Ring-opening (co)polymerization of γ-butyrolactone: a review
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT With increased environmental concerns and the rising demands for sustainable polymers, e.g., degradable polymers and chemically recyclable polymers, studies on ring-opening
polymerization (ROP) of cyclic esters have been developed in recent decades. Biorenewable five-membered _γ_-butyrolactone (_γ_BL) may be a desirable feedstock for the chemical synthesis of
poly(_γ_-butyrolactone) (P_γ_BL) or for the incorporation of _γ_BL units into polyester chains to modify their properties. Although _γ_BL is traditionally considered to be
“nonpolymerizable”, some progress was recently made concerning the ROP of _γ_BL. This mini-review is thus specifically focused on the ROP of _γ_BL and its copolymerization with other cyclic
esters. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS BIFUNCTIONAL AND RECYCLABLE POLYESTERS BY CHEMOSELECTIVE RING-OPENING
POLYMERIZATION OF A Δ-LACTONE DERIVED FROM CO2 AND BUTADIENE Article Open access 08 October 2024 SYNTHESIS OF FUNCTIONAL AND ARCHITECTURAL POLYETHERS VIA THE ANIONIC RING-OPENING
POLYMERIZATION OF EPOXIDE MONOMERS USING A PHOSPHAZENE BASE CATALYST Article 15 April 2021 CATIONIC RING-OPENING COPOLYMERIZATION OF A CYCLIC ACETAL AND Γ-BUTYROLACTONE: MONOMER SEQUENCE
TRANSFORMATION AND POLYMERIZATION–DEPOLYMERIZATION CONTROL BY VACUUMING OR TEMPERATURE CHANGES Article Open access 10 November 2023 INTRODUCTION Aliphatic polyesters are a fascinating class
of polymers considering their widespread applications in different areas due to their biodegradability and biocompatibility [1,2,3,4]. They can be obtained either by polycondensation of
diols with diacids, diesters, hydroxyesters or hydroxyacids or by ring-opening polymerization (ROP) of cyclic esters. The latter reaction has been demonstrated to be a powerful strategy to
synthesize polyesters with various macromolecular architectures and properties in a controlled manner [5,6,7,8]. Among the diverse types of lactones, the five-membered _γ_-butyrolactone
(_γ_BL), a renewable monomer derived from succinic acid that ranked as a top biomass-derived chemical, could be an alternative for the chemical synthesis of the biopolyester
poly(_γ_-butyrolactone) (P_γ_BL), a structural equivalent of poly(4-hydroxybutyrate) (P4HB), which is obtained from a bacterial fermentation process [9]. Moreover, it was demonstrated that
the incorporation of _γ_BL into polyesters resulted in enhanced biodegradability and flexibility [10]. Compared to the commonly utilized lactones with high strain energy, _γ_BL is
traditionally considered “nonpolymerizable” due to the low strain energy of the five-membered ring, which has a negative change in enthalpy (Δ_H_p) value that is too small to overcome the
large negative entropic change (Δ_S_p) associated with ROP [11,12,13,14]. It is then difficult to obtain a high-molar-mass P_γ_BL via a simple chemical synthesis process. As early as the
1930s to the 1950s, attempts to polymerize _γ_BL were unsuccessful [15, 16]. In the 1960s, oligomers of P_γ_BL were synthesized under extreme reaction conditions (e.g., 20,000 atm, 160 °C)
or through catalysis with a Lewis acid for long reaction times [17]. Thus, ROP of _γ_BL under mild conditions has remained a challenge in polymer synthesis for decades. In the last few
years, there has been a breakthrough in the ROP of _γ_BL, which can now be performed at low temperatures (below the ceiling temperature (_T_c)) and high monomer concentrations to yield
high-molar-mass P_γ_BL with linear or cyclic structures [18,19,20,21,22,23,24]. Ring-opening copolymerization (ROCP) of _γ_BL and other lactones with high ring strain energy (or cyclic
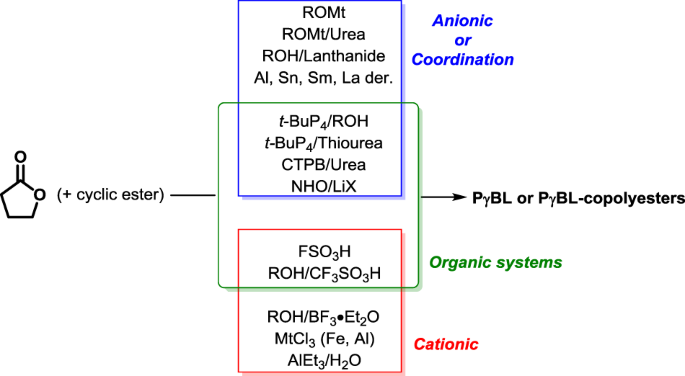
ethers) is another pathway to avoid the “nonpolymerizability” of _γ_BL (Fig. 1). Several examples have been described with different catalysts under varied conditions. In this mini-review,
we thus intend to summarize the studies and results on ROP and ROCP of _γ_BL. The different catalysts described in the literature will be presented, and recent progress will be emphasized. A
variety of metal-based systems and organic compounds are employed as catalysts/initiators in the ROP/ROCP of _γ_BL (Fig. 2), which can be classified as alkali metal bases, other
organometallics, organic bases, Lewis acids and Brønsted acids. Bimolecular catalytic systems consisting of both an acid (or hydrogen-donating) and a base (hydrogen-accepting) have also been
utilized for the synthesis of P_γ_BL. For the systems mentioned above, lanthanides, organic bases and different acid-base pairs have proven to be the most efficient initiator/catalyst
systems for ROP/ROCP of _γ_BL performed at a low temperature and high monomer concentration. RESULTS AND DISCUSSION ALKALI METAL DERIVATIVES AS INITIATORS Low reaction temperatures and high
monomer concentrations are favorable for the ROP of _γ_BL due to the low ceiling temperature (_T_c) of this monomer. The bulk polymerization of _γ_BL was shown to proceed at −40 °C using
potassium or sodium methoxide (MtOMe) with low monomer conversions of up to 25% after a few hours [23]. The use of tetrahydrofuran (THF) as a solvent at −50 °C yielded even worse results, as
only 4% conversion was achieved [25]. PEG-_b_-P_γ_BL block copolymers could also be prepared in low yields starting from a poly(ethylene glycol) macroinitiator deprotonated by NaH, followed
by polymerization of _γ_BL in a dichloromethane/THF (DCM/THF) mixture. This copolymerization actually proceeded at room temperature due to the presence of the second cyclic ester, whose
presence is mandatory to prevent back-biting reactions [26]. Lithium diisopropylamide in dioxane was also shown to copolymerize _ε_-caprolactone (_ε_CL) and _γ_BL at 25 °C with up to 26%
incorporation of the second monomer into the copolymer after ring opening [27]. Adding urea to NaOMe enhanced the reactivity of the active species, making the polymerization feasible in bulk
at −20 °C, with up to 70% monomer conversion in 2 h [23]. Using KOMe with urea in THF at −50 °C was less efficient in terms of monomer conversion [25]. In any case, the urea anion is
believed to activate both the alcohol initiator and _γ_BL before polymerization according to an anionic mechanism (Scheme 1). Such binary catalysts achieved better ROP control and monomer
conversion than alkali metal alkoxides alone. Moreover, alkaline urea-bearing electron-donating groups exhibit a lower activation barrier according to thermodynamic calculations and should
possess a higher reaction efficiency [23]. STANNOUS OR LANTHANIDE COMPOUNDS ASSOCIATED WITH ALCOHOLS Tin(II) octanoate (Sn(Oct)2) in the presence of ethanolamine was used to synthesize
poly(_ε_-caprolactone) including randomly distributed _γ_BL units (16 mol%). It was shown that both the –OH and –NH2 groups of the ethanolamine were linked to the Sn center, forming a new
complex able to perform the copolymerization initiated by those two functional groups (Scheme 2) [27]. This result suggests an anionic ring-opening polymerization process comparable to the
nucleophilic mechanism observed when alkali metal alkoxides are used as initiators. Similarly, ROP of _γ_BL, which employed benzyl alcohol (PhCH2OH, BnOH) as an initiator,
tri[_N_,_N_-bis(trimethylsilyl)amide] lanthanum(Ш) (La[N(SiMe3)2]3) as a catalyst, and a high concentration of _γ_BL, was achieved in THF at −40 °C with a yield of up to 43% in half a day
(Scheme 3) [18]. Linear and cyclic P_γ_BL structures were shown to coexist and to depend on the catalyst/initiator ratio. Intramolecular back-biting occurs in the La–OCH2Ph-initiated ROP,
but to a much lesser extent than in the ROP initiated by La–N(SiMe3)2 alone, which will be discussed in the next section. Effective copolymerization of _γ_BL with _ε_CL and _δ_-valerolactone
(_δ_VL) at low temperatures yielded a series of relatively high-molar-mass copolyesters with high levels of _γ_BL incorporation (8−84%) [28]. The copolyesters exhibited a random structure
with a high content of _γ_BL. ORGANOMETALLIC COMPOUNDS AND LANTHANIDES AS CATALYSTS Organometallic compounds and lanthanides were also proposed without any addition of protic species. A
coordination-insertion mechanism is thus expected. Tentative copolymerization of _γ_BL with glycolide or _β_-propiolactone (_β_PL) was performed using zinc chloride (ZnCl2) or aluminum
isopropoxide (Al(O_i_Pr)3) without real success [29, 30]. Using an aluminum isopropoxide trimer at room temperature, in the 1990s, Penczek and Duda prepared
poly(_ε_-caprolactone-_co_-_γ_-butyrolactone) copolymers with molar masses of up to 30,000 g/mol and containing up to 43 mol% of _γ_BL repeating units. Notably, the molar masses were
controlled by the concentrations of the consumed comonomers and the starting concentration of the aluminum initiator [31,32,33]. A tetraphenyl tin catalyst revealed its efficiency at 140 °C
for preparing poly(_γ_-butyrolactone-_co_-l-lactide) copolyesters with molar masses as high as 70,000 g/mol. The maximum _γ_BL content obtained was approximately 17% [10, 34]. Cyclic tin
alkoxide also showed some activity in the synthesis of _γ_BL-based copolymers with the same limitations [35]. l-Ethoxy-3-chlorotetrabutyldistannoxane was able to copolymerize _γ_BL with
_β_-butyrolactone (_β_BL) at 100 °C in bulk [36]. The amount of _γ_BL in the copolymer is highly dependent on the monomer feed ratio, ranging from 6 to 35% for a _β_BL/_γ_BL molar ratio
ranging from 90/10 to 10/90. The number-average molar mass (\(\overline {M_{\mathrm{n}}}\)) and polymerization yields decreased from 96,000 g/mol and 96% to 2700 g/mol and 13%, respectively,
with the increase in the amount of _γ_BL in the monomer feed (mol%) from 10 to 90%. The samarium(II) aryloxide complex Sm(OAr)2(THF)3 [37] or a samarium iodide-based complex [38] showed
some activity for the ROCP of _γ_BL and _ε_CL at 20 °C. The formation of _ε_CL-_γ_BL copolymers without _γ_BL blocks and limited _γ_BL conversion were observed with this system. Similar
conclusions were drawn when using the aluminum Schiff base complex HAPENAlO_i_Pr with calculated reactivity ratios equal to r_ε_CL = 19.4 and r_γ_BL = 0.11, confirming the presence of a long
sequence of _ε_CL units in the copolymer [27]. The ROP of _γ_BL using La[N(SiMe3)2]3 alone was also shown to synthesize P_γ_BL in a few percent yield when working at high monomer
concentrations and −40 °C (Scheme 4). A typical coordination-insertion mechanism for chain initiation and propagation steps was proposed with the formation of linear and cyclic polymers due
to intramolecular back-biting [18, 28]. ORGANIC BASES AND ACID-BASE PAIRS AS INITIATORS/CATALYSTS Since the description in 2001 of the first nucleophilic organocatalyzed ROP of lactide [39],
many organocatalytic systems have been studied for the ROP of cyclic esters [40]. The phosphazene base
1-_tert_-butyl-4,4,4-tris(dimethylamino)−2,2-bis-[tris(dimethylamino)phosphoranylidenamino]−2_λ_5,4_λ_5-catenadi(phosphazene) (_t_-BuP4) is one such system and has been shown to promote the
metal-free synthesis of P_γ_BL [19]. The polymerization is performed at high monomer concentrations and a low temperature (−40 °C). Two different mechanisms proposed by E. Chen and coworkers
differ in the use of an alcohol as an initiator or not. The superbase can directly initiate the polymerization by deprotonation of _γ_BL to generate a reactive enolate species. However, the
most efficient method is to deprotonate an alcohol such as BnOH by the organic base to obtain high-molar-mass polymers in high yields (Scheme 5). Linear and cyclic P_γ_BL were obtained in
both cases. ROCP of _γ_BL with _ε_CL and _δ_VL following the same approach was also successful [28]. Notably, the organic catalyst system based on _t_-BuP4/BnOH afforded copolyesters with
high _γ_BL incorporation (42−80%) at a relatively low _γ_BL/_ε_CL feed ratio (3 or 4/1). Cyclic trimeric phosphazene base (CTPB, Scheme 6) was also proposed as an organocatalyst for ROP of
_γ_BL [20]. CTPB is thought to be inactive in the absence of any alcohol, whereas it serves as a highly efficient system in the presence of BnOH in toluene at −60 °C to offer well-defined
P_γ_BL with a high monomer conversion (up to 98% in 4 h). The proposed mechanism is similar to the one involving the _t_-BuP4/BnOH initiating system (Scheme 5). The ion pair [BnO−⋅⋅⋅⋅CTPBH+]
can polymerize _γ_BL, and the bulky counterion seems to prevent the back-biting reaction and therefore produce only P_γ_BL with linear structures. In comparison to the alkali metal
alkoxides discussed previously, this system showed a better efficiency, probably due to its improved solubility in the reaction medium. Block copolymers of _γ_BL and L-lactide (LLA) were
prepared with the same phosphazene base via sequential ROP of _γ_BL and LLA [41]. CTPB/urea binary organocatalysts were also studied for the ROP of _γ_BL [25]. Using
1-cyclohexyl-3-(4-methoxyphenyl) urea, which consists of a urea moiety bearing unsymmetrical and electron-donating substituents (Scheme 7), yielded P_γ_BL contained linear and cyclic
structures with \(\overline {M_{\mathrm{n}}}\) up to 35,000 g/mol, four times higher than that produced by CTPB alone. The mechanism involved is similar to that described for alkali metal
alkoxide/urea systems (Scheme 1). A dual organocatalyst based on the combination of _t_-BuP4 and symmetrical thioureas bearing electron-donating groups was also demonstrated as an efficient
organocatalytic system to synthesize linear P_γ_BL with high polymerization rates (up to 80% monomer conversion in 4 h) at low temperatures (Scheme 8) [22]. _N_-Heterocyclic olefins (NHOs)
were also recently used for the polymerization of _γ_BL in bulk at −36 °C. Polyesters with a mixture of cyclic and linear structures were obtained only when an initiator (BnOH) was initially
added, meaning that a zwitterionic polymerization cannot occur [21]. A monomer conversion of 70% could be reached after 2 days, and polymers with \(\overline {M_{\mathrm{n}}}\) up to 7000
g/mol with a dispersity of approximately 1.8 were observed. The addition of a Lewis acid such as lithium halides (LiX) was shown to retard/decrease the back-biting reaction by
transesterification and therefore the formation of macrocycle polymers. Random copolyesters (with _ω_-pentadecalactone (ωPDL), _ε_CL and _δ_VL as comonomers) could also be prepared at low
polymerization temperatures in the presence of an alcohol as initiator with a _γ_BL content in the copolymers reaching 22% [42]. Interestingly, the polymerization could also occur without
any alcohol but only in the presence of a highly nucleophilic NHO, in agreement with a zwitterionic mechanism (Scheme 9) [21]. In the absence of any initiator, one can note that the
enolization of the lactone is favored over nucleophilic ring-opening when sterically hindered but strongly basic NHOs are used. The typical anionic polymerization mechanism observed was
proposed (Scheme 10) [21]. LEWIS ACIDS AND BRØNSTED ACIDS Cationic ROCP of _γ_BL have been far less studied than anionic or coordination-insertion polymerization. Nevertheless, the first
paper dealing with cationic ROP of _γ_BL was published in 1951 [43]. Indeed, Meerwein used tertiary oxonium salts (based on boron Lewis acids) to produce only dimers and trimers. Later,
triethylaluminum associated with water (AlEt3-H2O) revealed an effective catalytic system for the copolymerization of _γ_BL and _β_PL, whereas diethylzinc associated with water (ZnEt2-H2O)
was only able to achieve the homopolymerization of _β_PL [29]. Up to 29.5% of _γ_BL could be incorporated, and the reactivity ratios were determined to be _r__γ_BL = 0.36 and _r__β_PL = 18.
Copolymerization of 3,3-bis(chloromethyl)oxetane (BCMO) and _γ_BL was performed in toluene at room temperature with tin(IV) chloride (SnCl4) and boron trifluoride diethyl etherate (BF3•Et2O)
[44]. Alternating copolymers were produced in low yield. This is the only paper about copolymerization of _γ_BL and cyclic ether, as cyclic lactones are usually used as comonomers. In the
1980s, Kricheldorf showed that iron(III) chloride (FeCl3), aluminum(III) chloride (AlCl3), BF3•Et2O and fluorosulfonic acid (FSO3H) were able to catalyze the random copolymerization of _γ_BL
and GA at 60 °C in bulk with an incorporation of _γ_BL up to 30% with FeCl3 (Scheme 11) [30]. It was also shown that an increase in the amount of _γ_BL in the monomer feed significantly
decreased the polymerization yield. In contrast, ZnCl2, Al(O_i_Pr)3 and dibutyldimethoxytin (_n_Bu2Sn(OMe)2) were only able to achieve the homopolymerization of glycolide. Copolymerization
of _γ_BL and _β_BL was performed with BF3•Et2O, trifluoromethanesulfonic acid (CF3SO3H) or methyl trifluoromethanesulfonate in bulk at room temperature for 7 days, whereas AlCl3 and
antimony(III) fluoride (SbF3) were shown to be ineffective (Scheme 12) [45]. The combination of _γ_BL and _β_BL leads to a random poly(_γ_BL-_co_-_β_BL) (\(\overline {M_{\mathrm{n}}}\) =
1800–4400 g/mol, _Ð_ = 1.3–1.8), whose structure is identical to that of poly(hydroxy alkanoate)s produced by microorganisms. With BF3•Et2O, the incorporation of _γ_BL increases with the
feed ratio (_γ_BL/_β_BL) and reaches 56% at a molar ratio of 90/10 (_γ_BL/_β_BL). The determined reactivity ratios were equal to _r__γ_BL = 0.48 and _r__β_BL = 0.58. It is proposed that
adventitious water contained in the reaction medium served as the initiator by reacting with BF3-activated monomers. Lauryl alcohol was also used as an initiator. CF3SO3H was also shown to
catalyze the ROP of _γ_BL initiated by methanol under high pressure (800–1000 MPa) at 40 °C [46]. The \(\overline {M_{\mathrm{n}}}\) values of obtained the P_γ_BL were in the range of
6000–8000 g/mol (_Ð_ ≈ 1.5). Scandium trifluoromethanesulfonate also catalyzed the homopolymerization of _γ_BL under similar conditions. Copolymers of _γ_BL and _ε_CL were synthesized with
phosphoric acid at 200 °C after 3 days. Semicrystalline copolymers (\(\overline {M_{\mathrm{n}}}\) = 17,800 g/mol) with a melting temperature of 48 °C were obtained [47]. ENZYME-CATALYZED
ROP OF _Γ_BL More than 20 years ago, lipases were also employed as catalysts for the ROP of _γ_BL. For instance, P_γ_BL has been obtained with porcine pancreatic lipase or lipase PS30 from
_Pseudomonas cepacia_ after 18 days at 60 °C with a degree of polymerization (_DP_) of approximately 10 [48]. Copolymers with _ε_CL were also prepared but with a low incorporation of _γ_BL
[49]. More recently, the use of immobilized lipase B from _Candida antarctica_ in ionic liquids has led to the oligomerization of _γ_BL (_DP_ = 5) [50]. MISCELLANEOUS CATALYTIC SYSTEMS
Tin(IV) ion-exchanged montmorillonite has been used for the polymerization of _γ_BL and its copolymerization with _δ_VL at room temperature. For homopolymerization, dimers and trimers were
mainly obtained. For the copolymerization, _DP_ values of approximately 4–6 were obtained with a low incorporation of _γ_BL [51]. The copolymerization of _γ_BL with (di)ethylene glycol
catalyzed by activated clay in xylene under reflux was also studied. Oligomers were obtained and served as precursors for polyurethane synthesis [52]. It was also shown that even in the
absence of any catalyst, it was possible to copolymerize _γ_BL with l-lactic acid or glycolic acid at 200 °C in bulk [53, 54]. The molar masses were below 2500 g/mol for l-lactic acid and
below 5200 g/mol for glycolic acid with 10−20% _γ_BL incorporation in the copolymer. CONCLUSION The ROP of _γ_BL has been considered for a long time to be impossible or hardly possible.
Nevertheless, this review reports the recent progress that allows the control of the ROP process to yield P_γ_BL with a high-molar mass, especially with alkali metal alkoxide/urea systems,
under relatively mild reaction conditions. Anionic and coordination-insertion polymerizations have been studied more than cationic ones. Low reaction temperatures and high initial monomer
concentrations remain compulsory to perform the homopolymerization of _γ_BL. The ROCP of _γ_BL with other cyclic esters has been studied more than its homopolymerization. In this case, it
was shown that _γ_BL could be incorporated even if the polymerization was not performed at low temperature. REFERENCES * Longo JM, Sanford MJ, Coates GW. Ring-opening copolymerization of
epoxides and cyclic anhydrides with discrete metal complexes: structure–property relationships. Chem Rev. 2016;116:15167–97. CAS PubMed Google Scholar * Hillmyer MA, Tolman WB. Aliphatic
polyester block polymers: renewable, degradable, and sustainable. Acc Chem Res. 2014;47:2390–6. CAS PubMed Google Scholar * Schneiderman DK, Hillmyer MA. Aliphatic polyester block polymer
design. Macromolecules. 2016;49:2419–28. CAS Google Scholar * Gonçalves FAMM, Fonseca AC, Domingos M, Gloria A, Serra AC, JFJ Coelho. The potential of unsaturated polyesters in
biomedicine and tissue engineering: synthesis, structure-properties relationships and additive manufacturing. Prog Polym Sci. 2017;68:1–34. Google Scholar * Kamber NE, Jeong W, Waymouth RM,
Pratt RC, Lohmeijer BG, Hedrick JL. Organocatalytic ring-opening polymerization. Chem Rev. 2007;107:5813–40. CAS PubMed Google Scholar * Penczek S, Cypryk M, Duda A, Kubisa P, Slomkowski
S. Living ring-opening polymerizations of heterocyclic monomers. Prog Polym Sci. 2007;32:247–82. CAS Google Scholar * Jerome C, Lecomte P. Recent advances in the synthesis of aliphatic
polyesters by ring-opening polymerization. Adv Drug Deliv Rev. 2008;60:1056–76. CAS PubMed Google Scholar * Hu S, Zhao J, Zhang G, Schlaad H. Macromolecular architectures through
organocatalysis. Prog Polym Sci. 2017;74:34–77. CAS Google Scholar * Moore T, Adhikari R, Gunatillake P. Chemosynthesis of bioresorbable poly(gamma-butyrolactone) by ring-opening
polymerisation: a review. Biomaterials. 2005;26:3771–82. CAS PubMed Google Scholar * Nakayama A, Kawasaki N, Aiba S, Maeda Y, Arvanitoyannis I, Yamamoto N. Synthesis and biodegradability
of novel copolyesters containg γ-butyrolactone units. Polymer. 1998;39:1213–22. CAS Google Scholar * Houk K, Jabbari A, Hall H, Alemán C. Why δ-valerolactone polymerizes and
γ-butyrolactone does not. J Org Chem. 2008;73:2674–8. CAS PubMed Google Scholar * Dubois P, Coulembier O, Raquez J-M. _Handbook of Ring-Opening Polymerization_. 2009. Ch. 1, p. 1–51 *
Aleman C, Betran O, Casanovas J, Houk KN, Hall HK Jr. Thermodynamic control of the polymerizability of five-, six-, and seven-membered lactones. J Org Chem. 2009;74:6237–44. CAS PubMed
Google Scholar * Saiyasombat W, Molloy R, Nicholson TM, Johnson AF, Ward IM, Poshyachinda S. Ring strain and polymerizability of cyclic esters. Polymer. 1998;39:5581–5. CAS Google Scholar
* Carothers WH, Dorough GL, Natta FJv. Studies of polymerization and ring formation. X. The reversible polymerization of six-membered cyclic esters. J Am Chem Soc. 1932;54:761–72. CAS
Google Scholar * Hall HK, Schneider AK. Polymerization of cyclic esters, urethans, ureas and imides. J Am Chem Soc. 1958;80:6409–12. CAS Google Scholar * Korte F, Glet W.
Hochdruckreaktionen. II. Die polymerisation von γ-Butyrolacton und δ-Valerolactam bei hohen drücken. J Polym Sci Part B: Polym Lett. 1966;4:685–9. CAS Google Scholar * Hong M, Chen EY.
Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of gamma-butyrolactone. Nat Chem. 2016;8:42–9. CAS PubMed Google Scholar * Hong M, Chen
EY. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained gamma-butyrolactone. Angew Chem Int Ed Engl. 2016;55:4188–93. CAS PubMed Google
Scholar * Zhao N, Ren C, Li H, Li Y, Liu S, Li Z. Selective ring-opening polymerization of non-strained gamma-butyrolactone catalyzed by a cyclic trimeric phosphazene base. Angew Chem Int
Ed Engl. 2017;56:12987–90. CAS PubMed Google Scholar * Walther P, Frey W, Naumann S. Polarized olefins as enabling (co)catalysts for the polymerization of γ-butyrolactone. Polym Chem.
2018;9:3674–83. CAS Google Scholar * Zhang C-J, Hu L-F, Wu H-L, Cao X-H, Zhang X-H. Dual organocatalysts for highly active and selective synthesis of linear poly(γ-butyrolactone)s with
high molecular weights. Macromolecules. 2018;51:8705–11. CAS Google Scholar * Lin L, Han D, Qin J, Wang S, Xiao M, Sun L, et al. Nonstrained γ-butyrolactone to high-molecular-weight
poly(γ-butyrolactone): facile bulk polymerization using economical ureas/alkoxides. Macromolecules. 2018;51:9317–22. CAS Google Scholar * Olsen P, Odelius K, Albertsson AC. Thermodynamic
presynthetic considerations for ring-opening polymerization. Biomacromolecules. 2016;17:699–709. CAS PubMed PubMed Central Google Scholar * Shen Y, Zhao Z, Li Y, Liu S, Liu F, Li Z. A
facile method to prepare high molecular weight bio-renewable poly(γ-butyrolactone) using a strong base/urea binary synergistic catalytic system. Polym Chem. 2019;10:1231–7. CAS Google
Scholar * Shen Y, Zhang J, Zhao Z, Zhao N, Liu F, Li Z. Preparation of amphiphilic poly(ethylene glycol)- b-poly(gamma-butyrolactone) diblock copolymer via ring opening polymerization
catalyzed by a cyclic trimeric phosphazene base or alkali alkoxide. Biomacromolecules. 2019;20:141–8. CAS PubMed Google Scholar * Bhaw-Luximon A, Jhurry D, Motala-Timol S, Lochee Y.
Polymerization of ɛ-caprolactone and its copolymerization with γ-butyrolactone using metal complexes. Macromol Symposia. 2005;231:60–8. Google Scholar * Hong M, Tang X, Newell BS, Chen EYX.
“Nonstrained” γ-butyrolactone-based copolyesters: copolymerization characteristics and composition-dependent (thermal, eutectic, cocrystallization, and degradation) properties.
Macromolecules. 2017;50:8469–79. CAS Google Scholar * Tada K, Numata Y, Saegusa T, Furukawa J. Copolymerization of γ-butyrolactone and β-propiolactone. Makromol Chem. 1964;77:220–8. CAS
Google Scholar * Kricheldorf HR, Mang T, Jonté JM. Polylactones, 2 copolymerization of glycolide with β-propiolactone, γ-butyrolactone or δ-valerolactone. Makromol Chem. 1985;186:955–76.
CAS Google Scholar * Duda A, Penczek S, Dubois P, Mecerreyes D, Jérôme R. Oligomerization and copolymerization of γ-butyrolactone—a monomer known as unable to homopolymerize, 1.
Copolymerization with ɛ-caprolactone. Macromol Chem Phys. 1996;197:1273–83. CAS Google Scholar * Duda A, Biela T, Libiszowski J, Penczek S, Dubois P, Mecerreyes D, et al. Block and random
copolymers of ε-caprolactone. Polym Degrad Stab. 1998;59:215–22. CAS Google Scholar * Duda A, Libiszowski J, Mosnáček J, Penczek S. Copolymerization of cyclic esters at the living
polymer-monomer equilibrium. Macromol Symposia. 2005;226:109–20. CAS Google Scholar * Nakayama A, Kawasaki N, Arvanitoyannis I, Aiba S, Yamamoto N. Synthesis and biodegradation of
poly(γ-butyrolactone-co-l-lactide). J Environ Polym Degrad. 1996;4:205–11. CAS Google Scholar * Wei Z, Liu L, Qi M. Synthesis and characterization of homo- and co-polymers of
(R,S)-β-butyrolactone and γ-butyrolactone or β-valerolactone initiated with cyclic tin alkoxide. React Funct Polym. 2006;66:1411–9. CAS Google Scholar * Hori Y, Yamaguchi A, Hagiwara T.
Chemical synthesis of high molecular weight poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polymer. 1995;36:4703–5. CAS Google Scholar * Nishiura M, Hou Z, Koizumi T-a, Imamoto T, Wakatsuki
Y. Ring-opening polymerization and copolymerization of lactones by samarium(II) aryloxide complexes. Macromolecules. 1999;32:8245–51. CAS Google Scholar * Agarwal S, Xie X. SmI2/Sm-Based
γ-Buyrolactone−ε-Caprolactone Copolymers: Microstructural Characterization Using One- and Two-Dimensional NMR Spectroscopy. Macromolecules. 2003;36:3545–9. CAS Google Scholar * Nederberg
F, Connor EF, Möller M, Glauser T, Hedrick JL. New paradigms for organic catalysts: the first organocatalytic living polymerization. Angew Chem Int Ed Engl. 2001;40:2712–5. CAS PubMed
Google Scholar * Carlotti S, Peruch F. Cyclic monomers: epoxides, lactide, lactones, lactams, cyclic silicon-containing monomers, cyclic carbonates and others. In: Hadjichristidis N, Hirao,
A, editors. Anionic polymerization: principles, practice, strength, consequences, and applications. Japan: Springer; 2015. p. 191−305. Google Scholar * Shen Y, Zhang J, Zhao N, Liu F, Li
Z. Preparation of biorenewable poly(γ-butyrolactone)-b-poly(l-lactide) diblock copolyesters via one-pot sequential metal-free ring-opening polymerization. Polym Chem. 2018;9:2936–41. CAS
Google Scholar * Walther P, Naumann S. N-Heterocyclic olefin-based (co)polymerization of a challenging monomer: homopolymerization of ω-pentadecalactone and its copolymers with
γ-butyrolactone, δ-valerolactone, and ε-caprolactone. Macromolecules. 2017;50:8406–16. CAS Google Scholar * Meerwein H. Uber oxoniumverbindungen des Säure-ester und lactone. Angew Chem.
1951;63:480–1. Google Scholar * Ito K, Inoue T, Yamashita Y. Copolymerizations of 3.3-bis(chloromethyl)oxacyclobutane with β-propiolactone and γ-butyrolactone by lewis acids: “Two-state“
polymerization mechanism. Makromol Chem. 1970;139:153–64. CAS Google Scholar * Lee CW, Urakawa R, Kimura Y. Copolymerization of γ-butyrolactone and β-butyrolactone. Macromol Chem Phys.
1997;198:1109–20. CAS Google Scholar * Yamashita K, Yamamoto K, Kadokawa J-i. Acid-catalyzed ring-opening polymerization of γ-butyrolactone under high-pressure conditions. Chem Lett.
2014;43:213–5. CAS Google Scholar * Lin WJ. Comparison of thermal characteristics and degradation properties of epsilon-caprolactone copolymers. J Biomed Mater Res. 1999;47:420–3. CAS
PubMed Google Scholar * Nobes GAR, Kazlauskas RJ, Marchessault RH. Lipase-catalyzed ring-opening polymerization of lactones: a novel route to poly(hydroxyalkanoate)s. Macromolecules.
1996;29:4829–33. CAS Google Scholar * Dong H, Wang H-d, Cao S-g, Shen J-c. Lipase-catalyzed polymerization of lactones and linear hydroxyesters. Biotechnol Lett. 1998;20:905–8. CAS Google
Scholar * Gorke JT, Okrasa K, Louwagie A, Kazlauskas RJ, Srienc F. Enzymatic synthesis of poly(hydroxyalkanoates) in ionic liquids. J Biotechnol. 2007;132:306–13. CAS PubMed Google
Scholar * Kadokawa J, Iwasaki Y, Tagaya H. Ring-opening polymerization of lactones catalyzed by ion-exchanged clay montmorillonite. Green Chem. 2002;4:14–6. CAS Google Scholar * Miura H,
Tajima T, Nagata M, Royama T, Saito K, Hasegawa M. Synthesis of poly(ester ether)s by the reaction of .GAMMA.-butyrolactone with diols and their application to polyurthaues. Kobunshi
Ronbunshu. 1999;56:291–7. CAS Google Scholar * Fukuzaki H, Aiba Y, Yoshida M, Asano M, Kumakura M. Direct copolymerization of L-lactic acid with γ-butyrolactone in the absence of
catalysts. Die Makromol Chem. 1989;190:1553–9. CAS Google Scholar * Fukuzaki H, Yoshida M, Asano M, Aiba Y, Kumakura M. Direct copolymerization of glycolic acid with lactones in the
absence of catalysts. Eur Polym J. 1990;26:457–61. CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Univ. Bordeaux, CNRS, Bordeaux INP, LCPO, UMR 5629,
33600, Pessac, France Qilei Song, Frédéric Peruch & Stéphane Carlotti * Faculty of Materials Science and Engineering, South China University of Technology, 510640, Guangzhou, People’s
Republic of China Qilei Song, Junpeng Zhao & Guangzhao Zhang Authors * Qilei Song View author publications You can also search for this author inPubMed Google Scholar * Junpeng Zhao View
author publications You can also search for this author inPubMed Google Scholar * Guangzhao Zhang View author publications You can also search for this author inPubMed Google Scholar *
Frédéric Peruch View author publications You can also search for this author inPubMed Google Scholar * Stéphane Carlotti View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHORS Correspondence to Frédéric Peruch or Stéphane Carlotti. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of
interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Song, Q., Zhao, J., Zhang, G. _et al._ Ring-opening (co)polymerization of _γ_-butyrolactone: a review. _Polym J_ 52,
3–11 (2020). https://doi.org/10.1038/s41428-019-0265-5 Download citation * Received: 02 July 2019 * Revised: 24 August 2019 * Accepted: 25 August 2019 * Published: 25 September 2019 * Issue
Date: January 2020 * DOI: https://doi.org/10.1038/s41428-019-0265-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative