Cost-effectiveness of digital surveillance clinics with optical coherence tomography versus hospital eye service follow-up for patients with screen-positive maculopathy
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Annually 2.7 million individuals are offered screening for diabetic retinopathy (DR) in England. Spectral-Domain Optical Coherence Tomography (SD-OCT) has the potential
to relieve pressure on NHS services by correctly identifying patients who are screen positive for maculopathy on two-dimensional photography without evidence of clinically significant
macular oedema (CSMO), limiting the number of referrals to hospitals. We aim to assess whether the addition of SDOCT imaging in digital surveillance clinics is a cost-effective intervention
relative to hospital eye service (HES) follow-up. METHODS We used patient-level data from the Gloucestershire Diabetic Eye Screening Service linked to the local digital surveillance
programme and HES between 2012 and 2015. A model was used to simulate the progression of individuals with background diabetic retinopathy (R1) and diabetic maculopathy (M1) following DR
screening across the clinic pathways over 12 months. RESULTS Between January 2012 and December 2014, 696 people undergoing DR screening were found to have screen-positive maculopathy in at
least one eye for the first time, with a total of 766 eyes identified as having R1M1. The mean annual cost of assessing and surveillance through the SD-OCT clinic pathway was £101 (95% CI:
91–139) as compared with £177 (95%CI: 164–219) under the HES pathway. Surveillance under an SD-OCT clinic generated cost savings of £76 (95% CI: 70–81) per patient. CONCLUSIONS Our analysis
shows that SD-OCT surveillance of patients diagnosed as R1M1 at DR screening is not only cost-effective but generates considerable cost savings. SIMILAR CONTENT BEING VIEWED BY OTHERS A
HEALTH ECONOMIC PILOT STUDY COMPARING TWO DIABETIC RETINOPATHY SCREENING STRATEGIES Article Open access 06 July 2024 COST-EFFECTIVENESS OF INCORPORATING SELF-IMAGING OPTICAL COHERENCE
TOMOGRAPHY INTO FUNDUS PHOTOGRAPHY-BASED DIABETIC RETINOPATHY SCREENING Article Open access 24 August 2024 THE SCANNING CONFOCAL OPHTHALMOSCOPY FOR DIABETIC EYE SCREENING (CONCORDIA) STUDY
PAPER 1 Article Open access 08 October 2024 INTRODUCTION Diabetes places a great economic burden on society owing to its high prevalence and increased health-care expenditures and lost
productivity. Although the treatment of diabetes on its own is costly, its complications are the major contributors to health-care costs [1]. Among the main diabetes-related complications is
diabetic retinopathy (DR), which is an important cause of blindness in the working age population in the UK [2]. It is possible to treat sight threatening DR effectively [3, 4] and
cost-effectively [5] and screening using retinal photography has been shown to be cost-effective [6]. A common cause of sight-threatening retinopathy is diabetic macular oedema [7], which
can be treated effectively by either macular laser treatment or anti-vascular endothelial growth factor (VEGF) injections [8]. However, treatment is only recommended for patients with
clinically significant macular oedema (CSMO), with non-CSMO patients deriving little additional benefit from treatment [4]. In 2003, the NHS Diabetic Eye Screening Programme (NDESP) was
introduced in England which uses annual digital photography with pupil dilation [9, 10]. Until 2015, NDESP recommended all screen-positive patients identified with mild non-proliferative DR
and maculopathy (R1M1), moderate/severe non/pre-proliferative DR (R2M0, R2M1), or proliferative retinopathy (R3M0, R3M1) be referred to a hospital eye services (HES) for treatment
assessment. However, given the limitations of two-dimensional digital photographic retinal screening for maculopathy, less than a quarter of referred M1 patients were found to have CSMO in
need of treatment [11]. In January 2015, digital surveillance was included in the standard NDESP pathway for annual screening with a statement in the annual service specification “the
provider shall: refer people with diabetes to digital surveillance clinics that, in the opinion of the Clinical Lead, need more frequent review and do not require referral to the HES. This
should be done against local protocols based on best evidence and NDESP guidance, using appropriate technology. Surveillance clinics may interface with OCT assessment where this has been
agreed with commissioners of hospital eye services” and a pathway overview diagram which is unchanged in current documents [12, 13]. The digital surveillance pathway standards [14] were
updated in March 2018 to include a new standard (DES-PS-5) to ensure those in digital surveillance are seen within an appropriate time frame. No guidance has been given on grading criteria
used within digital surveillance or on the use of OCT which is currently considered ‘optional’. In June 2017, 2.7 million people were offered DR screening with 2.25 million uptake (82.2%)
[15], with an epidemic posing an ever-growing strain on the screening programme and resources in the wider NHS. One way to relieve pressure on NHS services would be to improve the
specificity of the current screening programme. Spectral domain optical coherence tomography (OCT) is an imaging technique that interprets reflected optical waves from a depth in the retina
of 2–3 mm to produce three-dimensional images with the potential to correctly identify patients without evidence of any oedema in the macular area or CSMO, therefore limiting the number of
referrals to hospitals and improving the specificity of the current screening programme [16]. Although SD-OCT imaging has been shown to be a useful adjunct in surveillance clinics for
screen-positive diabetic maculopathy [16], questions remain about its cost-effectiveness in the clinical setting given its high implementation costs. Using detailed data from the
Gloucestershire SD-OCT clinic surveillance programme, we aim to assess whether the addition of SD-OCT imaging surveillance in a community setting following digital retinal photography is a
cost-effective intervention when screening for CSMO relative to hospital eye service assessment and follow-up. METHODS PARTICIPANTS We used patient-level data from the Gloucestershire
Diabetic Eye Screening Service linked to the local digital surveillance programme and HES covering the period between 1st January 2009 and 31st December 2015. An anonymised cohort dataset of
individuals with incident R1M1 in one eye as detected by GDESP between 1st January 2012 and 31st December 2014 was analysed. To ensure that only incident R1M1 cases were considered in our
analysis, we excluded patients with previous history of R1M1 (that is, those individuals screened as R1M1 between January 2009 and December 2011). HES and SD-OCT clinic surveillance data
were analysed up to 31st December 2015, allowing for at least a full year of follow-up for all incident R1M1 cases. These data included basic demographics, DR screening encounters, grading
and referral outcomes, SDOCT clinic grading and referral outcomes, and HES grading, referral and treatment outcomes from two sources: * a. Gloucestershire Diabetic Retinopathy Eye Screening
Programme (GDESP) * i. A diabetes register for Gloucestershire provided by a regularly updated and collated data download from each Gloucestershire primary care General Practice. * ii. Every
DR screening encounter, grade, outcome and referral recorded in detail since 1998 and GDESP operational pathways and data requirements managed in accordance with National Screening
Programme specifications and standards. * a. Gloucestershire Hospital Eye Service (HES) * i. The HES data consists of an electronic medical record (EMR) for every patient attending an HES
appointment; * ii. Patient demographic Electronic Medical Record (EMR) is managed by an electronic interface with Gloucestershire Patient Administration System (PAS) and clinical episodes
are recorded, by medical and allied health professional staff at the time of a patient encounter. INTERVENTIONS UNDER STUDY We estimated the cost-effectiveness of two pathways of
surveillance for individuals with incident R1M1 grading detected in the diabetic screening programme: * Technician led digital surveillance clinic including SD-OCT in a community setting *
Ophthalmologist led HES clinic assessment and follow-up At the time of the analysis, the English National Screening Programme recommended annual screening for all patients with diabetes. The
technician led digital surveillance pathway consisted of two field mydriatic digital photography (macular and disc fields) followed by a macular SD-OCT using a Topcon OCT 2000. For the HES
clinical surveillance pathway, this consisted of a slit lamp biomicroscopy examination by an ophthalmologist and a macular SD-OCT using one of three SD-OCT machines used within the HES
(Heidelberg Spectralis, Zeiss Cirrhus or the Topcon OCT 2000). Patients attending HES who did not need treatment were followed up in a similar fashion to that observed in the SD-OCT clinic
surveillance programme in a community setting, with the exception that patients would continue to be assessed in HES. The comparison of the two pathways is shown in Online Appendix Figure
A1. GRADING CRITERIA The grading criteria used for retinopathy (R) and maculopathy (M) grades in the NHS Diabetic Eye Screening Programme are published [17] by NDESP. Online Appendix Table
A1 reports the grading criteria and assessment of image quality for the SD-OCT images. MODEL STRUCTURE A decision analytic model was developed to evaluate the impact of the two pathways of
surveillance under evaluation. Given the clinic pathways and the 1 year of time horizon used in the analysis, the most appropriate model was judged to be a decision tree which was developed
in Excel (Microsoft, Redmond, WA). Model structure and assumptions were informed by what was known about diabetic retinopathy, the clinic pathways of R1M1 individuals and discussions with
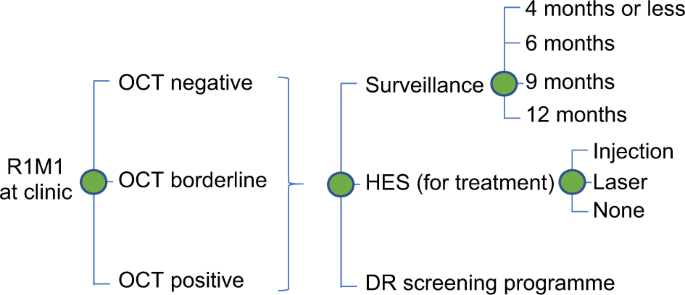
clinical experts and statisticians involved in the project. The model was used to simulate the progression of R1M1 individuals following DR screening across the clinic pathways over 12
months (Fig. 1). Nearly all individuals who were screen positive for R1M1 were referred to SD-OCT digital surveillance clinics with a very small number referred to HES. Hence, the R1M1
population was first divided into those that attended the SD-OCT/HES clinic appointments and those that did not. Of those attending SD-OCT clinic or HES, we further divided the R1M1
population according to the grading in the other eye at screening: R0/R1, R1M1 or R2-R3. Conditional on the grading in the other eye, we classified the R1M1 at screening according to their
possible grading at SD-OCT/HES clinic appointments: R0M0, R1M0, R1M1 and R2-R3. We then further divided these subgroups according to their SD-OCT results: negative, borderline and positive.
Conditional on the DR/OCT results, the individuals could be referred back to DR screening, to further surveillance (at the SD-OCT clinic or HES) or directly to HES for possible treatment of
maculopathy. If referred for further surveillance, the frequency of surveillance could be: 4 months or less, 6 months, 9 months or 12 months. If referred to HES for possible maculopathy
treatment, individuals might or might not receive treatment (VEGF injection or laser). Given the use of the same equipment in the digital surveillance pathway and the hospital eye services
(SD-OCT) and similar levels of DR/OCT grading expertise grading in both settings, we assumed that the grading, outcomes and frequency of referral would be identical in both models. COSTS
Following NICE recommendations, the perspective for the analysis was that of the UK NHS and the price year was 2015/16. Costs included: SD-OCT clinic surveillance appointment cost in a
community setting; HES outpatient appointment costs; capital cost of SD-OCT in a hospital setting, and hospital-based treatment costs for CSMO (VEGF injection and laser) (see Online Appendix
Table A2). The costs of SD-OCT surveillance clinics were obtained from a recent report evaluating community relative to hospital eye service follow-up for patients with age-related macular
degeneration. [18] These authors determined the costs of monitoring review in a community settings (£51.82 in 2013/14 prices) as well as the costs of SD-OCT equipment by review (£22.99 in
2013/14 prices). Outpatient consultation costs for ophthalmology were obtained from NHS Reference Costs 2015/16. [19] We costed the first outpatient appointment following DR screening as a
consultant led first appointment and the remaining appointments as consultant-led follow-up appointments. Under the HES pathway, we added the OCT equipment costs to outpatient appointment
costs. The annual costs associated with laser or VEGF injections for the treatment of CSMO were obtained from patient-level data from patients attending DR screening in Gloucestershire. [20]
STATISTICAL ANALYSIS We converted the Gloucestershire patient-level dataset into eye-level data so that we could follow the clinical pathway, grading and outcomes for the eye graded as R1M1
at screening over 12 months. These data were used to estimate the grade at screening in the other eye (R0-R1M0, R1M1 and R2-R3), the uptake of surveillance, the grading at community or HES
surveillance clinic, and the number of surveillance appointments attended during the 12 months following initial screening. For example, the uptake rate of the surveillance clinics
(community or HES-based) was determined by dividing the number of attenders, within 12 months of screening, out of all those referred at screening for HES or SD-OCT surveillance. We also
used three regression models to estimate: * 1. Probability of each referral outcome (i.e., referral back to DR screening, SD-OCT surveillance in a community or HES setting, HES direct
referral for possible treatment of maculopathy) following the SD-OCT surveillance clinic appointment (multinomial logit); * 2. Probability of the frequency of surveillance (i.e., 4 months or
less, 6 months, 9 months or 12 months) following SD-OCT surveillance clinic appointment (ordered logit); * 3. Probability of receiving treatment for CSMO (VEGF injection or laser). We
examined the following predictors: SD-OCT result (positive, borderline, negative), grading at surveillance clinic (R0M0, R1M0, R1M1), screening grade in other eye (R0-R1, R1M1) and other
ocular conditions (e.g., macular degeneration). A predictor was deemed to be statistically significant if _p_ < 0.05. Model fit was assessed using Pregibon’s link test. All analyses were
performed using STATA version 14 (StataCorp, College Station, TX). COST-EFFECTIVENESS ANALYSIS Following the assumption that the clinic pathways under evaluation would result in the same
patient grading results, OCT results, and subsequent referrals for treatment or further surveillance, the quality of life of the R1M1 individuals was assumed to be the same. As a result, we
estimated the incremental costs associated with surveillance in an SD-OCT clinic pathway relative to HES. Probabilistic sensitivity analysis (PSA) was performed where distributions were used
to represent the uncertainty in the model inputs [21]. Furthermore, to capture the uncertainty and correlations between the three regression models we re-estimated the same set of models
for each of the 1000 bootstraps (with replacement) of the sample dataset and saved the coefficients from the three regression models. SCENARIO ANALYSIS We explored the impact of the scenario
that once eyes were assessed at HES for the first time after diagnosis of R1M1 at screening they would no longer require further surveillance. We also explored no further surveillance for
SD-OCT negative results if clinic grading was R0M0 or R1M0 in both pathways. RESULTS GLOUCESTER COHORT STUDY AND MODEL INPUTS NUMBER OF EYES IDENTIFIED WITH R1M1 AT SCREENING Between January
2012 and December 2014, 696 people with diabetes screened for diabetic retinopathy were found to be screen positive for maculopathy (M1) in at least one eye for the first time. Of these,
622 (89%) had a diagnosis of R1M1 in one eye, 4 had R1M1 in one eye and moderate to proliferative retinopathy R2M1 in the other eye (1%) and 70 (10%) had R1M1 in both eyes (Table 1), with a
total of 766 eyes diagnosed as having R1M1. EYE REFERRAL ONCE IDENTIFIED WITH R1M1 AT SCREENING All 696 individuals identified as having R1M1 at screening were referred to either HES or
SD-OCT clinics, with 37 (5%) failing to attend their appointments within the first year after screening (Fig. 2). Of the remaining 659 patients, 652 (99%) were initially assessed at a
digital surveillance clinic with SD-OCT after their screening episode and 7 (1%) were assessed at HES. SD-OCT GRADING OF EYES IDENTIFIED WITH R1M1 AT SCREENING Table 2 presents the results
of the grading at the SD-OCT clinic, stratified by screen grade for the other eye, for the 652 patients attending an SD-OCT clinic because of diagnosis of R1M1 at screening. Of the 652
patients attending SD-OCT clinic, 13 (2%) patients had missing or incomplete data (e.g., absence of an OCT result). For the remaining 639 patients, 572 (81%) were diagnosed with R1M1 at
screening the other eye was diagnosed as R0/1M0 at screen (Table 2). At the first SD-OCT clinic visit, 171 (30%) of R1M1 patients at screening and with R0/1M0 in the other eye were found to
be R1M1 but with an SD-OCT negative result, and 54 (9%) as R1M1 with a positive result. There were 65 patients (10%) with R1M1 in both eyes at screening (that is. 130 eyes in total). Of
these patients, 2 (6%) had SD-OCT positive results in both eyes and 12 (18%) had SD-OCT positive results in one eye. Only two patients diagnosed as having R1M1 at screening were diagnosed
with R2/3 in the other eye. Overall, for the 639 patients, irrespective of diabetic retinopathy grading in the SD-OCT clinic, a total of 90 (14%) patients tested SD-OCT positive and 477
(66%) tested negative. REFERRAL OUTCOME FOLLOWING SD-OCT GRADING For the 639 patients with recorded SD-OCT results, outcome data were missing in 11 (2%) patients. For the remaining 628
patients, 118 (19%) were returned to the DR annual screening programme, 412 (66%) continued under SD-OCT clinic surveillance, and 98 (16%) were referred to HES. Patients with SD-OCT positive
results were significantly more likely to be referred to HES after controlling for SD-OCT clinic grading of the eye and the screen grading of the other eye (see Online Appendix Table A3).
Of the 412 patients under continued SD-OCT clinic surveillance, 4 (<1%) were followed up in SD-OCT clinic surveillance between 1 and 3 months, 174 (42%) every 6 months, 72 (17%) every 9
months and 162 (39%) were referred to annual SD-OCT clinic surveillance. Patients with SD-OCT negative results in one eye were significantly more likely to have longer surveillance intervals
than those with positive or borderline results after controlling for SD-OCT clinic grading of the eye and the screen grading of the other eye (see Online Appendix Table A4). Including the
initial SD-OCT clinic visit, the mean number of SD-OCT clinic visits for patients who were referred to SD-OCT clinic surveillance every 3, 6, 9 and 12 months was, respectively: 2.00 (S.D.
1.15); 1.95 (S.D. 0.44); 1.59 (S.D. 0.49); and 1.05 (S.D. 0.24) visits. TREATMENT FOR CSMO Of the 639 patients assessed at the SD-OCT clinic for suspected R1M1 and with SD-OCT results, 18
(3%) received treatment with laser or VEGF injection. Of these, 7 (39%) received VEGF injection and 11 (61%) received laser treatment. Results of the logistic regression (Online Appendix
Table A5) showed that a SD-OCT positive result was the only significant predictor of increased likelihood of treatment (odds ratio 36, 95% CI: 10–126). Therefore, the probability of
treatment given a SD-OCT positive result was 16%. COST COMPARISON BETWEEN SD-OCT AND HES SURVEILLANCE Online Appendix Table A2 reports the model inputs used to compare SD-OCT and HES
surveillance pathways. The mean annual cost of assessing and surveillance of an eye identified as R1M1 at DR screening through the SD-OCT clinic pathway was £101 (95% CI: 91–139) per patient
(Table 3). Results showed that the mean annual cost of assessing and surveillance of patient with at least one eye diagnosed as R1M1 at DR screening through the HES pathway was £177 (95%
CI: 164–219) per patient. As a result, the mean annual cost saving per patient with one identified as R1M1 in the DR screening programme and surveilled in the SD-OCT digital surveillance
pathway as opposed to a HES pathway was £76 (95% CI: 70–81), with these savings arising from less patients without maculopathy or negative SD-OCT results being referred to HES. Even if,
under the HES pathway, a patient with R1M1 was assessed at HES for the first time after diagnosis of R1M1 at screening and no longer require surveillance, the SD-OCT clinic pathway still
generated savings of £41 (95% CI: £37–£46) per eye assessed. Finally, assuming that there was no further surveillance for SD-OCT negative results if the clinic grading was R0M0 or R1M0
generated savings of £74 (95%CI: £67–£79) (Table 3). DISCUSSION Diabetic retinopathy screening is nationally mandated and has been shown to be cost-effective compared with no screening
[22,23,24]. There is also a clear understanding about the appropriateness of different screening intervals for patients at differing risks of developing sight threatening DR [20]. In
addition, studies have suggested that including an SD-OCT digital surveillance pathway could improve the overall efficiency of the DR screening programme [16], and its cost-effectiveness
[25]. This study, based on 652 patients and over 700 eyes diagnosed as screen positive for maculopathy with background DR, and referred almost entirely for surveillance in an SD-OCT clinic,
provides further evidence that surveillance of these patients in an SD-OCT clinic is not only cost-effective but generates substantial cost savings. We found that after the first
surveillance visit in an SD-OCT clinic, less than 20% of patients required referral to HES as they were found not to have macular oedema or CSMO and could be safely monitored at an SD-OCT
follow-up clinic or discharged back to screening if no longer screen positive for maculopathy. At a cost of £110 and £87 for a first and follow-up face-to-face consultant visit,
respectively, in ophthalmology, in addition to £24 for SD-OCT imaging, the costs of assessment at HES are considerable. By contrast, at a cost of £53 a visit including SD-OCT imaging, the
costs of an SD-OCT surveillance clinic are significantly lower. By reducing the number of patients referred to HES without CSMO and referring only those who will benefit from doing so, the
1-year cost savings associated with surveillance in an SD-OCT clinic rather than HES was £76 (95% CI: 70–81), that is. a saving of 41% compared with having all these eyes assessed in HES.
Despite our study being based on a large number of eyes diagnosed as R1M1 at screening, with individual patient record linkage of data on DR screening appointments, SD-OCT clinic
surveillance visits, HES appointments and eye treatments received, our study is not without limitations. First, we assumed that surveillance in an SD-OCT clinic would yield the same eye
outcomes as surveillance in HES, that is we assumed that the sensitivity of SD-OCT at detecting CSMO would be equal to that of HES assessment. Previous studies and literature reviews [16,
26], suggest that SD-OCT performs very well with very similar outcomes. Second, we only established the cost implications of the two surveillance programmes over 1-year and not over the
longer-term, which would most likely have resulted in larger cost savings by reviewing patients who are screen positive with R1M1 in digital surveillance clinics with SD-OCT as opposed to
HES. Finally, with no control group to compare the SD-OCT clinic surveillance programme with, we assumed that instead of attending the SD-OCT clinic for surveillance, patients attended HES
directly and were then followed-up in a similar fashion in HES to that observed in the Gloucestershire SD-OCT clinic surveillance programme. We tested the implications of this assumption in
our sensitivity analyses, which showed that even if eyes were assessed at HES for the first time after diagnosis of R1M1 at screening and no longer required surveillance, the SD-OCT clinic
pathway still generated significant cost savings. In conclusion, our analysis shows that SD-OCT digital surveillance of patients with diabetes, background diabetic retinopathy (R1) and
evidence of diabetic maculopathy (M1) at DR screening is not only cost-effective but generates considerable cost savings. SUMMARY WHAT WAS KNOWN BEFORE: * The NHS Diabetic Eye Screening
Programme have introduced digital surveillance clinics into their pathway for those patients who, in the opinion of the Clinical Lead, need more frequent review and do not require referral
to the HES. WHAT THIS STUDY ADDS: * This paper is the first to show that the use of OCT in the digital surveillance pathway of the English NHS Diabetic Eye Screening Programme is both
effective and cost-effective. REFERENCES * Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS
(UKPDS 84). Diabet Med: a J Br Diabet Assoc. 2015;32:459–66. Article CAS Google Scholar * Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England
and Wales in working age adults (16-64 years), 1999-2000 with 2009-2010. BMJ Open. 2014;4:e004015. Article Google Scholar * DRS. Photocoagulation treatment of proliferative diabetic
retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583–600. * ETDRS.
Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol.
1985;103:1796–806. Article Google Scholar * Savolainen EA, Lee QP. Diabetic retinopathy - need and demand for photocoagulation and its cost-effectiveness: evaluation based on services in
the United Kingdom. Diabetologia. 1982;23:138–40. Article CAS Google Scholar * James M, Turner DA, Broadbent DM, Vora J, Harding SP. Cost effectiveness analysis of screening for sight
threatening diabetic eye disease. BMJ. 2000;320:1627–31. Article CAS Google Scholar * Fong DS, Ferris FL 3rd, Davis MD, Chew EY. Causes of severe visual loss in the early treatment
diabetic retinopathy study: ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1999;127:137–41. Article CAS Google Scholar * Virgili G,
Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2012;12:CD007419.
* NICE. Type 1 diabetes in adults: diagnosis and management. Secondary Type 1 diabetes in adults: diagnosis and management 2015. www.nice.org.uk/guidance/ng17. * NICE. Type 2 diabetes in
adults: management. Secondary Type 2 diabetes in adults: management 2015. www.nice.org.uk/guidance/ng28. * Jyothi S, Elahi B, Srivastava A, Poole M, Nagi D, Sivaprasad S. Compliance with the
quality standards of National Diabetic Retinopathy Screening Committee. Prim Care Diabetes. 2009;3:67–72. Article Google Scholar * PHE. NHS public health functions agreement 2017-2018.
Service specification no.22. NHS Diabetic Eye Screening Programme. Secondary NHS public health functions agreement 2017-2018. Service specification no.22. NHS Diabetic Eye Screening
Programme 2017. www.england.nhs.uk/wp-content/uploads/2017/04/service-spec-22.pdf. * PHE. NHS Diabetic Eye Screening Programme Overview of patient pathway, grading pathway, surveillance
pathways and referral pathways. Secondary NHS Diabetic Eye Screening Programme Overview of patient pathway, grading pathway, surveillance pathways and referral pathways 2017.
www.gov.uk/government/uploads/system/uploads/attachment_data/file/648658/Diabetic_Eye_Screening_pathway_overviews.pdf. * PHE. NHS Diabetic Eye Screening Programme Pathway standards.
Secondary NHS Diabetic Eye Screening Programme Pathway standards 2018. * PHE. NHS Screening Programmes in England, 1 April 2016 to 31 March 2017. Secondary NHS Screening Programmes in
England, 1 April 2016 to 31 March 2017. 2017. www.gov.uk/government/uploads/system/uploads/attachment_data/file/661677/NHS_Screening_Programmes_in_England_2016_to_2017_web_version_final.pdf.
* Mackenzie S, Schmermer C, Charnley A, Sim D, Vikas T, Dumskyj M, et al. SDOCT Imaging to Identify Macular Pathology in Patients Diagnosed with Diabetic Maculopathy by a Digital
Photographic Retinal Screening Programme. PLoS One. 2011;6:e14811. Article CAS Google Scholar * PHE. NHS Diabetic Eye Screening Programme Grading definitions for referable disease.
Secondary NHS Diabetic Eye Screening Programme Grading definitions for referable disease 2017.
www.gov.uk/government/uploads/system/uploads/attachment_data/file/582710/Grading_definitions_for_referrable_disease_2017_new_110117.pdf. * Reeves BC, Scott LJ, Taylor J, Hogg R, Rogers CA,
Wordsworth S, et al. The Effectiveness, cost-effectiveness and acceptability of Community versus Hospital Eye Service follow-up for patients with neovascular age-related macular degeneration
with quiescent disease (ECHoES): a virtual randomised balanced incomplete block trial. Health Technol Assess (Rockv). 2016;20:1–120. Article Google Scholar * DH. NHS reference costs 2015
to 2016. Secondary NHS reference costs 2015 to 2016 2016. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016. * Scanlon PH, Aldington SJ, Leal J, Luengo-Fernandez R, Oke J,
Sivaprasad S, et al. Development of a cost-effectiveness model for optimisation of the screening interval in diabetic retinopathy screening. Health Technol Assess (Rockv). 2015;19:1–116.
Article Google Scholar * Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. Article CAS Google Scholar * Javitt JC, Aiello LP.
Cost-effectiveness of detecting and treating diabetic retinopathy. Ann Intern Med. 1996;124:164–9. Article CAS Google Scholar * Caro JJ, Ward AJ, O’Brien JA. Lifetime costs of
complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–81. Article Google Scholar * Fendrick AM, Javitt JC, Chiang YP. Cost-effectiveness of the screening and
treatment of diabetic retinopathy. What are the costs of underutilization? Int J Technol Assess Health Care. 1992;8:694–707. Article CAS Google Scholar * Prescott G, Sharp P, Goatman K,
Scotland G, Fleming A, Philip S, et al. Improving the cost-effectiveness of photographic screening for diabetic macular oedema: a prospective, multi-centre, UK study. Br J Ophthalmol.
2014;98:1042–9. Article Google Scholar * Jittpoonkuson T, Garcia PM, Rosen RB. Correlation between fluorescein angiography and spectral-domain optical coherence tomography in the diagnosis
of cystoid macular edema. Br J Ophthalmol. 2010;94:1197–200. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Steve Chave who collated all the data for
the analyses. FUNDING Project was funded by an unrestricted grant from Gloucestershire NHS Foundation Trust from funding received from Public Health England. AUTHOR INFORMATION Author notes
* These authors contributed equally: Jose Leal, Ramon Luengo-Fernandez. AUTHORS AND AFFILIATIONS * Health Economics Research Centre, Nuffield Department of Population Health, University of
Oxford, Oxford, UK Jose Leal & Ramon Luengo-Fernandez * Gloucestershire Retinal Research Group, Cheltenham General Hospital, Cheltenham, UK Irene M. Stratton, Angela Dale, Katerina
Ivanova & Peter H. Scanlon * Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, UK Peter H. Scanlon * School of Health and Social Care, University of
Gloucestershire, Cheltenham, UK Peter H. Scanlon Authors * Jose Leal View author publications You can also search for this author inPubMed Google Scholar * Ramon Luengo-Fernandez View author
publications You can also search for this author inPubMed Google Scholar * Irene M. Stratton View author publications You can also search for this author inPubMed Google Scholar * Angela
Dale View author publications You can also search for this author inPubMed Google Scholar * Katerina Ivanova View author publications You can also search for this author inPubMed Google
Scholar * Peter H. Scanlon View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Peter H. Scanlon. ETHICS DECLARATIONS
CONFLICT OF INTEREST JL, RLF, IMS, AD and KI declare no conflict of interest. PHS is Clinical Director for the English NHS Diabetic Eye Screening Programme. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Leal, J., Luengo-Fernandez, R., Stratton, I.M. _et al._ Cost-effectiveness of digital surveillance clinics with optical coherence tomography versus hospital eye service
follow-up for patients with screen-positive maculopathy. _Eye_ 33, 640–647 (2019). https://doi.org/10.1038/s41433-018-0297-7 Download citation * Received: 08 September 2018 * Revised: 11
October 2018 * Accepted: 21 October 2018 * Published: 30 November 2018 * Issue Date: April 2019 * DOI: https://doi.org/10.1038/s41433-018-0297-7 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative