Outcome of intravitreal avastin® injections in patients with macular oedema in uganda: a cohort study
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND To determine the outcome of Intravitreal Avastin (IVA) injections in patients with Macular Oedema (MO) in Uganda. METHODS We prospectively recruited patients presenting
with MO at the Department of Ophthalmology of Mbarara University of Science and Technology in Southern Uganda from November 2018 to April 2019. We treated them with intravitreal injection of
Bevacizumab (Avastin®) and followed them up for three consecutive months after the initial injection. We collected information on baseline clinical presentation and 3 month outcomes. We
performed a Student’s t-test to compare central macular thickness (CMT) and best corrected visual acuity (BCVA) at baseline and at 3 months after IVA injections. We performed linear
regression to test for predictors of change in CMT and BCVA at 3 months. RESULTS We enroled 32 patients (35 eyes) of which 29 patients (32 eyes) completed the follow up. The mean age was
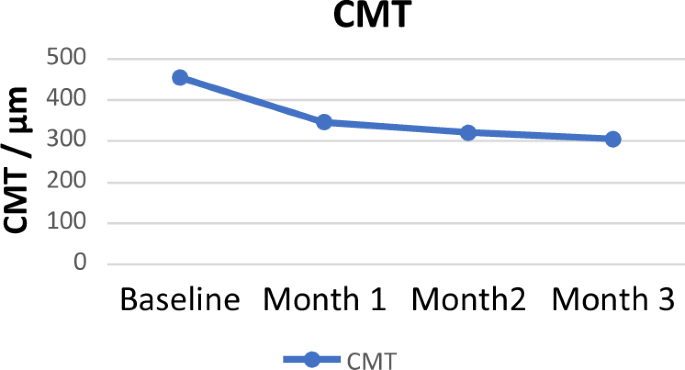
62.8 ± 11.8 years, and 53% were male. At 3 months after IVA, the mean CMT improved significantly from 426.90 ± 135.9 µm at baseline to 311.20 ± 134.80 µm (_p_ = 0.0008). The mean BCVA
improved from 0.70 ± 0.38 at baseline to 0.38 ± 0.36 logMAR units (_p_ = 0.003). The improvement in CMT and BCVA were more marked in patients who had Diabetic ME compared to other causes. A
high baseline CMT was a strong predictor of improvement in CMT at 3 months after IVA therapy. A worse baseline visual acuity was a predictor of improvement in vision at 3 months after IVA.
CONCLUSIONS IVA therapy results in anatomical and visual improvement at 3 months especially in patients with Diabetic MO. Having a high baseline CMT was a predictor of good CMT outcome at 3
months while a worse vision at baseline was a predictor of better visual outcome at 3 months. SIMILAR CONTENT BEING VIEWED BY OTHERS OUTCOMES IN PATIENTS WITH RETINAL VEIN OCCLUSION WITH
GOOD BASELINE VISUAL ACUITY Article 22 March 2023 EVALUATING INITIAL RESPONSES TO BROLUCIZUMAB IN PATIENTS UNDERGOING CONVENTIONAL ANTI-VEGF THERAPY FOR DIABETIC MACULAR EDEMA: A
RETROSPECTIVE, SINGLE-CENTER, OBSERVATIONAL STUDY Article Open access 05 July 2023 AN OPEN-SOURCE DATA SET OF ANTI-VEGF THERAPY IN DIABETIC MACULAR OEDEMA PATIENTS OVER 4 YEARS AND THEIR
VISUAL ACUITY OUTCOMES Article 26 June 2020 INTRODUCTION Macular Oedema (MO) is an abnormal thickening of the macula due to the accumulation of excess fluid in the extracellular space of the
retina [1]. MO occurs as a result of abnormal retinal vascular permeability and break down of the blood-retinal barrier mainly mediated by vascular endothelial growth factors (VEGF) and
other cytokines [2]. Weakened and blocked retinal vessels allow extravasation and accumulation of fluid within the retinal tissue at the macular area. It results in retinal hypoxia with
overexpression of VEGF, which in turn, will increase vascular permeability and enhance MO [1,2,3]. MO is caused by a variety of ophthalmic conditions such as diabetic retinopathy (DR),
retinal vein occlusion (RVO), intraocular inflammation (uveitis), and pseudophakia [1]. Diabetic macular oedema (DMO) is the commonest type of MO. Globally, the prevalence of DMO is
estimated at 7.48%. In Africa, it has been reported to be 3.2% in South Africa and 4.1% in Kenya [4]. These estimates are expected to rise further with the increasing prevalence of diabetes
mellitus (DM) and the increased life expectancy of DM patients [4]. MO may be treated with intravitreal injection of anti-VEGF drugs. Bevacizumab (Avastin®; Genentech, South San Francisco,
California) is a recombinant humanised monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human VEGF. It is an anti-VEGF drug approved by the Food and Drug
Administration for the treatment of metastatic colorectal, ovarian, and many other cancers [5]. Avastin is currently used as an off-label drug in intravitreal administration for the
treatment of wet age-related macular degeneration (ARMD), DMO, and cystoid macular oedema (CMO). CMO is a variant of MO which is most commonly caused by inflammatory processes within the eye
that cause multiple cyst-like (cystoid) areas of fluid to appear in the macula and cause oedema. Several studies have reported the effectiveness of Avastin in the treatment of MO caused by
retinal vascular disorders as well as ARMD [3, 6]. Avastin is widely used because it is widely available, relatively cheap and comparable to other approved anti-VEGF drugs (Ranibizumab and
Aflibercept) [7]. In Uganda, a modest proportion of patients with MO are treated with IVA. However, the response to this treatment in a predominantly black population has not been
systematically presented. The purpose of this study was to determine the outcomes of IVA injections in patients with MO in Uganda, three months after treatment, and to investigate predictors
of a good response. METHODS ETHICS STATEMENT This study adhered to the tenets of the Helsinki declaration. Approval of the study was obtained from the institutional ethical committee
(Ref:MUREC 1/7) and informed consent was obtained from all patients before enrolment. DESIGN This was a prospective cohort study conducted at Mbarara University and Referral Hospital Eye
Centre (MUHREC), Mbarara, South-Western Uganda. All patients presenting to MURHEC with MO from November 2018 to April 2019 were recruited. CASE DEFINITION MO was defined as a central macular
thickness (CMT) of above 250 µm or a perifoveal thickness of above 320 µm as determined by Optical Coherence Tomography ([OCT] [cirrus HD-OCT 500, carl Zeiss, Germany]). All patients who
had retinal changes were subjected to OCT screening for MO by any ophthalmologist in the clinic. Those who had MO were sent to the principal investigator for further examination and
assessment of eligibility criteria. INCLUSION We included all individuals aged 18 and above with OCT evidence of MO. EXCLUSION We excluded all patients with other retinal visual impairing
conditions such as glaucoma, optic atrophy, retinitis pigmentosa and macular dystrophies; all patients whose fundus assessment was impossible due to media opacity and those who had received
laser therapy. VARIABLES We collected data on demographics and also on past medical and ocular history. Random blood sugar was measured. All participants underwent full ocular examination
including Best Corrected Visual Acuity (BCVA) using Snellen’s chart and recorded as logMAR, intraocular pressure (IOP) measurement with Goldman applanation, slit lamp biomicroscopy and
dilated fundoscopy using a 90D lens. The OCT testing using a spectral-domain OCT (Cirrus HD-OCT 500, Carl Zeiss, Germany) was perfomed thereafter. The cause of MO was determined clinically,
after a dilated fundoscopy, by the principal investigator, and confirmed by a medical retina specialist. Participants were then treated with Avastin in the operating theatre. The main
outcome measures were CMT and BCVA at 3 months after the IVA injections. Vision was classified as “Normal vision” >6/9, “mild visual impairment (VI)” 6/12-6/18, “moderate VI” 6/18-6/60,
“severe VI” 6/60-3/60 and, “Blind” 3/60 [8]. IVA PROCEDURE In the operating room, tetracaine 1% was instilled in the patient’s eye. Then the eye was prepared with 5% povidone iodine
irrigation in the fornices. An injection of 1.25 mg/0.05 mL of Avastin was given using a tuberculin syringe and 30-gauge needle at 3.5 to 4 mm posterior to the limbus. A prophylactic topical
antibiotic (ciprofloxacin 0.3%, 1 drop qid) was given for five days after the IVA. FOLLOW UP All participants were reviewed monthly for three months. Full ocular examination including BCVA
and OCT testing were repeated on each visit. On each monthly visit, additional IVA injection was given to those whose MO had not resolved. DATA ANALYSIS Stata version 13.0 was used. Baseline
characteristics were presented in mean (SD) or proportions in a table. The causes of MO were calculated as proportions. A Student’s t-test was used to compare mean CMT and BCVA at the
baseline with those of 3 months after IVA treatment. For purposes of analysis, an improvement in CMT was taken as the difference between CMT at 3 months (cmt3) versus baseline (cmt0), i.e.
cmt0 - cmt3 to give a positive value if improved, zero if no change and a negative value if worsened. Improvement in vision was taken as the diferrence between logMAR vision at 3 months
(va3) versus baseline (va0), i.e. va0 - va3 to give a positive value if improved, zero if no change and a negative value if worsened. Univariable linear regression was used to analyse for
baseline factors associated with a change in CMT and vision at 3 months. Factors with a crude _p_ value of < 0.1 were included in the multivariable model and a back stepwise approach used
to retain factors with _p_ less than 0.05. RESULTS During the study period, we enroled 32 patients (35 eyes). Three patients were lost to follow up, and 29 (32 eyes) completed the study.
The baseline characteristics are given in Table 1. The mean age was 62.8 ± 11.8 years, 53% were male. Most of our participants were either diabetic (24/32) or hypertensive (20/32) and 13/32
were hypertensive and diabetic. The mean baseline CMT was 426.9 ± 135.9 µm and BCVA was 0.7 ± 0.38 logMAR. DR and RVO were the commonest causes of MO accounting for 53.1% and 25.0%
respectively. Overall, the mean CMT at 3 months decreased by 115.7 (±203.05) µm, (_p_ = 0.0008). A total of 11/32 (34.4%) achieved a CMT of less than or equal to 250 µm. Of these, one
required 2 injections and ten required 3. There was an improvement in BCVA in 22/32 (68.7%) eyes from the baseline with a mean difference of 0.34(±0.60) logMAR units (_p_ = 0.003). Of these,
16/22 (72.7%) had normal vision and 6/22 (27.3%) had moderate impairment. Evolution of CMT and BCVA is shown in Figs. 1 and 2. Changes in CMT and BCVA across the different pathologies are
presented in Table 2. A significant improvement in CMT was observed in patients with RVO while a significant improvement in BCVA was observed in patients with DR. Table 3 shows predictors of
a change in CMT after IVA. After adjusting for potential confounders, having a high CMT at baseline was associated with an improvement in CMT at 3 months (adjusted coefficient 0.86 [95% CI
0.48–1.24], _p_ < 0.001). Table 4 shows predictors of improvement in BCVA after IVA. After adjusting for confounders, having smoked and baseline BCVA were the strongest predictors of
improvement in vision at 3 months (adjusted coefficient 0.37 [95% CI 0.14–0.60], _p_ = 0.003) and (adjusted coefficient 1.18 [95% CI 0.86–1.50], _p_ < 0.001) respectively. We examined the
relationship between improvement in BCVA at 3 months and resolution of the CMT (Fig. 3). As CMT reduced, BCVA improved. DISCUSSION MO is a sight-threatening condition that affects most
commonly diabetic patients, and it is a leading cause of visual loss among working-age individuals globally [3, 9]. MO occurs in a wide variety of pathologic conditions and represents the
final common phenotype of several pathophysiologic processes that involve the damage of retinal vessels and disruption of the inner BRB [1, 10]. In this study, we found that MO was commonly
caused by DR (53.1%) and RVO (25.0%) as reported by many other studies [4, 11, 12]. Our study assessed the effect of IVA therapy for MO at three months. We found that overall, IVA therapy
resulted in both anatomical and functional improvement at three months after treatment. Our study was different from most in that is considered all causes of MO compared to other studies
that focused on outcomes of IVA in either DMO or RVO. When we disaggregated the effect of IVA by diagnosis, we found that with a modest change in CMT, IVA improved the BCVA significantly
among patients with DMO. Many studies have previously reported the good effect of IVA in DMO for both CMT and BCVA [3, 13,14,15]. However, some of those studies followed up patients for a
longer period (6months) [3, 9]. Improvement in vision is an important expectation among almost all patients with visual impairment that seek eye care services. In our setting, this
strengthened our evidence of benefits of IVA for treatment of DMO: it is effective, cheap, available and affordable to our population compared to other approved VEGF drugs. In many resource
limited settings in sub-Saharan Africa, using IVA could reduce the burden of visual impairment among DM patients presenting with MO, at least in the 3 months window that we studied. Although
we did not collect data on quality of life, we have reason to believe that improvement in vision could improve the quality of life of these patients. Our previous work among patients with
keratitis showed that an improvement in vision was associated with improved quality of life [16]. Conversely, although there was a marked improvement in CMT in patients with ME due to RVO,
this was not associated with a corresponding improvement in BCVA. Although both improvement in CMT and BCVA have been reported in other studies for RVO, this was not the case in our setting
[6, 17]. Of note, our study had few numbers of patients with RVO (eight patients) and five of them had central retinal vein occlusion (CRVO) which usually has a poor visual prognosis. We
also analysed for baseline factors associated with an improvement in CMT or BCVA at 3 months. Our study found that having a high baseline CMT was associated with an improvement of CMT at
three months after IVA injection. Patients who had a high baseline CMT could more likely achieve great improvement at three months of the treatment. Few studies that investigated predictors
of changes in CMT at three months post IVA therapy have reported age (<60 years), a low baseline CMT, presence of sub foveal fluid, OCT pattern of MO, and glycaemic control as significant
factors of change in CMT [6, 13, 18]. However, the factors influencing the change in CMT after IVA treatment for MO remain variable. There could be some other unknown individual-related
factors such as genetic, life style, race, environment which need to be identified through a large scale study. Our study found that baseline BCVA was the strongest predictor of visual
improvement at three months after IVA treatment. Patients who had severe visual impairement at baseline were more likely to have a marked improvement in vision at three months. Other studies
have reported duration of MO before the treatment, OCT pattern of MO and glycaemic control as predictors of improvement in vision. Previous laser treatment was reported as a negative
predictor of visual improvement [17, 18]. STRENGTHS AND LIMITATIONS OF THE STUDY DR is a rising problem in sub-Saharan Africa, the positive findings of the effect of IVA in improving vision
among patients with DMO provide some evidence for advocacy for access to treatment in such resource limited settings. However, it was generally a smaller study with a shorter follow up
period. CONCLUSION IVA resulted in significant visual and anatomical improvement in MO three months after treatment. Improvement in vision was most marked among patients with DMO, compared
to other causes of MO. In patients with RVO, however, although there was an improvement in CMT, there was no corresponding improvement in vision. A high baseline CMT was a strong predictor
of CMT improvement whereas a worse baseline vision was a strong predictor of visual improvement at three months after the treatment. RECOMMENDATIONS Based on these preliminary findings, a
larger scale study of the effectiveness and cost-effectiveness of Avastin for diabetic MO in Uganda is warranted to optimise patient selection. CHANGE HISTORY * _ 07 JUNE 2022 The original
online version of this article was revised to a Open Access. _ REFERENCES * Jack J Kanski BB. Kanski’s Clinical Ophthalmology, A Systematic Approach. 8 ed. Australia: Elsevier Saunders Ltd.;
2016. * Ollendorf DA, Colby JA, Pearson SD. Comparative effectiveness of anti-VEGF agents for diabetic macular edema. Int J Technol Assess Health Care. 2013;29:392–401. Article PubMed
Google Scholar * Tareen I-U-H, Rahman A, Mahar PS, Memon MS. Primary effects of intravitreal bevacizumab in patients with diabetic macular edema. Pak J Med Sci. 2013;29:1018–22. PubMed
PubMed Central Google Scholar * Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. Article Google
Scholar * Genentech. Avastin ® (Bevacizumab), Full prescribing informations for medical professionals. https://www.gene.com 2011. (Accessed 12 Oct 2018). * Wang M-Z, Feng K, Lu Y, Qian F,
Lu XR, Zang SW, et al. Predictors of short-term outcomes related to central subfield foveal thickness after intravitreal bevacizumab for macular edema due to central retinal vein occlusion.
Int J Ophthalmol. 2016;9:86–92. PubMed PubMed Central Google Scholar * Network DRCR. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl J Med. 2015;372:1193–203.
2015/03/26 Article Google Scholar * Organization WH. Change the definition of blindness. _Disponível no endereço eletrônico_
http://www.who.int/blindness/ChangetheDefinitionofBlindness.pdf.2008. * Arevalo JF, Sanchez JG, Fromow-Guerra J, Wu L, Berrocal MH, Farah ME, et al. Comparison of two doses of primary
intravitreal bevacizumab (Avastin) for diffuse diabetic macular edema: results from the Pan-American Collaborative Retina Study Group (PACORES) at 12-month follow-up. Graefes Arch Clin Exp
Ophthalmol. 2009;247:735–43. Article CAS PubMed Google Scholar * Johnson MW. Etiology and treatment of macular edema. Am J Ophthalmol. 2009;147:11–21. e11 Article PubMed Google Scholar
* Catier A, Tadayoni R, Paques M, Erginay A, Haouchine B, Gaudric A, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol.
2005;140:200–6. Article PubMed Google Scholar * Federation ID. International Diabetes Federation, IDF World Atlas, “The Global burden” _IDF_ 2019; 9:
https://www.google.com/search?q=Federation+ID.+International+Diabetes+Federation%2C+IDF+World+Atlas%2C+%22The+Global+burden%22+IDF+2019%3B+9&oq=Federation+ID.+International+Diabetes+Federation%2C+IDF+World+Atlas%2C+%22The+Global+burden%22+IDF+2019%3B+9&aqs=chrome..69i57.4714j0j7&sourceid=chrome&ie=UTF-8
(accessed 06 Dec 2020). * Bafaraj AN, Alshammari HS, Alshammari FS, Alibrahim AK, Masaud JS. Visual and anatomical outcomes after single injection of intravitreal bevacizumab (Avastin) in
patients with diabetic macular edema. Ann Int Med Den Res. 2017;3:07–10. 2017 Article Google Scholar * Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular
oedema: recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol. 2017;28:636–43. Article PubMed Google Scholar * Vyas S, Thapa R, Bajimaya S, Pradhan E, Paudyal
G. Anatomical and visual outcome of intravitreal bevacizumab (Avastin) in patients with diabetic macular edema. Nepal J Ophthalmol. 2016;8:54–61. Article PubMed Google Scholar * Arunga
S, Wiafe G, Habtamu E, Onyango J, Gichuhi S, Leck A, et al. The impact of microbial keratitis on quality of life in Uganda. BMJ Open Ophthalmol. 2019;4:000351. Article Google Scholar *
Hirose M, Matsumiya W, Honda S, Nakamura M. Efficacy and visual prognostic factors of intravitreal bevacizumab as needed for macular edema secondary to central retinal vein occlusion. Clin
Ophthalmol. 2014;8:2301–5. PubMed PubMed Central Google Scholar * Kim TK, Shin HY, Kim SY, Lee YC, Lee MY. Factors influencing intravitreal bevacizumab and triamcinolone treatment in
patients with diabetic macular edema. Eur J Ophthalmol. 2017;27:746–50. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors would like to appreciate Mr Johnson
Muiruri, Mr Bernard Nampa, Mr Grace Kansime and Ms Juliana Katushabe for helping in data collection. FUNDING RK was sponsored by a research grant from the Movimento Apostoico Cieccho (MAC)
through the medical department of the Catholic Diocese of Bukavu (BDOM), in South-Kivu Province of the Democratic Republic of Congo (DRC). The funding organisation was not involved in the
design, collection, analysis and review of this manuscript. The publication costs for this article were funded by The Queen Elizabeth Diamond Jubilee Trust through the Commonwealth Eye
Health Consortium. The funding body did not participate in the design of the study, data collection and analysis, interpretation of data and writing the manuscript. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Department of Ophthalmoogy, Mbarara University of Science and Technology, Mbarara, Uganda Rachel R. Kabunga, John Onyango, Sam Ruvuma & Simon Arunga *
International Centre for Eye Health, London School of Hygiene & Tropical Medicine, London, UK Simon Arunga Authors * Rachel R. Kabunga View author publications You can also search for
this author inPubMed Google Scholar * John Onyango View author publications You can also search for this author inPubMed Google Scholar * Sam Ruvuma View author publications You can also
search for this author inPubMed Google Scholar * Simon Arunga View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS RRK was responsible for
designing and writing the study protocol, screening eligible participants, conducting the study intervention and follow up of participants, entering and analyzing data, interpreting results,
updating reference lists and writing the final report of the study. JO contributed to designing and writing of the protocol, to interpreting result and updating the final report. SR
contributed to the designing of the study protocol, was responsible for supervising the screening process of eligible participants, supervising the procedure of intravitreal avastin
injection and participant’s follow ups, contributed to the final report writing. SA participated in designing and writing the study protocol, conducted the data analysis, contributed to
interpreting results, writing the final report and provided feedback on the report. CORRESPONDING AUTHOR Correspondence to Rachel R. Kabunga. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any
non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, and provide a link to the
Creative Commons license. You do not have permission under this license to share adapted material derived from this article or parts of it. The images or other third party material in this
article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons
license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kabunga, R.R., Onyango, J., Ruvuma, S. _et al._ Outcome
of intravitreal Avastin® injections in patients with macular oedema in Uganda: a cohort study. _Eye_ 36 (Suppl 1), 45–50 (2022). https://doi.org/10.1038/s41433-022-02006-5 Download citation
* Published: 19 May 2022 * Issue Date: May 2022 * DOI: https://doi.org/10.1038/s41433-022-02006-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative