI 0.70–1.17), as shown in Table 6. After additional adjustment for diabetes mellitus and smoking, there was a significantly low risk at CCr < 50 (OR = 0.54, CI
0.30–0.95). However, the significance disappeared after additional adjustment for hypertension (OR = 0.70, CI 0.42–1.14). Indeed, eGFR was inversely correlated with BMI (_r _= –0.072, _p_
< 0.0001), and CCr was positively correlated with BMI (_r _= 0.458, _p_ < 0.0001) when the relationship among eGFR, CCr and BMI was analyzed for all subjects (Supplementary figure 1).
Therefore, the discrepancy between the eGFR and CCr results might be caused by differences in the body weight in terms of the BMI of each classification group, as shown in Tables 3 and 5.
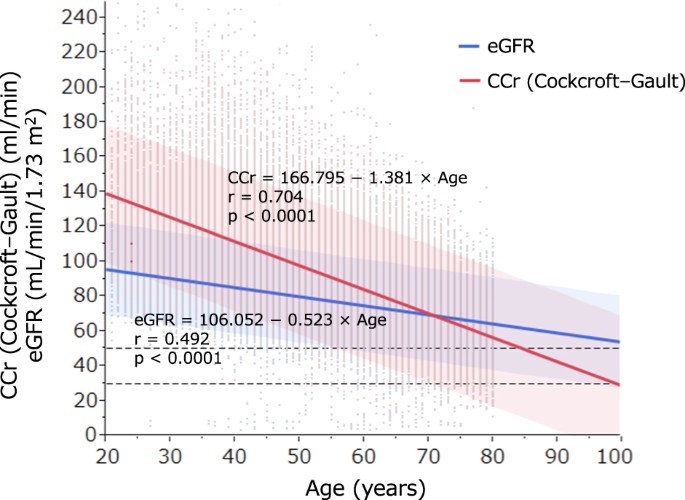
Finally, the associations between eGFR or CCr and age are shown in Fig. 1. Although the values of both indexes gradually decreased with age, CCr was higher than eGFR among participants
younger than 70 years. In contrast, CCr was lower than eGFR for those older than 70 years, and an inverse association was observed at age 70 years and younger. DISCUSSION This study
evaluated the prevalence of risk factors for AF by using a database of annual health check-up data for the general population living in central Japan. The multivariate logistic regression
analysis showed that male gender (OR = 3.65 vs. female) and age >65 years (OR = 2.52 vs.<65 years old) were independently associated with a high risk of AF in this study. The
prevalence of AF was 0.92% among participants aged ≥20 years. AF was rare in men <60 years old (0.29%) and in women <70 years old (0.14%), and the prevalence of AF was four times
higher in men than in women for the entire population (1.46% vs. 0.37%). Thus, AF is strongly associated with age and gender. The prevalences of AF for each decade increased in age for men
and women in the present study are comparable with those previously reported [16]. However, we found a higher prevalence of AF in men aged 60–80 years and women older than 80 years compared
with previous reports [20], which might reflect the one-decade difference between our study and the previous studies. In our country, the prevalence of AF has been predicted to rise with the
increased aging of the population [20]. BMI > 25 kg/m2 was also risk factor for AF (OR = 1.37) in this study. Obesity has been associated with an increased risk of AF; it was associated
with a 4–5% increased risk of AF over a mean follow-up of 14 year, independent of hypertension, diabetes mellitus and myocardial infarction in the Framingham Heart study [21]. This
relationship may be mediated by an increase in the left atrial diameter, which can be attenuated by weight loss [22]. Atrial stretch enhances the vulnerability of the atrium, which triggers
AF [23]. The Women’s Health study reported a linear relationship between BMI and AF, with a 5% increase in the risk of AF per 1-unit increase in BMI [24]. Although our data showed that
cardiac disease was a more frequent complication in both men and women with AF than in those without, cardiac disease was inferred from the questionnaire when patients selected more than one
item from the possible response categories (currently under treatment, under follow-up, completion of treatment and no treatment). Thus, participants may have been considering AF alone in
their response to the question regarding cardiac disease. For this reason, the rate of comorbidity with cardiac disease may be high for AF patients of both genders compared with previous
studies. In addition to cardiac disease, the complications of hypertension and diabetes mellitus were more than two times more common in both men and women with AF compared with those
without AF. Moreover, the multivariate logistic regression analysis demonstrated that hypertension (OR = 1.14 vs. normotension) was independently associated with a high risk of AF. It has
also been reported that hypertension is the most prevalent risk factor for AF [25,26,27] and is frequently complicated by AF [20]. Of note, the stepwise regression analysis of the continuous
variables revealed that SBP at the time of health examination (OR = 1.07) was independently associated with a high risk of AF in this study. The Cardio-Sis trial demonstrated that new-onset
AF occurred less often in the blood pressure tight control group (SBP < 130 mmHg) than in the usual control group (SBP < 140 mmHg) after 2 years of treatment in hypertensive patients
without diabetes mellitus [28]. Taken together, these data suggest that inadequate antihypertensive treatment is unable to prevent AF. Diabetes mellitus and other risk factors were also
comparable with previous reports [20]. Diabetes mellitus (OR = 2.38, _p_ < 0.0001) was a significant risk factor for AF in the simple logistic regression analysis but not in the
multivariate logistic regression analysis. It is possible that other confounding variables were more strongly associated with AF. In general, it has been reported that reverse epidemiology,
that is, the cholesterol paradox, does exist between the lipid profile and AF; that is, low levels of HDL cholesterol and LDL cholesterol or triglycerides are positively related to an
increase in AF [29, 30]. The mechanism of this relationship was suggested by Suzuki [31]. Although hyperlipidemia data were collected via a questionnaire, there was no association between
lipid profile factors, such as HDL and LDL cholesterol and triglyceride levels, at the time of the health examination and the prevalence of AF in this study (data are not shown). Although
the GFR is estimated with CCr in practice, the two indexes are strictly different. The Cockcroft–Gault formula (CCr), which uses age, body weight, serum creatinine and gender, has been
proposed [16]. At present, the United States Food and Drug Administration recommends that drug dosage be adjusted according to CCr in patients with impaired renal function, and it does not
approve of using eGFR in place of CCr for the renal dosing of drugs [8]. Previously, serum creatinine was measured by the Jaffe method, which yielded higher results than the current
enzymatic assay, which causes slight overestimation, especially at eGFR > 60 ml/min/1.73 m2 [32]. In contrast, Lindeman et al. [33] reported that the mean decrease in real CCr with age
was 0.75 ml/min/year, which is smaller than the predicted decrease for CCr (1.0 ml/min/year). Thus, CCr tends to underestimate real CCr in elderly subjects. In fact, however, our data
demonstrated that the CCr was lower than the eGFR in patients older than 70 years, whereas the inverse relationship was observed for those younger than 70 years. Moreover, the CCr value
varies greatly depending on body weight [16]. In the present study, a gradual decrease in body weight was associated with lower categories of CCr (Table 5). In contrast, the overall analysis
revealed that high BMI, which was positively correlated with CCr, was one of the most important risk factors for AF in our study. Therefore, the lower CCr category was associated with lower
body weight and female gender, which might explain the low prevalence of AF in these groups. Of note, CCr is an estimate of CCr and not GFR, and its ability to estimate GFR was suboptimal,
especially in cases of advanced kidney disease that included tubular creatinine secretion [34, 35]. Therefore, Levey et al. [36] published a new formula based on patients who had a GFR <
60 ml/min/1.73 m2 from the Modification of Diet in Renal Disease (MDRD) study. The formula was found to be superior to the Cockcroft–Gault formula for estimating GFR. However, the MDRD study
included primarily whites and African Americans. Matsuo et al. [37] reported that eGFRs obtained using the isotope-dilution mass spectrometry-traceable four-variable MDRD study equation
were significantly higher than GFRs based on inulin clearance in Japanese patients; they calculated a correction coefficient of 0.808 for the MDRD study equation and developed a new Japanese
equation for GFR estimation. Although the best overall index of renal function is the GFR [38], measuring the GFR is cumbersome and time consuming. eGFR is currently used by most clinical
laboratories as a reliable and convenient index for the diagnosis and evaluation of CKD. CKD is staged by levels of eGFR according to the KDIGO clinical practice guideline for CKD [15]. The
eGFR was calculated by adjusting for the standard body surface area (1.73 m2), and the value differs from the real value when the body weight or/and height deviate from standard values.
However, the mean body weight and height for the three eGFR categories in this study were almost equal (Table 3). Thus, the OR of having AF was significantly high in the lowest category of
eGFR (≤59) compared with the highest category of eGFR (≥90) after adjustment for age, gender, diabetes mellitus and smoking in the multivariate logistic regression analysis (OR = 2.10, CI
1.21–3.86, Table 4). Recently, Ohyama et al. [39] also reported that eGFR ≤ 59 ml/min/m2 was strongly associated with AF after adjustment for age, gender, diabetes mellitus, smoking,
hypertension and cardiac disease in the multivariate logistic regression analysis of the community-based population from Gunmma prefecture. The discrepancies between our results and those of
the studies cited above may be explained by the differences in the participants’ age (56.5 vs. 53.2 years old) and/or gender (male: 48.2% vs. 62.0%) and/or the number of subjects (20,091
vs. 85,414), any of which could have an effect on the prevalence of AF. Another possible explanation is that the prevalence of AF in the high GFR group was too small (6/3,148 subjects,
0.19%) and presented a bias in the latter study compared with our study (44/15,491 subjects, 0.28%). In our study, eGFR but not CCr was significantly associated with AF, a correlation that
has been previously suggested in our country [40, 41]. However, this significant association disappeared after adjustment of the confounding variables, such as cardiac disease and
hypertension, in the multivariate regression analysis. Thus, these variables were considered closely connected with eGFR. Both AF and flutter were defined as AF in this study because only 13
patients (1.3%) were diagnosed with atrial flutter by the automated computerized analysis. Moreover, atrial flutter and fibrillation sometimes coexist as they are the electrical consequence
of the same arrhythmogenic substrate. The frequency of coexistence of these two arrhythmias is not easily predictable because both are often silent. Randomized controlled trials of AF have
also included patients with atrial flutter [42, 43]. LIMITATIONS Various limitations apply to the present study. First, this was a cross-sectional observation study. Thus, the mechanism
linking the prevalence of AF and risk factors for AF was not known. Second, the presence of AF was based on only a single standard 12-lead electrocardiographic recording. Almost of all cases
of paroxysmal AF were excluded from this study. Third, information regarding medical histories of risk factors for AF was only collected by questionnaire at the annual health check-up;
because the actual medical history was difficult to determine in some cases, information might be limited, which would have caused a bias in the results. Fourth, some patients taking
medications were included in this cohort, which may have influenced the prevalence of AF. Finally, although the eGFR equation used in the MDRD study was developed mainly with CKD patients,
its accuracy was still moderate, particularly in patients with GFR > 60 ml/min/1.73 m2 [36]; however, the calculated eGFR is lower when the MDRD equation is applied to healthy subjects.
To improve this weak point, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula, which uses a different estimation equation based on the value of serum creatinine, has
been devised in the United States [44]. However, the estimated error of the eGRF calculated using the modified CKD-EPI formula was greater than that calculated using the modified MDRD
formula in Japanese subjects with a GFR < 60 ml/min/1.73 m2 [45]. Thus, the eGFR calculated by the CKD-EPI formula is not suitable for Japanese subjects at present. CONCLUSION The
prevalence of AF has increased with the increasing age of the population in central Japan. Cardiac disease, gender, aging, hyperlipidemia, obesity, hypertension (especially in SBP) and renal
dysfunction were strong risk factors for AF. The identification of renal dysfunction as a morbidity risk for AF suggests that eGFR should be used instead of CCr (Cockcroft–Gault formula) in
the general population in this area. REFERENCES * Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national
implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. Article PubMed CAS Google
Scholar * Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults.
Circulation. 1997;96:2455–61. Article PubMed CAS Google Scholar * Shang W, Li L, Huang S, Zeng R, Huang L, Ge S, Xu G. Chronic kidney disease and the risk of new-onset atrial
fibrillation: a meta-analysis of prospective cohort studies. PLoS ONE. 2016;11:e0155581. Article PubMed PubMed Central CAS Google Scholar * Sarnak MJ, Levey AS, Schoolwerth AC, Coresh
J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular
Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the
American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–65.
Article PubMed CAS Google Scholar * Olesen JB, Lip GY, Kamper AL, Hommel K, Køber L, Lane DA, Lindhardsen J, Gislason GH, Torp-Pedersen C. Stroke and bleeding in atrial fibrillation with
chronic kidney disease. N Engl J Med. 2012;367:625–35. Article PubMed CAS Google Scholar * Harder S. Renal profiles of anticoagulants. J Clin Pharmacol. 2012;52:964–75. Article PubMed
CAS Google Scholar * Harel Z, Sholzberg M, Shah PS, Pavenski K, Harel S, Wald R, Bell CM, Perl J. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with
CKD. J Am Soc Nephrol. 2014;25:431–42. Article PubMed PubMed Central CAS Google Scholar * Dowling TC, Matzke GR, Murphy JE, Burckart GJ. Evaluation of renal drug dosing: prescribing
information and clinical pharmacist approaches. Pharmacotherapy. 2010;30:776–86. Article PubMed CAS Google Scholar * Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M,
Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation:
insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–30. Article PubMed CAS Google Scholar * Apostolakis S, Guo Y, Lane DA, Buller H, Lip GY. Renal function and outcomes in
anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur Heart J. 2013;34:3572–9. Article PubMed CAS Google Scholar * Piccini JP, Stevens SR, Chang Y, Singer
DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM, ROCKET AF Steering Committee and Investigators. .
Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban
Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk
factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–32. Article PubMed CAS Google Scholar * Abe M, Ogawa H, Ishii M, Masunaga N, Esato M, Chun YH, Tsuji H, Wada H,
Hasegawa K, Lip GY, Akao M. Relation of stroke and major bleeding to creatinine clearance in patients with atrial fibrillation (from the Fushimi AF Registry). Am J Cardiol. 2017;119:1229–37.
Article PubMed CAS Google Scholar * Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J
Med. 2004;351:1296–305. Article PubMed CAS Google Scholar * Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing
the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. Article PubMed CAS Google Scholar * Kidney
Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int.
2013;3(Supple):19–62. Google Scholar * Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. Article PubMed CAS Google Scholar *
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC,
Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. Article PubMed CAS
Google Scholar * Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf
RM, ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. Article PubMed CAS Google Scholar * Granger CB, Alexander JH,
McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S,
Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators.
Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. Article PubMed CAS Google Scholar * Inoue H, Fujiki A, Origasa H, Ogawa S, Okumura K, Kubota
I, Aizawa Y, Yamashita T, Atarashi H, Horie M, Ohe T, Doi Y, Shimizu A, Chishaki A, Saikawa T, Yano K, Kitabatake A, Mitamura H, Kodama I, Kamakura S. Prevalence of atrial fibrillation in
the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–7. Article PubMed Google Scholar * Wang TJ, Parise H, Levy D, D’Agostino RB
Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. Article PubMed CAS Google Scholar * Garza CA, Pellikka PA, Somers VK,
Sarr MG, Seward JB, Collazo-Clavell ML, Oehler E, Lopez-Jimenez F. Major weight loss prevents long-term left atrial enlargement in patients with morbid and extreme obesity. Eur J
Echocardiogr. 2008;9:587–93. Article PubMed PubMed Central Google Scholar * Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial
fibrillation. Circulation. 2000;101:2200–5. Article PubMed CAS Google Scholar * Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and
short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55:2319–27. Article PubMed PubMed Central
Google Scholar * Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart
Study. JAMA. 1994;271:840–804. Article PubMed CAS Google Scholar * Grundvold I, Skretteberg PT, Liestøl K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Upper normal blood
pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension. 2012;59:198–204. Article PubMed CAS Google Scholar * Son MK, Lim NK,
Cho MC, Park HY. Incidence and risk factors for atrial fibrillation in Korea: the National Health Insurance Service Database (2002-2010). Korean Circ J. 2016;46:515–21. Article PubMed
PubMed Central Google Scholar * Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G, Cardio-Sis investigators. Usual
versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–33. Article PubMed Google
Scholar * Watanabe H, Tanabe N, Yagihara N, Watanabe T, Aizawa Y, Kodama M. Association between lipid profile and risk of atrial fibrillation. Circ J. 2011;75:2767–74. Article PubMed CAS
Google Scholar * Iguchi Y, Kimura K, Shibazaki K, Aoki J, Kobayashi K, Sakai K, Sakamoto Y. Annual incidence of atrial fibrillation and related factors in adults. Am J Cardiol.
2010;106:1129–33. Article PubMed Google Scholar * Suzuki S. “Cholesterol paradox” in atrial fibrillation. Circ J. 2011;75:2749–50. Article PubMed CAS Google Scholar * Schmidt RL,
Straseski JA, Raphael KL, Adams AH, Lehman CM. A risk assessment of the Jaffe vs enzymatic method for creatinine measurement in an outpatient population. PLoS ONE. 2015;10:e0143205. Article
PubMed PubMed Central CAS Google Scholar * Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc. 1985;33:278–85.
Article PubMed CAS Google Scholar * Delanaye P, Pottel H, Botev R, Inker LA, Levey AS. Con: should we abandon the use of the MDRD equation in favour of the CKD-EPI equation? Nephrol Dial
Transplant. 2013;28:1396–403. Article PubMed Google Scholar * Hallan SI, Gansevoort RT. Moderator’s view: estimating glomerular filtration rate--the past, present and future. Nephrol
Dial Transplant. 2013;28:1404–6. Article PubMed Google Scholar * Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate
from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. Article PubMed CAS Google Scholar * Matsuo S, Imai
E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR
from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. Article PubMed CAS Google Scholar * Smith HW. The kidney: structure and functional in health and disease. New York:
Oxford University Press Inc.; 1951. pp. 1–1049. Google Scholar * Ohyama Y, Imai M, Kurabayashi M. Estimated glomerular filtration rate and proteinuria are separately and independently
associated with the prevalence of atrial fibrillation in general population. PLoS ONE. 2013;8:e79717. Article PubMed PubMed Central CAS Google Scholar * Iguchi Y, Kimura K, Kobayashi K,
Aoki J, Terasawa Y, Sakai K, Uemura J, Shibazaki K. Relation of atrial fibrillation to glomerular filtration rate. Am J Cardiol. 2008;102:1056–9. Article PubMed Google Scholar * Watanabe
H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am
Heart J. 2009;158:629–36. Article PubMed Google Scholar * Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R,
Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P,
Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. Article
PubMed CAS Google Scholar * Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko
A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R,
Yusuf S, AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. Article PubMed CAS Google Scholar * Levey AS,
Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions.
Am J Kidney Dis. 2010;55:622–7. Article PubMed PubMed Central Google Scholar * Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration
(CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–38. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study
was a joint effort of many investigators and staff members whose contribution is gratefully acknowledged. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Cardiology and
Nephrology, Dokkyo Medical University, 880 Mibu, Tochigi, 321-0293, Japan Yutaka Yonezawa & Shigeo Horinaka * Tochigi Public Health Service Association, 3337-1 Komanyu, Utsunomiya,
Tochigi, 320-8503, Japan Chiaki Shirakawa & Yoshio Kogure Authors * Yutaka Yonezawa View author publications You can also search for this author inPubMed Google Scholar * Shigeo Horinaka
View author publications You can also search for this author inPubMed Google Scholar * Chiaki Shirakawa View author publications You can also search for this author inPubMed Google Scholar
* Yoshio Kogure View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Shigeo Horinaka. ETHICS DECLARATIONS CONFLICT OF
INTEREST The authors declare that they have no conflict of interest. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY TABLE 1(PPTX 46 KB) SUPPLEMENTARY FIGURE 1(PPTX 90 KB) RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yonezawa, Y., Horinaka, S., Shirakawa, C. _et al._ Estimated glomerular filtration ratio is a better index than
creatinine clearance (Cockcroft–Gault) for predicting the prevalence of atrial fibrillation in the general Japanese population. _Hypertens Res_ 41, 451–459 (2018).
https://doi.org/10.1038/s41440-018-0032-6 Download citation * Received: 29 May 2017 * Revised: 16 September 2017 * Accepted: 27 September 2017 * Published: 20 March 2018 * Issue Date: June
2018 * DOI: https://doi.org/10.1038/s41440-018-0032-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative